Abstract
Accumulating studies have highlighted the great potential of postbiotics in alleviating diseases and protecting host health. Compared with traditional functional foods (such as probiotics and prebiotics), postbiotics have the advantages of a single composition, high physiological activity, long shelf life, easy absorption, and high targeting, etc. The development of postbiotics has led to a wide range of potential applications in functional food and drug development. However, the lack of clinical trial data, mechanism analyses, safety evaluations, and effective regulatory frameworks has limited the application of postbiotic products. This review describes the definition, classification, sources, and preparation methods of postbiotics, the progress and mechanism of preclinical and clinical research in improving host diseases, and their application in food. Strengthen understanding of the recognition and development of related products to lay a theoretical foundation.
Keywords: Postbiotics, Polysaccharides, Safety evaluation
Graphical abstract

Highlights
-
•
Postbiotics have high physiological activity, and high targeting.
-
•
There are lack clinical trial data, and safety evaluations of postbiotic products.
-
•
More research should be conducted to understand the mechanisms of action in the host.
1. Introduction
The human gut contains many complex microbial communities that cooperate to maintain a dynamic balance and play an important role in maintaining human health. Many studies have demonstrated a strong association between the gut microbiome composition and various diseases. On the one hand, the occurrence of some diseases is often accompanied by changes in the structure of intestinal flora (Abate et al., 2022). Besides, intestinal microbes can affect disease and health via various mechanisms, such as affecting host metabolism and regulating the immune system (Hosomi et al., 2022; Le Roy et al., 2022). These effects may be largely attributed to the composition and associated metabolites. These health-beneficial non-living components are named “postbiotics” and mainly include bacteria-free supernatants, short-chain fatty acids (SCFAs), bacterial lysates, exopolysaccharides, vitamins, enzymes, peptides, etc. (Table 1).
Table 1.
Main types of postbiotics.
| Experiment Type | Source | Type | Model | Disease | Effect | Main Mechanism | References |
|---|---|---|---|---|---|---|---|
| Cell experiment | L. rhamnosus KCTC 12202BP | Protein | DLD-1 cell | Colorectal cancer | It suppresses the growth of colorectal cancer. | The P8 protein in the nucleus binds directly to the intron region of the GSK3β gene, resulting in dysregulation of GSK3β transcription and cell cycle arrest in colorectal cancer cells. | An, Ahn, Kwon, Kwak, Heo, Kim, et al., 2023 |
| Animal experiment | B. animalis subsp. lactis BPL1 | Lipoteichoic acid | Caenorhabditis elegans | Hyperglycaemic conditions | It reduces fat deposition. | Fat deposition is reduced through insulin-like signaling pathways and maintained under hyperglycemic conditions. | Balaguer, Enrique, Llopis, Barrena, Navarro, Álvarez, et al., 2022 |
| Animal experiment | A. muciniphila MucT | Extracellular vesicles and pasteurized cells | C57BL/6 mice | Obesity | It inhibits obesity and its associated inflammatory response. | It inhibits the expression of lipid metabolism-related genes, and downregulates the expression of adipose inflammatory genes. | Ashrafian, Keshavarz Azizi Raftar, Lari, Shahryari, Abdollahiyan, Moradi, et al., 2021 |
| Cell experiment | B. longum, C. butyricum, and L. plantarum WCFS1 | Extracellular vesicles | DC2.4 cells and RAW264.7 cells | Immune dysregulation-related diseases | It promotes the secretion of inflammatory cytokines by immune cells. | It promotes the mRNA expression levels of TNF-α and IL-6. | Morishita, Horita, Higuchi, Marui, Katsumi, & Yamamoto, 2021 |
| Cell experiment | L. paracasei VL8 | Extracellular polysaccharides | RAW264.7 macrophages | Immune dysregulation-related diseases | It modulates immune response. | It exerts an immunomodulatory effect by promoting the phagocytic activity of RAW7.8 cells and increasing their secretion of nitric oxide. | Liu, Mao, Zhang, Chitrakar, Huang, Wang, et al., 2022 |
| Cell experiment | E. coli | Bacteriocin | L. monocytogenes ATCC 15,313 | Bacterial infection | Kill L. monocytogenes | SICs of Bacteriocin can inhibit the formation of biofilm through reduce cell adhesion, exopolypolysaccharide production, quorum sensing, and virulence gene expression. | Qiao, Zhang, Wang, Liu, Shan, Yi, et al., 2022 |
| Cell and animal experiments | - | Short chain fatty acids | Human gastric cancer cell lines and the human gastric epithelial cell line. GPR109A−/−and WT C57BL/6J mice. | Gastric cancer | Gastric cancer | Butyrate enhanced CD8+ T cell cytotoxicity via GPR109A/HOPX, thus inhibiting gastric cancer carcinogenesis. | Yu, Ou, Wang, Li, Ren, Xie, et al., 2024 |
| Cell experiment | L. plantarum S1 | Inanimate microorganisms | Peripheral blood mononuclear cells | Inflammation | Anti-inflammatory and antioxidant | Scavenge DPPH-free radicals, reduce TNF-α levels in immune cells, and DNA damage to immune cells caused by hydrogen peroxide. | Kostelac, Geric, Gajski, & Frece, 2022 |
| Cell experiments | B. longum CECT-7347 | Inanimate microorganisms | Human colonic epithelial cells | Inflammation | Anti-inflammatory and antioxidant | Enhance resistance to H2O2 oxidative stress, inhibit pro-inflammatory cytokines and NF-κB activation. | Martorell, Alvarez, Llopis, Navarro, Ortiz, Gonzalez, et al., 2021 |
| Animal experiment | L. plantarum RG14 | Cell-free supernatant | Post-weaning lambs | Oxidative stress | It enhances antioxidant capacity and protect the intestinal barrier. | The glutathione peroxidase in serum and rumen was increased, and the expressions of liver antioxidase genes and tight junction proteins were up-regulated. | Izuddin, Humam, Loh, Foo, & Samsudin, 2020 |
| Animal experiment | L. acidophilus, L. plantarum, B. lactis and B. breve | Cell-free supernatant | BALB/c mice | Colitis | Antioxidant and immune regulation | The levels of nitric oxide, TNF-α, malondialdehyde, myeloperoxidase, and the expression of NF-κB p65, iNOS, and COX2 in the ileum and colon were down-regulated. | Samer, Toumi, Soufli, & Touil-Boukoffa, 2022 |
Note: L, Lactobacillus; B, Bifidobacterium; C, Clostridium; A, Akkermansia; E, Escherichia; SIC, sub-minimum inhibitory concentrations.
Compared to classic functional foods (such as probiotics and prebiotics), postbiotics show stronger stability and wider applications owing to their inanimate activity that cannot be replicated. Postbiotics can improve intestinal health by enhancing the intestinal epithelial barrier, inhibiting pathogens, and regulating immune responses (Ma et al., 2023), demonstrating their great potential for protecting host health. Therefore, postbiotics can be added to some functional foods instead of probiotics or prebiotics, which are more stable in transport and storage. They are conducive to expanding the market of functional foods. In addition, postbiotics also show great potential in the pharmaceutical industry due to their strong biological activity.
However, the development of intervention strategies for host diseases is often limited by the lack of clarity regarding their occurrence and mechanisms of action. Elucidating the mechanism of postbiotic use in disease intervention is conducive to their clinical application. At present, the application of postbiotics lacks sufficient safety evaluations and clinical data, which limits their application and promotion. This review introduces the main types, sources, and techniques used to prepare and identify postbiotics. Preclinical and clinical data on the role of postbiotics in alleviating disease and their mechanisms are described. The present situation and existing problems in the safety evaluation of postbiotics are summarized to lay a theoretical foundation for the future application of postbiotics.
2. Definition and main sources
2.1. Definition
In recent years, postbiotics as a new concept has gradually attracted people's attention because of its unique advantages. In 2021, the International Scientific Association for Probiotics and Prebiotics (ISAPP) provided a clear definition of postbiotics as preparations of non-living microorganisms and/or their components that confer host health benefits (Salminen et al., 2021). In addition, it proposes the following scope: postbiotics must come from known microorganisms, and undefined microorganisms cannot be used to prepare postbiotics. Postbiotic preparations must satisfy the molecular characteristics of microorganisms of known origin, inanimate, and inactivated processes, and be beneficial to host health. Therefore, postbiotic preparations' composition and safety assessments should be clarified.
2.2. Source
2.2.1. Probiotics
According to the existing literature, postbiotics are mainly derived from certain known microorganisms and their components, such as traditional probiotics represented by Lactobacillus and Bifidobacterium, next-generation probiotics represented by Bacteroides and Akkermansia, and other intestinal microorganisms: 1) Traditional probiotics: Lactobacillus and Bifidobacterium, as important representatives of traditional probiotics, have been widely recognized for their protective effects on host health, and this probiotic effect is largely derived from their bacterial components or metabolites. Lactobacillus can produce a variety of postbiotics, such as exopolysaccharides (EPS) (Wang et al., 2022). In contrast to Lactobacillus, the metabolites of Bifidobacterium show great differences, mainly including unsaturated fatty acids, small molecule neurotransmitters, etc. (Li, Liu, et al., 2022). 2) Next-generation probiotics: The next generation of probiotics, represented by Bacteroides and Akkermansia, are also important sources of postbiotics. Capsular polysaccharides, metabolized hydrolases, and SCFAs of Bacteroides appear to be an important material for their physiological functions. Capsular polysaccharide A (PSA) from Bacteroides fragilis can alleviate abnormal voriconazole metabolism accompanied by the inhibition of toll-like receptor 4 (TLR4)/ nuclear factor kappa-B (NF-κB) pathway (Wang et al., 2021). β-carboline alkaloid harmaline of Akkermansia muciniphila could indirectly modulate the virus-induced inflammatory response via regulating secondary bile acid metabolism (Xie et al., 2023). Butyrate from Faecalibacterium prausnitzii can improve chronic kidney disease via the GPR (G protein-coupled receptor)-4 (Li, Xu, et al., 2022).
According to the existing literature, postbiotics are mainly derived from certain known microorganisms and their components, such as traditional probiotics represented by Lactobacillus and Bifidobacterium, next-generation probiotics represented by Bacteroides and Akkermansia, and other intestinal microorganisms: 1) Traditional probiotics: Lactobacillus and Bifidobacterium, as important representatives of traditional probiotics, have been widely recognized for their protective effects on host health, and this probiotic effect is largely derived from their bacterial components or metabolites. Lactobacillus can produce a variety of postbiotics, such as exopolysaccharides (EPS) (Wang, Xu, Xu, Ma, Zhang, Wang et al., 2022). In contrast to Lactobacillus, the metabolites of Bifidobacterium show great differences, mainly including unsaturated fatty acids, small molecule neurotransmitters, etc. (Li, Liu, et al., 2022). 2) Next-generation probiotics: The next generation of probiotics, represented by Bacteroides and Akkermansia, are also important sources of postbiotics. Capsular polysaccharides, metabolized hydrolases, and SCFAs of Bacteroides appear to be an important material for their physiological functions. Capsular polysaccharide A (PSA) from Bacteroides fragilis can alleviate abnormal voriconazole metabolism accompanied by the inhibition of toll-like receptor 4 (TLR4)/ nuclear factor kappa-B (NF-κB) pathway (Wang et al., 2021). β-carboline alkaloid harmaline of Akkermansia muciniphila could indirectly modulate the virus-induced inflammatory response via regulating secondary bile acid metabolism (Xie et al., 2023). Butyrate from Faecalibacterium prausnitzii can improve chronic kidney disease via the GPR (G protein-coupled receptor)-4 (Li, Xu, et al., 2022).
2.2.2. Others
Other intestinal strains can also produce postbiotics that are beneficial to the host's health. Healthy components can be isolated from grains, fermented foods, and animal feces, and such postbiotic components are derived from the complex microbial composition of the host. The microbiota in kefir grains is mainly composed of lactic acid bacteria, yeasts, and acetic acid bacteria. Exopolysaccharides produced by microorganisms isolated from kefir grains enhance the relative abundance of Bacteroidetes and produce acetate, propionate, and butyrate (Tan et al., 2022).
Notably, the same species of microorganisms also showed significant differences in their postbiotic abilities, which may be due to their molecular level or external environmental influences. For example, EPS116, the EPS of Lactobacillus plantarum NCU116, promotes intestinal homeostasis by regulating intestinal stem cell proliferation and differentiation and altering intestinal microbiota (Zhou et al., 2021). When Momordica charantia L. was used as a nutrient source, L. plantarum NCU116 can reduce obesity by regulating gut microbiota and serum metabolites (Wen et al., 2021). These results deepen the understanding of the differences in metabolites produced by microorganisms, which can vary greatly when the same microorganism uses different nutrients, and highlight the influence of the external environment (especially nutrient elements) on metabolites. Therefore, while studying the source of postbiotics, we should not only pay attention to the microorganisms themselves but also to the external environmental influences on microbial survival.
3. Preparation
The preparation of postbiotics consists of several major steps: culture of microorganisms, cell lysis, centrifugation of lysates, and preservation of the products. The quantity and variety of postbiotic products are largely determined by many factors such as strain type, fermentation medium, and cracking method. Therefore, the postbiotic preparation method affects the collection effect and product type of the postbiotics.
3.1. Extraction
Commonly used methods for cracking microorganisms are heat treatment, enzyme treatment, solvent extraction, and ultrasonic treatment, as well as some emerging technologies such as ohmic heating, pulsed light, high-pressure treatment, cold plasma technology, and supercritical carbon dioxide. After the cells were lysed, the metabolites, cell membranes, cell walls, and other components within the cell were released. To separate the unlyzed cells from the metazoic metabolites, the obtained solution was centrifuged or ultrafiltered. Considering the variety and structure of postbiotics, a suitable extraction method is the key to preparing different postbiotics.
3.1.1. Conventional solvent extraction
Extraction of postbiotics, such as EPS, bacteriocins, and SCFAs, is usually performed using solvents. Streptococcus mutans is one of the main bacteriocins-producing bacteria. Because of defects in the extracellular peptide production and extraction processes, only a few bacteriocins produced by S. mutans have been extracted and purified. Cheng et al. (2020) proposed a method for extracting bacteriocins from S. mutans; chloroform was added to the bacteriocin-containing culture supernatant for extraction and dissolved by centrifugation and filtration with a filter with a low protein-binding membrane. EPS of bacteria is extracted by trichloroacetic acid: After liquefaction of modified phosphoric acid buffer, TCA, KOH, and pre-cooled anhydrous ethanol were gradually added for crude extraction to neutralize filtrate and precipitate EPS (Jivkova et al., 2022). This method can be used to extract EPS from most bacterial strains.
Gu et al. (2021) proposed for the first time to extract SCFAs under alkaline conditions and reduce the volatility of SCFAs by adding NaOH to the sample. SCFAs are then extracted and derived using N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide. Compared with traditional solvent extraction methods of n-hexane or diethyl ether, SCFAs can be extracted and derived without any assistance such as ultrasonication. This method reduces the analysis load and analysis time of SCFAs. Traditional solvent extraction is also used to extract postbiotic components such as menaquinones (Lee et al., 2022).
3.1.2. Ultrasonic-assisted extraction
Ultrasound is a frequency greater than the threshold for human hearing detection, according to the different frequencies, ultrasonic can be divided into low-intensity ultrasonic and high-intensity ultrasonic. Constant high-intensity sound waves destabilize the resulting bubbles, causing them to collapse, and the resulting high temperatures and pressures affect biological materials and tissues (Shen et al., 2023), which produces sufficient energy to break down the cell, facilitating the release of cell contents such as EPS, cell wall fragments, SCFAs, peptides, and vitamins, thereby facilitating the transfer of postbiotic components to the extraction solvent. Ultrasound-assisted extraction is a low-cost, reproducible, simple, and effective method, usually performed under mild conditions without damaging the properties and functions of the bioactive molecules.
3.1.3. Ohmic heating assisted extraction
Ohmic heating refers to the method by which a material generates heat through the resistance generated by the current flow when a voltage is applied. Compared with traditional heat treatment methods, the ohmic heating process converts electrical energy into heat energy, heating faster and more evenly, and causing less thermal damage to the product, which is more conducive to protecting the components in the cell, the electric field during ohmic heating directly or indirectly affects the cell wall, resulting in the exudation of intracellular substances such as amino acids, proteins, nucleic acids, and coenzymes. Ohmic heating has been used to prepare non-living microbial probiotics. For example, the optimal conditions for the preparation of inactivated microorganisms by ohmic heating of Lactobacillus acidophilus LA05, Lacticaseibacillus casei 01, and Bifidobacterium animalis Bb 12, and found that compared with conventional heat treatment, there was less damage to membrane integrity (Barros et al., 2021). As a new technology, ohmic heating is mainly used to inactivation microorganisms through the electroporation effect caused by the thermal effect and electric field on the cell membrane (Kubo et al., 2020). However, ohmic heating can also cause nonthermal damage to cells. Electroporation can be caused by the presence of electric fields during heating, thereby increasing the permeability of cell membranes, promoting cell death, and diffusion of cell contents (Barros et al., 2021; Park & Kang, 2013). Additional effects of current and electrical frequency on microorganisms during ohmic heating need to be clarified to facilitate the optimization of the extraction process.
3.1.4. Supercritical carbon dioxide assisted extraction
Supercritical fluids are promoted as a new, green, and environmentally friendly process that helps solve some of the drawbacks of traditional processes, such as the heavy use of toxic organic solvents and high energy consumption. It causes microbial lysis and death by reducing intracellular pH, modifying cell membranes, and inactivating metabolic enzymes in cells. Bifidobacterium lactis suspensions obtained by supercritical carbon dioxide treatment can increase albumin and creatinine levels and reduce HDL cholesterol levels in Wistar male rats (Almada et al., 2021). Supercritical carbon dioxide can extract bioactive molecules from substrates. After the extraction process was completed, the pressure was released, and the supercritical carbon dioxide became carbon dioxide gas and volatilized, leaving only the extract. However, the polarity of supercritical carbon dioxide is very low. Therefore, when extracting polar or moderately polar compounds, it is necessary to add another solvent to improve polarity (Qamar et al., 2021).
3.2. Identification
Identification is an important step in the preparation of postbiotics. First, to ensure that there were no living cells in the prepared postbiotic products, a negative cell viability test should be performed (Vale et al., 2023). Due to the complexity of the epigenetic structure, its quantitative and qualitative analysis usually needs to be achieved by using some techniques, such as chromatography, Fourier transforms infrared absorption spectrometry, spectrophotometry, nuclear magnetic resonance, and spectroscopy (Moradi et al., 2021).
4. The effect of postbiotics on alleviating disease
Postbiotics are classified according to structure, elemental composition, proteins, vitamins, lipids, organic acids, and different complex molecules (Rafique et al., 2023), including non-living microorganisms, cell-free supernatants, cell wall fragments, SCFAs, vitamins, enzymes, extracellular polysaccharides, various peptides, amino acids, and fermentation by-products, etc.
4.1. SCFAs
SCFAs are defined as a group of fatty acids with fewer than six carbons, mainly formic acid, acetic acid, propionic acid, butyric acid, and valeric acid, etc., are volatile fatty acid metabolites produced by the anaerobic microbial fermentation of dietary fiber that host enzymes cannot digest in the cecum and colon. The production of SCFAs is related to the properties and structure of dietary fibers (Fattahi et al., 2020). SCFAs play important roles in regulating the gut microbiome, energy metabolism, diet, and weight control.
As the most widely studied postbiotic, SCFAs exert their physiological functions in a variety of ways (Fig. 1): 1) An energy source for cells. SCFAs are absorbed by cells (Silva et al., 2020), most of which are used as their energy source. 2) Immune regulation. SCFAs induce autophagy apoptosis through a variety of pathways: sodium butyrate could activate endoplasmic reticulum stress-mediated apoptotic pathway in K562/ADR cells (Jia et al., 2019). Another study showed that butyrate enhanced the efficacy of the cancer chemotherapy drug oxaliplatin by modulating CD8+ T cell function via the IL-12 signaling pathway (He et al., 2021). 3) Protect the intestinal barrier. SCFAs improve host intestinal barrier function by reducing intestinal permeability and promoting the expression of tight junction proteins claudin, occludin, and ZO-related genes in the intestine (Ma et al., 2022). Butyrate recovers epithelial barrier function under inflammatory conditions by activating GPR41/FFA3 and GPR109a/HCA2 to induce independent IgA secretion by T cells in the colon (Isobe et al., 2020).
Fig. 1.
Mechanism of short-chain fatty acids to protect human health and related diseases.
Butyrate and propionate alleviate inflammation by reducing the pro-inflammatory factors secreted by mature dendritic cells (Nastasi et al., 2015). SCFAs promote the secretion of GLP-1 and PYY by binding to GPR41 and GPR43 (Holst, 2007). Propionate can act as a substrate for gluconeogenic reactions and promote leptin secretion, thereby increasing satiety and reducing appetite. GLP-1 promotes insulin secretion by the pancreas to improve blood sugar levels (Cani et al., 2013). Butyrate is the main energy source for colon cells and improves cardiac function after myocardial infarction by promoting the polarization of M2 macrophages; inhibiting the expression of NE, NGF, and pro-inflammatory factors; and promoting the secretion of anti-inflammatory factors (Jiang et al., 2020). SCFAs, such as butyrate, improve the intestinal epithelial barrier in a variety of ways by activating GPR41 and GPR109A to promote T-cell secretion of lgA to restore the epithelial barrier under inflammatory conditions (Isobe et al., 2020). Acetate coordinates neutrophils and ILC3s through FFAR2 to restore the intestinal damage caused by C. difficile (Fachi et al., 2020). Propionate inhibits the accumulation of body fat and liver fat by activating PPARα and related protein genes through the AMPK pathway (Yang et al., 2023).
Note: GLP-1, glucagon-like peptide-1; PYY, peptide tyrosine tyrosine; GPR41, G protein-coupled receptor 41; GPR43, G protein-coupled receptor 43; NE, norephinephrine; NGF, nerve growth factor; IgA, SIgA; FFAR2, free fatty acid receptor 2; C. difficile, Clostridium difficile.
4.2. Bacteriocin
Bacteriocins are bioactive antimicrobial peptides capable of killing or inhibiting gram-negative and Gram-positive pathogens (Kumariya et al., 2019). Bacteriocins can be divided into three categories. Class I bacteriocins are small heat-stable peptides with high posttranslational modification. Class II bacteriocins include small peptides heat-stable, heat-labile proteins with a high molecular weight are classified as class III bacteriocins (Negash & Tsehai, 2020). They can inhibit or kill pathogens in different ways. Class II bacteriocin BM1829 inhibits the growth of Escherichia coli and Staphylococcus aureus by disrupting the integrity of cell membranes and inducing cell cycle stagnation in the R phase (Yan et al., 2021). Class III bacteriocins Y19–2 inhibit the growth of pathogens by destroying bacterial cell walls and affecting the synthesis of bacterial nucleic acids (Fu et al., 2022).
The physiological functions of bacteriocins include the following: 1) Inhibit the growth of pathogenic bacteria. Bacteriotin produced by Pediococcus acidilactici HW01 can inhibit biofilm formation of Pseudomonas aeruginosa and virulence factors, such as pyocyanin, protease, and rhamnolipid, to exert an antibacterial effect (Lee et al., 2020). The novel bactericin acidicin P from P. acidilactici LAC5–17 can inhibit Listeria monocytogenes by inducing severe penetration and depolarization of the cell plasma membrane and causing significant changes in the cell morphology and ultrastructure of Listeria monocytogenes (Xia et al., 2023). 2) Alleviating of intestinal diseases. Bacteriocins have a positive role in alleviating colorectal cancer, plantaricin JLA-9, plantaricin W, lactococcin A, and lactococcin MMFII, can reduce inflammation associated with colorectal cancer by interacting with the COX2 protein, which promotes colorectal cancer, and by regulating the NLRP3 and NF-kB pathways of the inflammasome complex (Patra et al., 2022). 3) Metabolic regulation. Bacteriocin PJ4, produced by Lactobacillus helveticus PJ4, ameliorates obesity by reducing adipose tissue weight and normalizing levels of IL-6, TNF-a, resistin, and adiponectin (Bai et al., 2020). In summary, bacteriocins are ideal substitutes for antibiotics because of their strong antibacterial activity and positive roles in various inflammatory diseases. Class II bacteriocins have great potential for alleviating obesity and colorectal cancer. However, there are relatively few studies on class III bacteriocins.
4.3. Eps
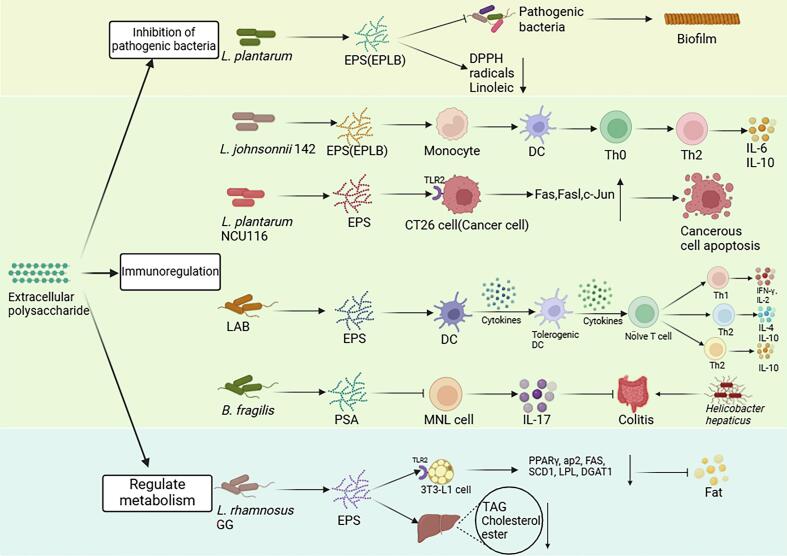
EPS are high molecular weight extracellular carbohydrate polymers secreted by microorganisms, which are generally divided into homopolysaccharides and heteropolysaccharides, and polysaccharides are branched or unbranched, consisting of a single glucose or fructose, and are divided into α-D-glucan, β-D-glucan, fructan, and polygalactose. EPS has been found to have beneficial anti-inflammatory, immunomodulatory, and antimicrobial effects in humans (Abdalla et al., 2021; Angelin & Kavitha, 2020) (Fig. 2): 1) Inhibit the proliferation of pathogenic bacteria. The EPS produced by microorganisms can fight pathogens by impeding biofilm formation. EPS produced by Enterococcus durans DU1 showed antibiofilm activity against Yersinia enterocolitica, S. aureus, and Bacillus cereus (Soliemani et al., 2022). The EPS of L. plantarum 12 also exerts antibacterial effects by inhibiting the biofilm formation of Shigella flexneri (Song et al., 2020). 2) Immune regulation. EPS produced by Lactobacillus pentosus LZ-R-17 plays an immunomodulatory role by enhancing the activity of RAW264.7 cells and macrophages, enhancing phagocytosis and the secretion of TNF-α, Il-1β, IL-6, and IL-10 (You et al., 2020). EPS derived from Lactobacillus pantheris TCP102 can significantly induce Ana-1 cells and peritoneal macrophages to produce nitric oxide, TNF-α, and IL-6, inhibit the proliferation of HCT-116, BCG-803, and A-2780 cells, and thus exert their immune enhancement function (Sheng et al., 2022). 3) Regulate of metabolism. EPS from L. plantarum L-14 inhibited adipogenesis through TLR2 and AMPK signaling pathways (Lee et al., 2021).
Fig. 2.
Mechanism of exopolysaccharides (EPS) to protect human health and related diseases.
EPS secreted by microorganisms plays an active role in the inhibition of pathogenic bacteria, immune regulation, and metabolic regulation through various mechanisms. EPS produced by LAB plays an immunomodulatory role by promoting the differentiation of immune cells and altering intestinal microbiota (Zhou et al., 2021). The EPS from L. plantarum can inhibit biofilm formation by pathogenic bacteria, thereby inhibiting bacterial proliferation (Mahdhi et al., 2017). L. plantarum-derived EPS induces apoptosis in colon cancer cells by upregulating TLR2 and promoting the expression of cancer cell apoptosis genes (Zhou et al., 2017). B. fragilis-derived PSA prevents Helicobacter hepaticus-induced colitis by inhibiting IL-17 secretion (Mazmanian et al., 2008). EPS from LGG relies on TLR2 to inhibit fat production and reduce liver triacylglycerol and cholesterol ester levels (Zhang et al., 2016).
Note: L. plantarum, Lactobacillus plantarum; LAB, Lactic acid bacteria; TLR2, Toll-like receptor-2; B. fragilis, Bacteroides fragilis; PSA, polysaccharide A; LGG, Lactobacillus rhamnosus GG.
4.4. Peptidoglycan
Peptidoglycan is an important component of bacterial cell walls. Peptidoglycans typically consist of variable-length glycan chains that are cross-linked via oligopeptide bridges to give cells the ability to respond to environmental stress. During bacterial growth and death, peptidoglycans are continuously synthesized, remodeled, and repaired to adapt to cell division and growth (Galinier et al., 2023; Weaver et al., 2023). As new peptidoglycans are continuously synthesized, old peptidoglycans are shed into the environment (Galinier et al., 2023) and have positive effects on host health: 1) Immunoregulation. Peptidoglycans can be modulated in a variety of ways, such as stimulating different cytokine release and Th1/ Th2-led immune responses during humoral or cellular immune responses to induce specific immunity, and PGN recognition proteins, nucleotide oligomeric domain-like receptors, C-type lectin receptors, and toll-like receptors to activate non-specific immune regulation (Sun et al., 2022). Muramyl dipeptide, a peptidoglycan, induces tolerant dendritic cells the generation of cytokine GM-CSF to regulate immunological tolerance (Prescott et al., 2020). 2) Anti-inflammatory. Peptidoglycans exert their anti-inflammatory effects mainly by influencing the expression of pro-inflammatory cytokines. Lactobacillus rhamnosus CRL1505 and its peptidoglycan can enhance resistance to Streptococcus pneumoniae infection by normalizing serum levels of TNF-α, IL-1β, and IL-6 (Salva et al., 2021).
4.5. Lipoteichoic acid
Lipoteichoic acid is an important component of Gram-positive cell walls, which exerts its anti-inflammatory effects primarily by modulating immune response. Lipoteichoic acid of L. plantarum can promote TNF-α production and induce the phosphorylation of NF-κB-p65, p-38, and JNK (Jung et al., 2022). Lipoteichoic acid from L. paracasei D2–5 has been shown to alleviate metabolic and cognitive impairment in obese elderly mice fed high fat by stimulating Muc2 expression by activating the toll-like receptor 2/p2-MAPK (TLR-38/p2-MAPK)/NF-κB pathway (Wang et al., 2020). Lipoteichoic acid produced by Bifidobacterium animalis subsp. lactis BPL1 has shown therapeutic potential in metabolic syndromes, such as obesity and diabetes, by reducing fat deposition via an insulin-like signaling pathway (IGF-1) (Balaguer et al., 2022).
4.6. Inanimate microorganism
Non-living microorganisms can be obtained by a variety of methods, such as acid treatment, heat treatment, ultrasonic treatment, freezing treatment, and ultraviolet irradiation, but the most common method is heating treatment. Inanimate microorganisms have been shown to have numerous health benefits for their hosts, including anti-inflammatory, anti-cancer, and metabolic disease relief. 1) Anti-inflammation. Inactivated L. plantarum S1 exhibits free radical scavenging activity and exerts anti-inflammatory and antioxidant effects by reducing levels of TNF-α in immune cells and DNA damage to immune cells caused by hydrogen peroxide (Kostelac et al., 2022). 2) Anti-cancer. A recent study showed that the heat-suppressing Lactobacillus brevis KU15176 can exert anti-proliferation effects on human gastric adenocarcinoma gastric cancer cell lines by regulating the expression of apoptosis-related genes such as Bax, Caspase-3, and Caspase-9, and increasing the effective apoptosis rate and caspase activity (Hwang et al., 2022). 3) Neuroprotective effect. Studies have shown that heat-killed Lactococcus lactis KC24 can exert neuroprotective effects by promoting the expression of brain-derived neurofactor (BDNF) in HT-29 cells and BDNF expression in SH-SY5Y cells under oxidative stress, as well as reducing the Bax/Bcl-2 ratio associated with apoptosis (Lim et al., 2020).
4.7. Cell-free supernatant
The cell-free supernatant of microorganisms refers to a collection of bioactive metabolites secreted by microorganisms. Cell-free supernatants from a variety of microorganisms have been shown to have antioxidant, antibacterial, antitumor, and metabolic disease-relieving effects. 1) Anti-inflammation. Cell-free supernatants from probiotics can alleviate colitis by down-regulating the levels of nitric oxide, TNF-α, malonaldehyde, and myeloperoxidase, as well as the expression of NF-κB p65, iNOS, and COX2 in the ileum and colon (Samer et al., 2022). 2) Antibacterial. The cell-free supernatant of L. gasseri GM18 can improve the efficacy of Cronobacter sakazakii and Listeria monocytogenes infections by disrupting or preventing biofilm formation (Singh et al., 2020). 3) Anti-tumor. The cell-free supernatants of L. paracasei SD1 and L. rhamnosus SD11 exerted anti-colorectal cancer activity by inhibiting the expression of pro-inflammatory factors in Caco-2 cells and the growth of Caco-2 cells (Pahumunto & Teanpaisan, 2023). 4) Relieve metabolic diseases. Cell-free supernatants of B. bifidum DS0908 and B. longum DS0950 ameliorated obesity by promoting thermoproduction and improving lipid profiles, insulin sensitivity, and glucose metabolism without altering food intake (Rahman et al., 2023).
4.8. Others
Other postbiotic components such as extracellular vesicles, surface proteins, vitamins, enzymes, and amino acids play beneficial roles in host health. Extracellular vesicles are lipid bilayers derived from cell membranes and contain polysaccharides, proteins, nucleic acids, and enzymes. Extracellular vesicles of LGG exert anti-colorectal cancer activity by inducing carcinoembryonic antigen gene expression and protein synthesis and inhibiting the proliferation of HT-29 and SW-480 cells (Keyhani et al., 2022).
Recent studies have shown that probiotic-derived proteins can be used as anticancer biotherapeutics. The probiotics-derived P8 protein is transported to the nucleus by KPNA3 and insertin and binds to the GSK3β intron, disrupting its transcription. Secondly, cytoplasmic P8 binds specifically to GSK3β, preventing its inactivation by the protein kinase AKT/CK1ε/PKA, thereby inducing cell cycle arrest in colorectal cancer cells (An et al., 2023). The Pediococcus pentosaceus-derived P8 protein is safe to consume orally in rodent and marmoset models (An et al., 2021). Vitamin D is an essential substance for maintaining human life activities, and the host lacks its synthesis pathway; therefore, it must be supplemented through an exogenous diet. Some strains have been shown to synthesize vitamins. Folic acids are antioxidants that play important roles in DNA synthesis, methylation, and repair (Żółkiewicz et al., 2020). Treating HFD-fed and STZ-induced T2DM mice with yogurt containing fermented GABA-producing Streptococcus thermophilus improved glucose tolerance and insulin sensitivity (Li et al., 2020).
5. Safety evaluation
Safety evaluation is an important prerequisite for the development of functional food. Clinical trials have shown that fermented foods containing postbiotics are beneficial to human health and do not adversely affect the host: A double-blind controlled trial randomly divided 215 fully IF-fed infants under 14 days of age into two groups: milk powder containing 26% fermented formula containing postbiotics extracted during the Lactobacillus fermentation process or control group until 17 weeks of age. There are no statistical differences in adverse events or gastrointestinal tolerances between the randomised infant formula groups, concluding that partially fermented IF with postbiotics is safe and well tolerated in healthy full-term infants (Vandenplas et al., 2020). The results of a systematic review and meta-analysis showed that the addition of postbiotics to infant formula could increase the concentration of stool SIgA with no adverse reactions (Liang et al., 2024). These clinical trials have shown that postbiotics can improve human health without negatively affecting the host, demonstrating the potential value of postbiotics in treating human diseases and maintaining human health.
However, the existing safety evaluation data mainly focus on a few types such as inactivated probiotics, and the research on other postbiotics components is still scarce. Future research should focus on conducting clinical studies on more types of postbiotics to demonstrate the beneficial effects of postbiotics in improving host health, and to evaluate the safety of postbiotics to lay the foundation for the development of functional foods.
6. The application in food
At present, researchers are not only concerned about its effects on host health, but also about its application prospects in food. Yeasts can provide a variety of beneficial postbiotics such as polyamines, acetic acids, cinnamic acids, proteases, bioactive proteins, B vitamins, and phenolic compounds (Chan & Liu, 2022), some of which can significantly improve the bioavailability of nutrients. Purified phytase from Bifidobacterium longum spp. infantis and Bifdobacterium pseudocatenulatum could reduce phytate content and raise inositol triphosphate levels in cereal combinations (Tomasik & Tomasik, 2020). Shafipour Yordshahi et al. (2020) developed an antimicrobial packaging nanopaper for minced meat by incorporating lyophilised powder of cell-free supernatant of L. plantarum ATCC 14917 into bacterial nanofibrillated cellulose, which was able to effectively inhibit Listeria monocytogenes in minced meat without affecting the organoleptic properties of the meat.
The use of postbiotics to prevent microbial spoilage and extend the shelf life of food. The combination of lyophilized and inactivated P. acidilactici (6%) and EDTA (0.02 M) inhibited psychrotrophs, mold-yeast, and Pseudomonas spp. over the storage (Incili et al., 2023). Lactarius volemus Fr. polysaccharide extract can significantly increase the production of essential amino acids in yogurt and the water retention capacity of lactic acid bacteria, shorten the fermentation period of yogurt, and extend the shelf life of yogurt (Huang et al., 2020). Organic acids, bacteriocins, EPS, and other postbiotics extracted from various probiotics have been proven to inhibit the growth of pathogens and spoilage microorganisms, which have been used for preservation and preservative of dairy products, meat, poultry, seafood, fruits, and vegetables (Sharafi et al., 2023). In summary, food as a medium for the production of postbiotics is a field full of opportunities. Notably, there are great challenges in industrial production. Postbiotics not only inhibit pathogenic microorganisms but also show strong inhibitory activity against spoilage microorganisms, which provides another idea for food production and preservation.
7. Prospects
Because of their inanimate nature, postbiotics present less risk of use, and their preparation, storage, and transportation are also less challenging; therefore, their prospects of use are broader. Currently, it is used in food production, preservation, and functional food development. However, the lack of a complete regulatory framework and sufficient safety evaluation data also limits the development and application of postbiotics: 1) Some clinical trials have proved that postbiotics are well tolerated in healthy people, but some people with compromised immune systems should avoid using postbiotics to avoid adverse reactions. 2) Many clinical trials have demonstrated the positive impact of inanimate microorganisms on the host, and there is still a large gap in clinical research on microbial metabolites and cell components. 3) The results of some clinical trials need to be carefully evaluated because the products used contain prebiotics, and their functional composition is unclear. Therefore, to promote the development of postbiotics, future studies should focus on 1) Reasonable animal models or clinical trials to explore the effects of postbiotics in immunodeficient populations. 2) More research should be conducted to understand the mechanisms of action in the host. 3) Future studies should be conducted to determine the safe dose range for postbiotic use to enhance safety evaluations.
Funding statement
This work was supported by the Fundamental Research Funds for the Central Universities (SWU-KQ22050 and SWU-KQ22076).
CRediT authorship contribution statement
Li Wei: Writing – original draft, Investigation. Botao Wang: Writing – original draft, Investigation. Junying Bai: Visualization, Investigation. Yuyan Zhang: Visualization, Investigation. Cuiping Liu: Writing – review & editing, Supervision. Huayi Suo: Writing – review & editing, Supervision. Chen Wang: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Abate M., Vos E., Gonen M., Janjigian Y.Y., Schattner M., Laszkowska M.…Strong V.E. A novel microbiome signature in gastric cancer: A two independent cohort retrospective analysis. Annals of Surgery. 2022;276(4):605–615. doi: 10.1097/SLA.0000000000005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdalla A.K., Ayyash M.M., Olaimat A.N., Osaili T.M., Al-Nabulsi A.A., Shah N.P., Holley R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Food Microbiology. 2021;12 doi: 10.3389/fmicb.2021.664395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almada C.N., Almada-Erix C.N., Roquetto A.R., Santos-Junior V.A., Cabral L., Noronha M.F.…Sant’Ana A.S. Paraprobiotics obtained by six different inactivation processes: Impacts on the biochemical parameters and intestinal microbiota of Wistar male rats. International Journal of Food Sciences and Nutrition. 2021;72(8):1057–1070. doi: 10.1080/09637486.2021.1906211. [DOI] [PubMed] [Google Scholar]
- An B.C., Ahn J.Y., Kwon D., Kwak S.H., Heo J.Y., Kim S.…Chung M.J. Anti-cancer roles of probiotic-derived P8 protein in colorectal cancer cell line DLD-1. International Journal of Molecular Sciences. 2023;24(12):9857. doi: 10.3390/ijms24129857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B.C., Yoon Y.S., Park H.J., Park S., Kim T.Y., Ahn J.Y.…Chung M.J. Toxicological evaluation of a probiotic-based delivery system for P8 protein as an anti-colorectal cancer drug. Drug Design, Development and Therapy. 2021;15:4761–4793. doi: 10.2147/DDDT.S319930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin J., Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. International Journal of Biological Macromolecules. 2020;162:853–865. doi: 10.1016/j.ijbiomac.2020.06.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian F., Keshavarz Azizi Raftar S., Lari A., Shahryari A., Abdollahiyan S., Moradi H.R.…Siadat S.D. Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microbial Cell Factories. 2021;20(1):219. doi: 10.1186/s12934-021-01709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Kumar S., Verma S., Seshadri S. Bacteriocin PJ4 from probiotic Lactobacillus reduced adipokine and inflammasome in high fat diet induced obesity. 3. Biotech. 2020;10(8):355. doi: 10.1007/s13205-020-02317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer F., Enrique M., Llopis S., Barrena M., Navarro V., Álvarez B., Chenoll E., Ramón D., Tortajada M., Martorell P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microbial Biotechnology. 2022;15(3):805–816. doi: 10.1111/1751-7915.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros C.P., Pires R.P.S., Guimaraes J.T., Abud Y.K.D., Almada C.N., Pimentel T.C.…Cruz A.G. Ohmic heating as a method of obtaining paraprobiotics: Impacts on cell structure and viability by flow cytometry. Food Research International. 2021;140 doi: 10.1016/j.foodres.2020.110061. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Everard A., Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Current Opinion in Pharmacology. 2013;13(6):935–940. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Chan M.Z.A., Liu S.-Q. Fortifying foods with synbiotic and postbiotic preparations of the probiotic yeast, Saccharomyces boulardii. Current Opinion in Food Science. 2022;43:216–224. [Google Scholar]
- Cheng M., Gong S.-G., Levesque C.M. Rapid isolation and purification of secreted Bacteriocins from Streptococcus mutans and other lactic acid bacteria. Bio-Protocol. 2020;10(22) doi: 10.21769/BioProtoc.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachi J.L., Sécca C., Rodrigues P.B., Mato F.C.P.D., Di Luccia B., Felipe J.D.S.…Sampaio U. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. Journal of Experimental Medicine. 2020;217(3) doi: 10.1084/jem.20190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi Y., Heidari H.R., Khosroushahi A.Y. Review of short-chain fatty acids effects on the immune system and cancer. Food Bioscience. 2020;38 [Google Scholar]
- Fu Y., Zhao D., Wang L., Jiang G., Liu X. A broad-spectrum novel bacteriocin produced by Lactobacillus sakei in Nanjing steamed roast duck: Purification, antimicrobial characteristics, and antibacterial mechanisms. Food Bioscience. 2022;50 [Google Scholar]
- Galinier A., Delan-Forino C., Foulquier E., Lakhal H., Pompeo F. Recent advances in peptidoglycan synthesis and regulation in Bacteria. Biomolecules. 2023;13(5):720. doi: 10.3390/biom13050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Jasbi P., Patterson J., Jin Y. Enhanced detection of short-chain fatty acids using gas chromatography mass spectrometry. Current Protocols. 2021;1(6):e177. doi: 10.1002/cpz1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Fu L., Li Y., Wang W., Gong M., Zhang J.…Guo X. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metabolism. 2021;33(5):988–+. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- Holst J.J. The physiology of glucagon-like peptide 1. Physiological Reviews. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Hosomi K., Saito M., Park J., Murakami H., Shibata N., Ando M., Nagatake T., Konishi K., Ohno H., Tanisawa K., Mohsen A., Chen Y.-A., Kawashima H., Natsume-Kitatani Y., Oka Y., Shimizu H., Furuta M., Tojima Y., Sawane K.…Kunisawa J. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nature Communications. 2022;13(1):4477. doi: 10.1038/s41467-022-32015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhao S., Yao K., Liu D., Peng X., Huang J., Huang Y., Li L. Physicochemical, microbiological, rheological, and sensory properties of yoghurts with new polysaccharide extracts from Lactarius volemus Fr. Using three probiotics. International Journal of Dairy Technology. 2020;73(1):168–181. [Google Scholar]
- Hwang C.H., Lee N.K., Paik H.D. The anti-cancer potential of heat-killed Lactobacillus brevis KU15176 upon AGS cell lines through intrinsic apoptosis pathway. International Journal of Molecular Sciences. 2022;23(8):4073. doi: 10.3390/ijms23084073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incili G.K., Akgol M., Karatepe P., Kanmaz H., Kaya B., Tekin A., Hayaloglu A.A. Inhibitory effect of bioactive compounds derived from freeze-dried paraprobiotic of Pediococcus acidilactici against food-borne pathogens: In-vitro and food model studies. Food Research International. 2023;170 doi: 10.1016/j.foodres.2023.113045. [DOI] [PubMed] [Google Scholar]
- Isobe J., Maeda S., Obata Y., Iizuka K., Nakamura Y., Fujimura Y., Kimizuka T., Hattori K., Kim Y.-G., Morita T., Kimura I., Offermanns S., Adachi T., Nakao A., Kiyono H., Takahashi D., Hase K. Commensal-bacteria-derived butyrate promotes the T-cell-independent IgA response in the colon. International Immunology. 2020;32(4):243–258. doi: 10.1093/intimm/dxz078. [DOI] [PubMed] [Google Scholar]
- Izuddin W.I., Humam A.M., Loh T.C., Foo H.L., Samsudin A.A. Dietary postbiotic Lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants (Basel) 2020;9(3):250. doi: 10.3390/antiox9030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Zheng Y., Guo Y., Chen K. Sodium butyrate and panobinostat induce apoptosis of chronic myeloid leukemia cells via multiple pathways. Molecular Genetics & Genomic Medicine. 2019;7(5) doi: 10.1002/mgg3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Huang X., Tong Y., Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Canadian Journal of Physiology and Pharmacology. 2020;98(6):391–399. doi: 10.1139/cjpp-2019-0531. [DOI] [PubMed] [Google Scholar]
- Jivkova D., Sathiyanarayanan G., Harir M., Hertkorn N., Schmitt-Kopplin P., Sanhaji G., Fochesato S., Berthomieu C., Heyraud A., Achouak W., Santaella C., Heulin T. Production and characterization of a novel exopolysaccharide from Ramlibacter tataouinensis. Molecules. 2022;27(21):7172. doi: 10.3390/molecules27217172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B.-J., Kim H., Chung D.-K. Differential immunostimulatory effects of lipoteichoic acids isolated from four strains of Lactiplantibacillus plantarum. Applied Sciences-Basel. 2022;12(3):954. [Google Scholar]
- Keyhani G., Mahmoodzadeh Hosseini H., Salimi A. Effect of extracellular vesicles of Lactobacillus rhamnosus GG on the expression of CEA gene and protein released by colorectal cancer cells. Iranian Journal of Microbiology. 2022;14(1):90–96. doi: 10.18502/ijm.v14i1.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostelac D., Geric M., Gajski G., Frece J. Probiotic and paraprobiotic derivates exhibit anti-inflammatory and genoprotective effects during induced stress. Journal of Applied Microbiology. 2022;133(2):819–829. doi: 10.1111/jam.15595. [DOI] [PubMed] [Google Scholar]
- Kubo M.T., Siguemoto É.S., Funcia E.S., Augusto P.E., Curet S., Boillereaux L., Sastry S.K., Gut J.A. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Current Opinion in Food Science. 2020;35:36–48. [Google Scholar]
- Kumariya R., Garsa A.K., Rajput Y.S., Sood S.K., Akhtar N., Patel S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microbial Pathogenesis. 2019;128:171–177. doi: 10.1016/j.micpath.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Le Roy T., de Hase E.M., Van Hul M., Paquot A., Pelicaen R., Regnier M.…Cani P.D. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut. 2022;71(3):534–543. doi: 10.1136/gutjnl-2020-323778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.-H., Kim B.S., Kang S.-S. Bacteriocin of Pediococcus acidilactici HW01 inhibits biofilm formation and virulence factor production by Pseudomonas aeruginosa. Probiotics and Antimicrobial Proteins. 2020;12(1):73–81. doi: 10.1007/s12602-019-09623-9. [DOI] [PubMed] [Google Scholar]
- Lee J., Park S., Oh N., Park J., Kwon M., Seo J., Roh S. Oral intake of Lactobacillus plantarum L-14 extract alleviates TLR2- and AMPK-mediated obesity-associated disorders in high-fat-diet-induced obese C57BL/6J mice. Cell Proliferation. 2021;54(6) doi: 10.1111/cpr.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Hu X., Stuckey D.C. Optimised “green solvent” extraction of long-chain menaquinones (vitamin K2) from wet Lactococcus lactis biomass. Separation and Purification Technology. 2022;287 [Google Scholar]
- Li B., Liu K., Kwok L.-Y., Guo S., Bai L., Yang X., Chen Y. Development of a non-target metabolomics-based screening method for elucidating metabolic and probiotic potential of bifidobacteria. Innovative Food Science & Emerging Technologies. 2022;77 [Google Scholar]
- Li H.B., Xu M.L., Xu X.D., Tang Y.Y., Jiang H.L., Li L.…Yang T. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circulation Research. 2022;131(9):e120–e134. doi: 10.1161/CIRCRESAHA.122.320184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Chen L., Zhu X., Lu Z., Lu Y. Effect of γ-aminobutyric acid-rich yogurt on insulin sensitivity in a mouse model of type 2 diabetes mellitus. Journal of Dairy Science. 2020;103(9):7719–7729. doi: 10.3168/jds.2019-17757. [DOI] [PubMed] [Google Scholar]
- Liang X., Li Y., Zhao Z., Ding R., Sun J., Chi C. Safety and efficacy of adding postbiotics in infant formula: A systematic review and meta-analysis. Pediatric Research. 2024;95(1):43–51. doi: 10.1038/s41390-023-02813-w. [DOI] [PubMed] [Google Scholar]
- Lim S.M., Lee N.K., Paik H.D. Potential neuroprotective effects of heat-killed Lactococcus lactis KC24 using SH-SY5Y cells against oxidative stress induced by hydrogen peroxide. Food Science and Biotechnology. 2020;29(12):1735–1740. doi: 10.1007/s10068-020-00830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mao K., Zhang N., Chitrakar B., Huang P., Wang X., Yang B., Sang Y. Structural characterization and immunomodulatory effects of extracellular polysaccharide from Lactobacillus paracasei VL8 obtained by gradient ethanol precipitation. Journal of Food Science. 2022;87(5):2034–2047. doi: 10.1111/1750-3841.16153. [DOI] [PubMed] [Google Scholar]
- Ma J., Piao X., Mahfuz S., Long S., Wang J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Animal Nutrition. 2022;9:159–174. doi: 10.1016/j.aninu.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Tu H., Chen T. Postbiotics in human health: A narrative review. Nutrients. 2023;15(2):291. doi: 10.3390/nu15020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdhi A., Leban N., Chakroun I., Chaouch M.A., Hafsa J., Fdhila K.…Majdoub H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microbial Pathogenesis. 2017;109:214–220. doi: 10.1016/j.micpath.2017.05.046. [DOI] [PubMed] [Google Scholar]
- Martorell P., Alvarez B., Llopis S., Navarro V., Ortiz P., Gonzalez N., Balaguer F., Rojas A., Chenoll E., Ramón D., Tortajada M. Heat-treated Bifidobacterium longum CECT-7347: A whole-cell postbiotic with antioxidant, anti-inflammatory, and gut-barrier protection properties. Antioxidants (Basel) 2021;10(4):536. doi: 10.3390/antiox10040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Moradi M., Molaei R., Guimarães J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme and Microbial Technology. 2021;143 doi: 10.1016/j.enzmictec.2020.109722. [DOI] [PubMed] [Google Scholar]
- Morishita M., Horita M., Higuchi A., Marui M., Katsumi H., Yamamoto A. Characterizing different probiotic-derived extracellular vesicles as a novel adjuvant for immunotherapy. Molecular Pharmaceutics. 2021;18(3):1080–1092. doi: 10.1021/acs.molpharmaceut.0c01011. [DOI] [PubMed] [Google Scholar]
- Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., Biagi E., Andersen M.H., Brigidi P., Ødum N., Litman T. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Scientific Reports. 2015;5(1):16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash A.W., Tsehai B.A. Current applications of bacteriocin. International Journal of Microbiology. 2020;2020:4374891. doi: 10.1155/2020/4374891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahumunto N., Teanpaisan R. Anti-cancer properties of potential probiotics and their cell-free supernatants for the prevention of colorectal cancer: An in vitro study. Probiotics and Antimicrobial Proteins. 2023;15(5):1137–1150. doi: 10.1007/s12602-022-09972-y. [DOI] [PubMed] [Google Scholar]
- Park I.K., Kang D.H. Effect of electropermeabilization by ohmic heating for inactivation of Escherichia coli O157: H7, Salmonella enterica serovar typhimurium, and Listeria monocytogenes in buffered peptone water and apple juice. Applied and Environmental Microbiology. 2013;79(23):7122–7129. doi: 10.1128/AEM.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra S., Sahu N., Saxena S., Pradhan B., Nayak S.K., Roychowdhury A. Effects of probiotics at the interface of metabolism and immunity to prevent colorectal cancer-associated Gut inflammation: A systematic network and meta-analysis with molecular docking studies. Food Microbiology. 2022;13 doi: 10.3389/fmicb.2022.878297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D., Maisonneuve C., Yadav J., Rubino S.J., Girardin S.E., Philpott D.J. NOD2 modulates immune tolerance via the GM-CSF-dependent generation of CD103+ dendritic cells. Proceedings of the National Academy of Sciences. 2020;117(20):10946–10957. doi: 10.1073/pnas.1912866117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar S., Torres Y.J.M., Parekh H.S., Falconer J.R. Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies: A review. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2021;1167 doi: 10.1016/j.jchromb.2021.122581. [DOI] [PubMed] [Google Scholar]
- Qiao Z., Zhang L., Wang X., Liu B., Shan Y., Yi Y.…Lü X. Antibiofilm effects of Bacteriocin BMP32r on Listeria monocytogenes. Probiotics and Antimicrobial Proteins. 2022;14(6):1067–1076. doi: 10.1007/s12602-021-09863-8. [DOI] [PubMed] [Google Scholar]
- Rafique N., Jan S.Y., Dar A.H., Dash K.K., Sarkar A., Shams R.…Hussain S.Z. Promising bioactivities of postbiotics: A comprehensive review. Journal of Agriculture and Food Research. 2023;14:100708. [Google Scholar]
- Rahman M.S., Lee Y., Park D.S., Kim Y.S. Bifidobacterium bifidum DS0908 and Bifidobacterium longum DS0950 culture-supernatants ameliorate obesity-related characteristics in mice with high-fat diet-induced obesity. Journal of Microbiology and Biotechnology. 2023;33(1):96–105. doi: 10.4014/jmb.2210.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M.…Vinderola G. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews Gastroenterology & Hepatology. 2021;18(9):649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salva S., Kolling Y., Ivir M., Gutiérrez F., Alvarez S. The role of immunobiotics and postbiotics in the recovery of immune cell populations from respiratory mucosa of malnourished hosts: Effect on the resistance against respiratory infections. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.704868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samer A., Toumi R., Soufli I., Touil-Boukoffa C. Cell-free probiotic supernatant (CFS) treatment alleviates indomethacin-induced enterocolopathy in BALB/c mice by down-modulating inflammatory response and oxidative stress: Potential alternative targeted treatment. Inflammopharmacology. 2022;30(5):1685–1703. doi: 10.1007/s10787-022-00996-y. [DOI] [PubMed] [Google Scholar]
- Shafipour Yordshahi A., Moradi M., Tajik H., Molaei R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. International Journal of Food Microbiology. 2020;321 doi: 10.1016/j.ijfoodmicro.2020.108561. [DOI] [PubMed] [Google Scholar]
- Sharafi H., Divsalar E., Rezaei Z., Liu S.Q., Moradi M. The potential of postbiotics as a novel approach in food packaging and biopreservation: A systematic review of the latest developments. Critical Reviews in Food Science and Nutrition. 2023:1–31. doi: 10.1080/10408398.2023.2253909. [DOI] [PubMed] [Google Scholar]
- Shen L., Pang S., Zhong M., Sun Y., Qayum A., Liu Y., Rashid A., Xu B., Liang Q., Ma H., Ren X. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrasonics Sonochemistry. 2023;101 doi: 10.1016/j.ultsonch.2023.106646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S., Fu Y., Pan N., Zhang H., Xiu L., Liang Y., Liu Y., Liu B., Ma C., Du R., Wang X. Novel exopolysaccharide derived from probiotic Lactobacillus pantheris TCP102 strain with immune-enhancing and anticancer activities. Frontiers in Microbiology. 2022;13:1015270. doi: 10.3389/fmicb.2022.1015270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Frontiers in Endocrinology (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Kaur R., Singh B.P., Rokana N., Goel G., Puniya A.K., Panwar H. Impairment of Cronobacter sakazakii and Listeria monocytogenes biofilms by cell-free preparations of lactobacilli of goat milk origin. Folia Microbiologica (Praha) 2020;65(1):185–196. doi: 10.1007/s12223-019-00721-3. [DOI] [PubMed] [Google Scholar]
- Soliemani O., Salimi F., Rezaei A. Characterization of exopolysaccharide produced by probiotic Enterococcus durans DU1 and evaluation of its anti-biofilm activity. Archives of Microbiology. 2022;204(7):419. doi: 10.1007/s00203-022-02965-z. [DOI] [PubMed] [Google Scholar]
- Song Y., Sun M., Feng L., Liang X., Song X., Mu G., Tuo Y., Jiang S., Qian F. Antibiofilm activity of Lactobacillus plantarum 12 exopolysaccharides against Shigella flexneri. Applied and Environmental Microbiology. 2020;86(15) doi: 10.1128/AEM.00694-20. e00694–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Liu X., Li X. Peptidoglycan-based immunomodulation. Applied Microbiology and Biotechnology. 2022;106(3):981–993. doi: 10.1007/s00253-022-11795-4. [DOI] [PubMed] [Google Scholar]
- Tan L.L., Ngiam J.J., Sim E.S.Z., Conway P.L., Loo S.C.J. Liquorilactobacillus satsumensis from water kefir yields α-glucan polysaccharides with prebiotic and synbiotic qualities. Carbohydrate Polymers. 2022;290 doi: 10.1016/j.carbpol.2022.119515. [DOI] [PubMed] [Google Scholar]
- Tomasik P., Tomasik P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Applied Sciences-Basel. 2020;10(4):1470. [Google Scholar]
- Vale A.D.S., Pereira G.V.D.M., de Oliveira A.C., Neto D.P.D.C., Herrmann L.W., Karp S.G.…Soccol C.R. Production, formulation, and application of postbiotics in the treatment of skin conditions. Fermentation-Basel. 2023;9(3):264. [Google Scholar]
- Vandenplas Y., de Halleux V., Arciszewska M., Lach P., Pokhylko V., Klymenko V.…Porcel Rubio R. A partly fermented infant formula with postbiotics including 3′-GL, specific oligosaccharides, 2’-FL, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: A double-blind, randomised, controlled, multi-country trial. Nutrients. 2020;12(11):3560. doi: 10.3390/nu12113560. On Behalf Of The Voyage Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Ahmadi S., Nagpal R., Jain S., Mishra S.P., Kavanagh K.…Yadav H. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. Elegans to mice. Geroscience. 2020;42(1):333–352. doi: 10.1007/s11357-019-00137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xu M., Xu D., Ma K., Zhang C., Wang G., Dong M., Li W. Structural and prebiotic activity analysis of the polysaccharide produced by Lactobacillus helveticus SNA12. Carbohydrate Polymers. 2022;296 doi: 10.1016/j.carbpol.2022.119971. [DOI] [PubMed] [Google Scholar]
- Wang X., Ye C., Xun T., Mo L., Tong Y., Ni W., Huang S., Liu B., Zhan X., Yang X. Bacteroides fragilis polysaccharide A ameliorates abnormal voriconazole metabolism accompanied with the inhibition of TLR4/NF-κB pathway. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.663325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A., Taguchi A., Dörr T. Masters of misdirection: Peptidoglycan glycosidases in bacterial growth. Journal of Bacteriology. 2023;205(3) doi: 10.1128/jb.00428-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J.-J., Li M.-Z., Gao H., Hu J.-L., Nie Q.-X., Chen H.-H., Zhang Y.-L., Xie M.-Y., Nie S.-P. Polysaccharides from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorate metabolic disorders and gut microbiota change in obese rats. Food & Function. 2021;12(6):2617–2630. doi: 10.1039/d0fo02600j. [DOI] [PubMed] [Google Scholar]
- Xia T., Teng K., Liu Y., Guo Y., Huang F., Tahir M.…Zhong J. A novel two-component bacteriocin, acidicin P, and its key residues for inhibiting Listeria monocytogenes by targeting the cell membrane. Microbiology Spectrum. 2023;11(4) doi: 10.1128/spectrum.05210-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Li H., Zhang X., Yang T., Yue M., Zhang Y.…Liu W. Akkermansia muciniphila protects mice against an emerging tick-borne viral pathogen. Nature Microbiology. 2023;8(1):91–106. doi: 10.1038/s41564-022-01279-6. [DOI] [PubMed] [Google Scholar]
- Yan H., Lu Y., Li X., Yi Y., Wang X., Shan Y.…Lu X. Action mode of bacteriocin BM1829 against Escherichia coli and Staphylococcus aureus. Food Bioscience. 2021;39 [Google Scholar]
- Yang X., Zhang M., Liu Y., Wei F., Li X., Feng Y., Jin X., Liu D., Guo Y., Hu Y. Inulin-enriched megamonas funiformis ameliorates metabolic dysfunction-associated fatty liver disease by producing propionic acid. npj Biofilms and Microbiomes. 2023;9(1):84. doi: 10.1038/s41522-023-00451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X., Yang L., Zhao X., Ma K., Chen X., Zhang C., Wang G., Dong M., Rui X., Zhang Q., Li W. Isolation, purification, characterization and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan kefir. International Journal of Biological Macromolecules. 2020;158:408–419. doi: 10.1016/j.ijbiomac.2020.05.027. [DOI] [PubMed] [Google Scholar]
- Yu X., Ou J., Wang L., Li Z., Ren Y., Xie L., Chen Z., Liang J., Shen G., Zou Z., Zhao C., Li G., Hu Y. Gut microbiota modulate CD8(+) T cell immunity in gastric cancer through butyrate/GPR109A/HOPX. Gut Microbes. 2024;16(1):2307542. doi: 10.1080/19490976.2024.2307542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhou Z., Li Y., Zhou L., Ding Q., Xu L. Isolated exopolysaccharides from Lactobacillus rhamnosus GG alleviated adipogenesis mediated by TLR2 in mice. Scientific Reports. 2016;6(1):36083. doi: 10.1038/srep36083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Hong T., Yu Q., Nie S., Gong D., Xiong T., Xie M. Exopolysaccharides from Lactobacillus plantarum NCU116 induce c-Jun dependent Fas/Fasl-mediated apoptosis via TLR2 in mouse intestinal epithelial cancer cells. Scientific Reports. 2017;7(1):14247. doi: 10.1038/s41598-017-14178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhang D., Qi W., Hong T., Xiong T., Wu T., Geng F., Xie M., Nie S. Exopolysaccharides from Lactobacillus plantarum NCU116 facilitate intestinal homeostasis by modulating intestinal epithelial regeneration and microbiota. Journal of Agricultural and Food Chemistry. 2021;69(28):7863–7873. doi: 10.1021/acs.jafc.1c01898. [DOI] [PubMed] [Google Scholar]
- Żółkiewicz J., Marzec A., Ruszczyński M., Feleszko W. Postbiotics-a step beyond pre- and probiotics. Nutrients. 2020;12(8):2189. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.