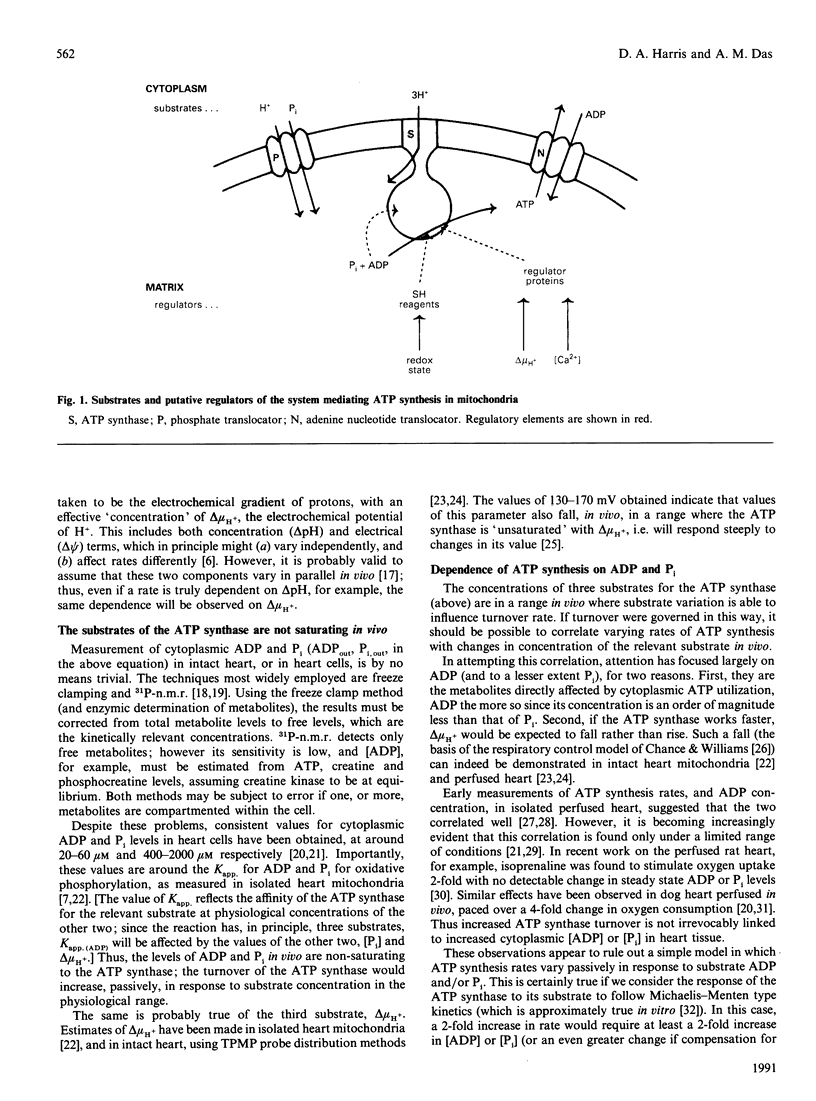
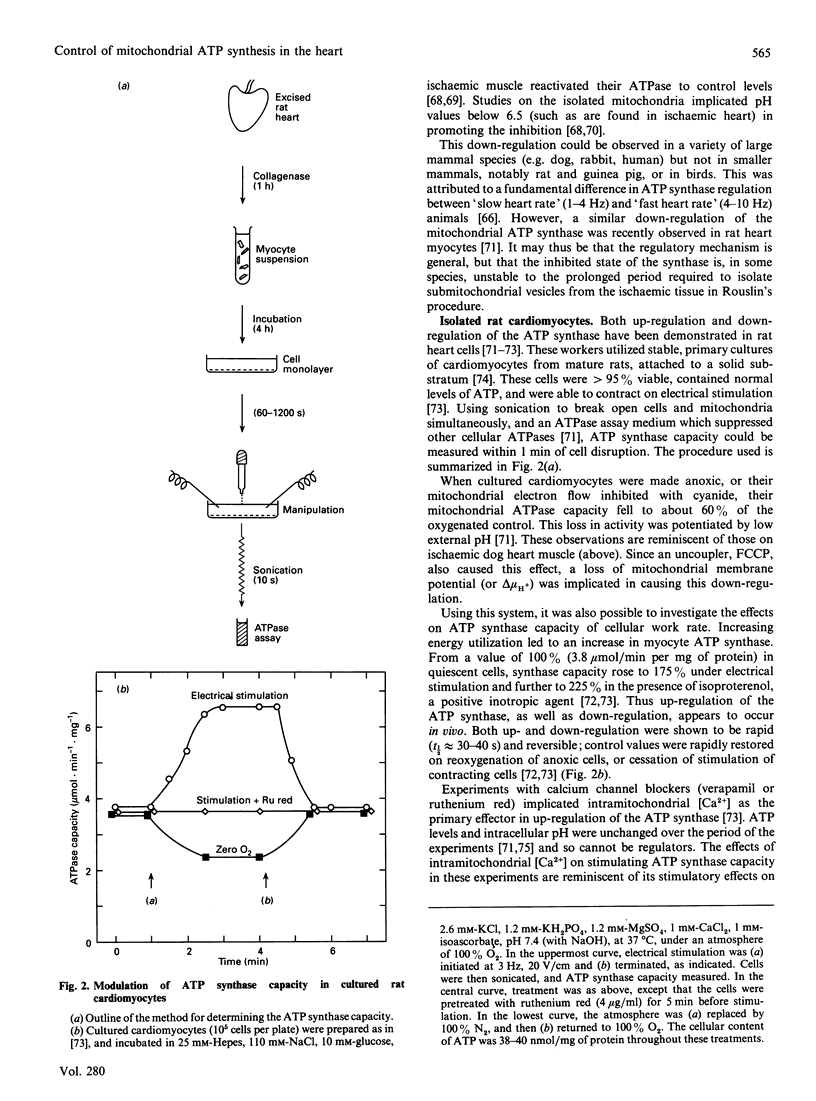
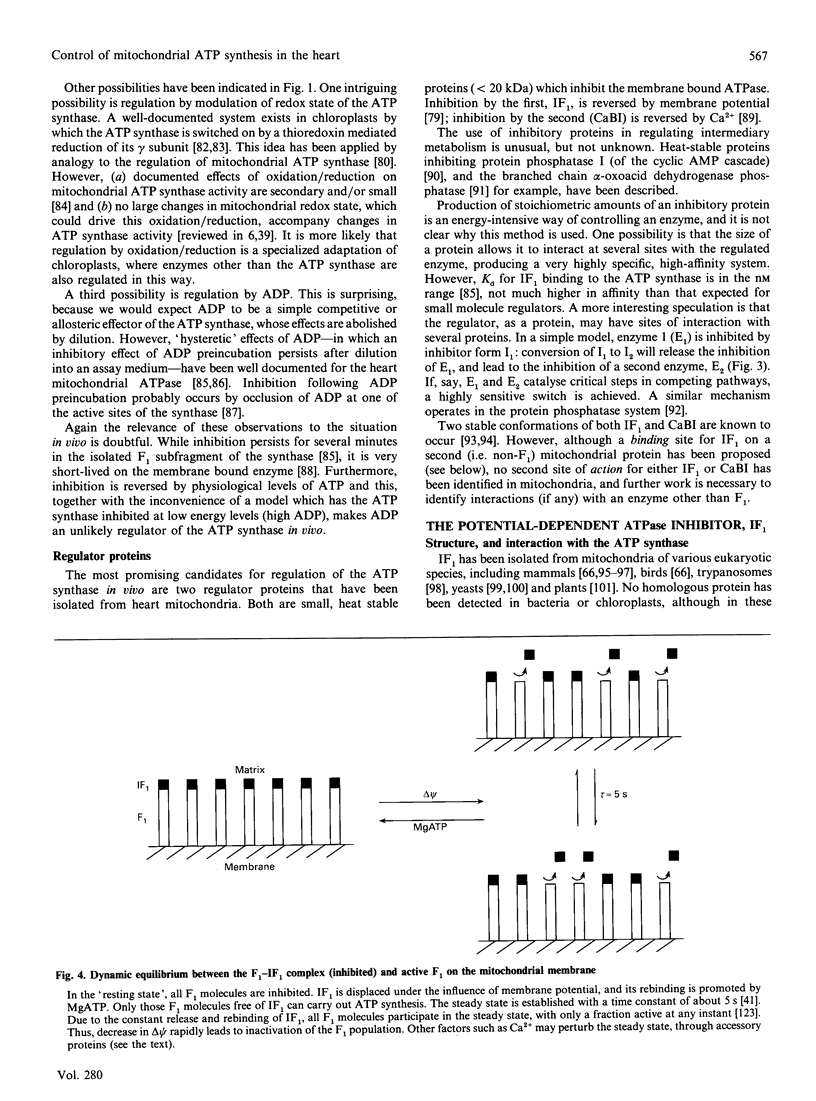
Full text
PDF




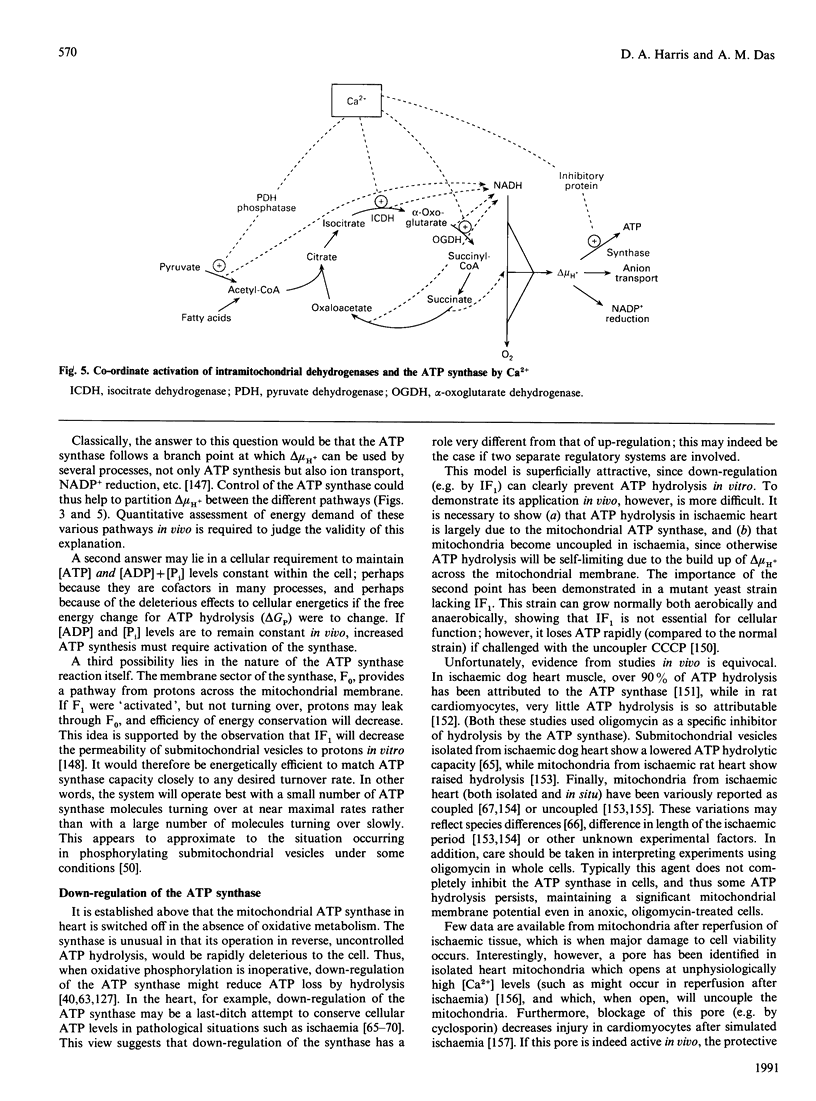



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsen R., McClung J. A., Moudrianakis E. N. Electrophoretic microheterogeneity and subunit composition of the 13S coupling factors of oxidative and photosynthetic phosphorylation. Biochemistry. 1975 Apr 22;14(8):1727–1735. doi: 10.1021/bi00679a027. [DOI] [PubMed] [Google Scholar]
- Andralojc P. J., Harris D. A. Promotion and inhibition of catalytic cooperativity of the Ca2+-dependent ATPase activity of spinach chloroplast coupling factor 1 (CF1). Biochim Biophys Acta. 1990 Mar 15;1016(1):55–62. doi: 10.1016/0005-2728(90)90006-p. [DOI] [PubMed] [Google Scholar]
- Audinet C., Dianoux A. C., Vignais P. V. Immunological studies on the beef heart natural ATPase inhibitor: localization of an antigenic determinant in the inhibitor molecule. Biochem Biophys Res Commun. 1986 May 29;137(1):364–371. doi: 10.1016/0006-291x(86)91219-2. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Balaban R. S. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990 Mar;258(3 Pt 1):C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Beltrán C., Tuena de Gómez-Puyou M., Darszon A., Gómez-Puyou A. Simultaneous synthesis and hydrolysis of ATP regulated by the inhibitor protein in submitochondrial particles. Eur J Biochem. 1986 Oct 1;160(1):163–168. doi: 10.1111/j.1432-1033.1986.tb09953.x. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Murphy M. P. Control of electron flux through the respiratory chain in mitochondria and cells. Biol Rev Camb Philos Soc. 1987 May;62(2):141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Niggli V., Malmström K., Caroni P. Calmodulin in natural and reconstituted calcium transporting systems. Ann N Y Acad Sci. 1980;356:258–266. doi: 10.1111/j.1749-6632.1980.tb29616.x. [DOI] [PubMed] [Google Scholar]
- Catia Sorgato M., Lippe G., Seren S., Ferguson S. J. Partial uncoupling, or inhibition of electron transport rate, have equivalent effects on the relationship between the rate of ATP synthesis and proton-motive force in submitochondrial particles. FEBS Lett. 1985 Feb 25;181(2):323–327. doi: 10.1016/0014-5793(85)80285-4. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Kent J., McCully K., Nioka S., Clark B. J., Maris J. M., Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak B. V., Dukhovich V. F., Khodjaev EYu The effect of the natural protein inhibitor on H+-ATPase hepatoma 22a mitochondria. FEBS Lett. 1987 May 11;215(2):300–304. doi: 10.1016/0014-5793(87)80166-7. [DOI] [PubMed] [Google Scholar]
- Chernyak B. V., Khodjaev EYu, Kozlov I. A. The oxidation of sulfhydryl groups in mitochondrial F1-ATPase decreases the rate of its inactivation by the natural protein inhibitor. FEBS Lett. 1985 Aug 5;187(2):253–256. doi: 10.1016/0014-5793(85)81253-9. [DOI] [PubMed] [Google Scholar]
- Cintrón N. M., Pedersen P. L. A protein inhibitor of the mitochondrial adenosine triphosphatase complex of rat liver. Purification and characterization. J Biol Chem. 1979 May 10;254(9):3439–3443. [PubMed] [Google Scholar]
- Classen J. B., Mergner W. J., Costa M. ATP hydrolysis by ischemic mitochondria. J Cell Physiol. 1989 Oct;141(1):53–59. doi: 10.1002/jcp.1041410109. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Crompton M., Costi A. A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J. 1990 Feb 15;266(1):33–39. doi: 10.1042/bj2660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Humphreys J. S., Reed L. J. A potent, heat-stable protein inhibitor of [branched-chain alpha-keto acid dehydrogenase]-phosphatase from bovine kidney mitochondria. Proc Natl Acad Sci U S A. 1986 Jan;83(2):285–289. doi: 10.1073/pnas.83.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res. 1990 May;24(5):411–417. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Control of mitochondrial ATP synthase in rat cardiomyocytes: effects of thyroid hormone. Biochim Biophys Acta. 1991 Jun 5;1096(4):284–290. doi: 10.1016/0925-4439(91)90064-g. [DOI] [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Defects in regulation of mitochondrial ATP synthase in cardiomyocytes from spontaneously hypertensive rats. Am J Physiol. 1990 Oct;259(4 Pt 2):H1264–H1269. doi: 10.1152/ajpheart.1990.259.4.H1264. [DOI] [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Regulation of the mitochondrial ATP synthase in intact rat cardiomyocytes. Biochem J. 1990 Mar 1;266(2):355–361. doi: 10.1042/bj2660355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Reversible modulation of the mitochondrial ATP synthase with energy demand in cultured rat cardiomyocytes. FEBS Lett. 1989 Oct 9;256(1-2):97–100. doi: 10.1016/0014-5793(89)81725-9. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro A., Penin F., Julliard J. H., Godinot C., Gautheron D. C. IF1 inhibition of mitochondrial F1-ATPase is correlated to entrapment of four adenine- or guanine-nucleotides including at least one triphosphate. Biochem Biophys Res Commun. 1988 May 16;152(3):1319–1325. doi: 10.1016/s0006-291x(88)80429-7. [DOI] [PubMed] [Google Scholar]
- Dianoux A. C., Hoppe J. Complete amino-acid sequence of the natural ATPase inhibitor from the mitochondria of the yeast Candida utilis. Eur J Biochem. 1987 Feb 16;163(1):155–160. doi: 10.1111/j.1432-1033.1987.tb10749.x. [DOI] [PubMed] [Google Scholar]
- Dianoux A. C., Tsugita A., Klein G., Vignais P. V. Effects of proteolytic fragmentations on the activity of the mitochondrial natural ATPase inhibitor. FEBS Lett. 1982 Apr 19;140(2):223–228. doi: 10.1016/0014-5793(82)80899-5. [DOI] [PubMed] [Google Scholar]
- Doussiere J., Ligeti E., Brandolin G., Vignais P. V. Control of oxidative phosphorylation in rat heart mitochondria. The role of the adenine nucleotide carrier. Biochim Biophys Acta. 1984 Aug 31;766(2):492–500. doi: 10.1016/0005-2728(84)90265-2. [DOI] [PubMed] [Google Scholar]
- Dreyfus G., Gómez-Puyou A., Iuena de Gómez-Puyou M. Electrochemical gradient induced displacement of the natural ATPase inhibitor protein from mitochondrial ATPase as directed by antibodies against the inhibitor protein. Biochem Biophys Res Commun. 1981 May 15;100(1):400–406. doi: 10.1016/s0006-291x(81)80110-6. [DOI] [PubMed] [Google Scholar]
- Duncan T. M., Parsonage D., Senior A. E. Structure of the nucleotide-binding domain in the beta-subunit of Escherichia coli F1-ATPase. FEBS Lett. 1986 Nov 10;208(1):1–6. doi: 10.1016/0014-5793(86)81519-8. [DOI] [PubMed] [Google Scholar]
- Dunn S. D., Zadorozny V. D., Tozer R. G., Orr L. E. Epsilon subunit of Escherichia coli F1-ATPase: effects on affinity for aurovertin and inhibition of product release in unisite ATP hydrolysis. Biochemistry. 1987 Jul 14;26(14):4488–4493. doi: 10.1021/bi00388a047. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Bogucka K., Wojtczak L. Homeostasis of the protonmotive force in phosphorylating mitochondria. Biochim Biophys Acta. 1984 Dec 18;767(3):540–547. doi: 10.1016/0005-2728(84)90053-7. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Regulation of cellular energy metabolism. J Membr Biol. 1982;70(1):1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- Ernster L., Juntti K., Asami K. Mechanisms of energy conservation in the mitochondrial membrane. J Bioenerg. 1973 Jan;4(1):149–159. doi: 10.1007/BF01516053. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Harris D. A., Radda G. K. The adenosine triphosphatase-inhibitor content of bovine heart submitochondrial particles. Influence of the inhibitor on adenosine triphosphate-dependent reactions. Biochem J. 1977 Feb 15;162(2):351–357. doi: 10.1042/bj1620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Rosenwasser E., Penefsky H. S., Pullman M. E. Amino acid sequence of the protein inhibitor of mitochondrial adenosine triphosphatase. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7403–7407. doi: 10.1073/pnas.78.12.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- From A. H., Petein M. A., Michurski S. P., Zimmer S. D., Uğurbil K. 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett. 1986 Oct 6;206(2):257–261. doi: 10.1016/0014-5793(86)80992-9. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hashimoto T., Yoshida Y., Miura R., Yamano T., Tagawa K. pH-Induced conformational change of ATPase inhibitor from yeast mitochondria. A proton magnetic resonance study. J Biochem. 1983 Jan;93(1):189–196. doi: 10.1093/oxfordjournals.jbchem.a134153. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Gibbs C. The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol. 1985 Aug;17(8):727–731. doi: 10.1016/s0022-2828(85)80034-1. [DOI] [PubMed] [Google Scholar]
- Giesen J., Kammermeier H. Relationship of phosphorylation potential and oxygen consumption in isolated perfused rat hearts. J Mol Cell Cardiol. 1980 Sep;12(9):891–907. doi: 10.1016/0022-2828(80)90058-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez J. C., Harris D. A. A thermodynamic analysis of the interaction between the mitochondrial coupling adenosine triphosphatase and its naturally occurring inhibitor protein. Biochem J. 1978 Dec 15;176(3):967–975. doi: 10.1042/bj1760967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser M., Cardon J., Rosen G., Boyer P. D. Demonstration and quantitation of catalytic and noncatalytic bound ATP in submitochondrial particles during oxidative phosphorylation. J Biol Chem. 1979 Nov 10;254(21):10649–10653. [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Guerrieri F., Zanotti F., Che Y. W., Scarfò R., Papa S. Inactivation of the mitochondrial ATPase inhibitor protein by chemical modification with diethylpyrocarbonate. Biochim Biophys Acta. 1987 Jul 22;892(3):284–293. doi: 10.1016/0005-2728(87)90232-5. [DOI] [PubMed] [Google Scholar]
- Gómez-Puyou A., de Gómez-Puyou M. T., Ernster L. Inactive to active transitions of the mitochondrial ATPase complex as controlled by the ATPase inhibitor. Biochim Biophys Acta. 1979 Aug 14;547(2):252–257. doi: 10.1016/0005-2728(79)90008-2. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Harris D. A. Azide as a probe of co-operative interactions in the mitochondrial F1-ATPase. Biochim Biophys Acta. 1989 May 8;974(2):156–162. doi: 10.1016/s0005-2728(89)80368-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A., von Tscharner V., Radda G. K. The ATPase inhibitor protein in oxidative phosphorylation. The rate-limiting factor to phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1979 Oct 10;548(1):72–84. doi: 10.1016/0005-2728(79)90188-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Yoshida Y., Tagawa K. Binding properties of 9K protein to F1-ATPase: a counterpart ligand to the ATPase inhibitor. J Biochem. 1987 Oct;102(4):685–692. doi: 10.1093/oxfordjournals.jbchem.a122106. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Yoshida Y., Tagawa K. Purification and properties of factors in yeast mitochondria stabilizing the F1F0-ATPase-inhibitor complex. J Biochem. 1984 Jan;95(1):131–136. doi: 10.1093/oxfordjournals.jbchem.a134576. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Yoshida Y., Tagawa K. Regulatory proteins of F1F0-ATPase: role of ATPase inhibitor. J Bioenerg Biomembr. 1990 Feb;22(1):27–38. doi: 10.1007/BF00762843. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Yoshida Y., Tagawa K. Simultaneous bindings of ATPase inhibitor and 9K protein to F1F0-ATPase in the presence of 15K protein in yeast mitochondria. J Biochem. 1990 Jul;108(1):17–20. doi: 10.1093/oxfordjournals.jbchem.a123154. [DOI] [PubMed] [Google Scholar]
- Hassinen I. E. Mitochondrial respiratory control in the myocardium. Biochim Biophys Acta. 1986;853(2):135–151. doi: 10.1016/0304-4173(86)90008-x. [DOI] [PubMed] [Google Scholar]
- Hatase O., Tokuda M., Itano T., Matsui H., Doi A. Purification and characterization of calmodulin from rat liver mitochondria. Biochem Biophys Res Commun. 1982 Jan 29;104(2):673–679. doi: 10.1016/0006-291x(82)90689-1. [DOI] [PubMed] [Google Scholar]
- Heineman F. W., Balaban R. S. Control of mitochondrial respiration in the heart in vivo. Annu Rev Physiol. 1990;52:523–542. doi: 10.1146/annurev.ph.52.030190.002515. [DOI] [PubMed] [Google Scholar]
- Herweijer M. A., Berden J. A., Kemp A., Slater E. C. Inhibition of energy-transducing reactions by 8-nitreno-ATP covalently bound to bovine heart submitochondrial particles: direct interaction between ATPase and redox enzymes. Biochim Biophys Acta. 1985 Aug 28;809(1):81–89. doi: 10.1016/0005-2728(85)90170-7. [DOI] [PubMed] [Google Scholar]
- Herweijer M. A., Berden J. A., Slater E. C. Uncoupler-inhibitor titrations of ATP-driven reverse electron transfer in bovine submitochondrial particles provide evidence for direct interaction between ATPase and NADH:Q oxidoreductase. Biochim Biophys Acta. 1986 Apr 24;849(2):276–287. doi: 10.1016/0005-2728(86)90034-4. [DOI] [PubMed] [Google Scholar]
- Holzhütter H. G., Henke W., Dubiel W., Gerber G. A mathematical model to study short-term regulation of mitochondrial energy transduction. Biochim Biophys Acta. 1985 Nov 27;810(2):252–268. doi: 10.1016/0005-2728(85)90140-9. [DOI] [PubMed] [Google Scholar]
- Husain I., Harris D. A. ATP synthesis and hydrolysis in submitochondrial particles subjected to an acid-base transition. Effects of the ATPase inhibitor protein. FEBS Lett. 1983 Aug 22;160(1-2):110–114. doi: 10.1016/0014-5793(83)80947-8. [DOI] [PubMed] [Google Scholar]
- Husain I., Jackson P. J., Harris D. A. Interaction between F1-ATPase and its naturally occurring inhibitor protein. Studies using a specific anti-inhibitor antibody. Biochim Biophys Acta. 1985 Jan 23;806(1):64–74. doi: 10.1016/0005-2728(85)90082-9. [DOI] [PubMed] [Google Scholar]
- Ichikawa N., Yoshida Y., Hashimoto T., Ogasawara N., Yoshikawa H., Imamoto F., Tagawa K. Activation of ATP hydrolysis by an uncoupler in mutant mitochondria lacking an intrinsic ATPase inhibitor in yeast. J Biol Chem. 1990 Apr 15;265(11):6274–6278. [PubMed] [Google Scholar]
- Jackson P. J., Harris D. A. Sites of protein-protein interaction on the mitochondrial F1-ATPase inhibitor protein. Biochem J. 1986 Apr 15;235(2):577–583. doi: 10.1042/bj2350577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. J., Harris D. A. The mitochondrial ATP synthase inhibitor protein binds near the C-terminus of the F1 beta-subunit. FEBS Lett. 1988 Feb 29;229(1):224–228. doi: 10.1016/0014-5793(88)80832-9. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Moreadith R. W., Vandegaer K. M. Mitochondrial respiratory control. Evidence against the regulation of respiration by extramitochondrial phosphorylation potentials or by [ATP]/[ADP] ratios. J Biol Chem. 1982 Mar 10;257(5):2397–2402. [PubMed] [Google Scholar]
- Jacobus W. E. Respiratory control and the integration of heart high-energy phosphate metabolism by mitochondrial creatine kinase. Annu Rev Physiol. 1985;47:707–725. doi: 10.1146/annurev.ph.47.030185.003423. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Hill M. L., Mayer S. E. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res. 1981 Oct;49(4):892–900. doi: 10.1161/01.res.49.4.892. [DOI] [PubMed] [Google Scholar]
- Kalashnikova TYu, Milgrom YaM, Postanogova N. V. The complex of mitochondrial F1-ATPase with the natural inhibitor protein is unable to catalyze single-site ATP hydrolysis. FEBS Lett. 1988 Mar 28;230(1-2):163–166. doi: 10.1016/0014-5793(88)80663-x. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett. 1987 Sep 14;221(2):270–276. doi: 10.1016/0014-5793(87)80939-0. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Intracellular pH and inorganic phosphate content of heart in vivo: a 31P-NMR study. Am J Physiol. 1988 Jul;255(1 Pt 2):H189–H196. doi: 10.1152/ajpheart.1988.255.1.H189. [DOI] [PubMed] [Google Scholar]
- Kauppinen R. Proton electrochemical potential of the inner mitochondrial membrane in isolated perfused rat hearts, as measured by exogenous probes. Biochim Biophys Acta. 1983 Oct 31;725(1):131–137. doi: 10.1016/0005-2728(83)90232-3. [DOI] [PubMed] [Google Scholar]
- Khodjaev EYu, Komarnitsky F. B., Capozza G., Dukhovich V. F., Chernyak B. V., Papa S. Activation of a complex of ATPase with the natural protein inhibitor in submitochondrial particles. FEBS Lett. 1990 Oct 15;272(1-2):145–148. doi: 10.1016/0014-5793(90)80469-y. [DOI] [PubMed] [Google Scholar]
- Kingsley-Hickman P., Sako E. Y., Andreone P. A., St Cyr J. A., Michurski S., Foker J. E., From A. H., Petein M., Ugurbil K. 31P NMR measurement of ATP synthesis rate in perfused intact rat hearts. FEBS Lett. 1986 Mar 17;198(1):159–163. doi: 10.1016/0014-5793(86)81204-2. [DOI] [PubMed] [Google Scholar]
- Koretsky A. P., Katz L. A., Balaban R. S. The mechanism of respiratory control in the in vivo heart. J Mol Cell Cardiol. 1989 Feb;21 (Suppl 1):59–66. doi: 10.1016/0022-2828(89)90838-9. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Jeffries F. M., Radda G. K. Kinetic control of mitochondrial ATP synthesis. Biochemistry. 1986 Nov 18;25(23):7667–7675. doi: 10.1021/bi00371a058. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Strzelecki T., Strzelecka D., Koch C. Regulation of the uncoupling protein in brown adipose tissue. J Biol Chem. 1986 Jan 5;261(1):298–305. [PubMed] [Google Scholar]
- Lippe G., Sorgato M. C., Harris D. A. Kinetics of the release of the mitochondrial inhibitor protein. Correlation with synthesis and hydrolysis of ATP. Biochim Biophys Acta. 1988 Mar 30;933(1):1–11. doi: 10.1016/0005-2728(88)90050-3. [DOI] [PubMed] [Google Scholar]
- Lippe G., Sorgato M. C., Harris D. A. The binding and release of the inhibitor protein are governed independently by ATP and membrane potential in ox-heart submitochondrial vesicles. Biochim Biophys Acta. 1988 Mar 30;933(1):12–21. doi: 10.1016/0005-2728(88)90051-5. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Hase T., Hashimoto T., Tagawa K. Amino acid sequence of an intrinsic inhibitor of mitochondrial ATPase from yeast. J Biochem. 1981 Oct;90(4):1159–1165. doi: 10.1093/oxfordjournals.jbchem.a133568. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Hatefi Y. Kinetic modalities of ATP synthesis. Regulation by the mitochondrial respiratory chain. J Biol Chem. 1986 Oct 25;261(30):14031–14038. [PubMed] [Google Scholar]
- Matthews P. M., Bland J. L., Gadian D. G., Radda G. K. The steady-state rate of ATP synthesis in the perfused rat heart measured by 31P NMR saturation transfer. Biochem Biophys Res Commun. 1981 Dec 15;103(3):1052–1059. doi: 10.1016/0006-291x(81)90915-3. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Williams S. R., Seymour A. M., Schwartz A., Dube G., Gadian D. G., Radda G. K. A 31P-NMR study of some metabolic and functional effects of the inotropic agents epinephrine and ouabain, and the ionophore R02-2985 (X537A) in the isolated, perfused rat heart. Biochim Biophys Acta. 1982 Apr 29;720(2):163–171. doi: 10.1016/0167-4889(82)90008-8. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- McGuinness O., Crompton M. Cyclosporin and mitochondrial dysfunction. Biochem Soc Trans. 1990 Oct;18(5):883–884. doi: 10.1042/bst0180883. [DOI] [PubMed] [Google Scholar]
- Mela-Riker L. M., Bukoski R. D. Regulation of mitochondrial activity in cardiac cells. Annu Rev Physiol. 1985;47:645–663. doi: 10.1146/annurev.ph.47.030185.003241. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Bisson R. ATP binding to bovine heart cytochrome c oxidase. A photoaffinity labelling study. Biochem J. 1986 Feb 15;234(1):241–243. doi: 10.1042/bj2340241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hogue B. A., Hansford R. G. Influence of NAD-linked dehydrogenase activity on flux through oxidative phosphorylation. Biochem J. 1990 Jun 1;268(2):421–428. doi: 10.1042/bj2680421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XII. Purification and properties of an inhibitor isolated from chloroplast coupling factor 1. J Biol Chem. 1972 Dec 10;247(23):7657–7662. [PubMed] [Google Scholar]
- Neubauer S., Hamman B. L., Perry S. B., Bittl J. A., Ingwall J. S. Velocity of the creatine kinase reaction decreases in postischemic myocardium: a 31P-NMR magnetization transfer study of the isolated ferret heart. Circ Res. 1988 Jul;63(1):1–15. doi: 10.1161/01.res.63.1.1. [DOI] [PubMed] [Google Scholar]
- Norling B., Tourikas C., Hamasur B., Glaser E. Evidence for an endogenous ATPase inhibitor protein in plant mitochondria. Purification and characterization. Eur J Biochem. 1990 Mar 10;188(2):247–252. doi: 10.1111/j.1432-1033.1990.tb15396.x. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M. The relation between the internal phosphorylation potential and the proton motive force in mitochondria during ATP synthesis and hydrolysis. J Biol Chem. 1984 Aug 25;259(16):10004–10011. [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- Panchenko M. V., Vinogradov A. D. Interaction between the mitochondrial ATP synthetase and ATPase inhibitor protein. Active/inactive slow pH-dependent transitions of the inhibitor protein. FEBS Lett. 1985 May 20;184(2):226–230. doi: 10.1016/0014-5793(85)80611-6. [DOI] [PubMed] [Google Scholar]
- Pansini A., Guerrieri F., Papa S. Control of proton conduction by the H+ -ATPase in the inner mitochondrial membrane. Eur J Biochem. 1978 Dec;92(2):545–551. doi: 10.1111/j.1432-1033.1978.tb12776.x. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Hullihen J. Inhibitor peptide of mitochondrial proton adenosine triphosphatase. Neutralization of its inhibitory action by calmodulin. J Biol Chem. 1984 Dec 25;259(24):15148–15153. [PubMed] [Google Scholar]
- Petronilli V., Azzone G. F., Pietrobon D. Analysis of mechanisms of free-energy coupling and uncoupling by inhibitor titrations: theory, computer modeling and experiments. Biochim Biophys Acta. 1988 Mar 9;932(3):306–324. doi: 10.1016/0005-2728(88)90167-3. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Schwartz P., Spahr R., Hütter J. F., Spieckermann P. G. Anoxic injury of adult cardiac myocytes. Basic Res Cardiol. 1985;80 (Suppl 1):37–41. doi: 10.1007/978-3-662-11041-6_6. [DOI] [PubMed] [Google Scholar]
- Power J., Cross R. L., Harris D. A. Interaction of F1-ATPase, from ox heart mitochondria with its naturally occurring inhibitor protein. Studies using radio-iodinated inhibitor protein. Biochim Biophys Acta. 1983 Jul 29;724(1):128–141. doi: 10.1016/0005-2728(83)90034-8. [DOI] [PubMed] [Google Scholar]
- Pérez J. A., Ferguson S. J. Kinetics of oxidative phosphorylation in Paracoccus denitrificans. 2. Evidence for a kinetic and thermodynamic modulation of F0F1-ATPase by the activity of the respiratory chain. Biochemistry. 1990 Nov 20;29(46):10518–10526. doi: 10.1021/bi00498a014. [DOI] [PubMed] [Google Scholar]
- Rilo M. C., Cataldi de Flombaum M. A., Stoppani A. O. Isolation of the peptide inhibitor of H+-ATP synthase from Crithidia fasciculata and Trypanosoma cruzi. Biochem Int. 1989 Feb;18(2):447–454. [PubMed] [Google Scholar]
- Rouslin W., Broge C. W. Factors affecting the reactivation of the mitochondrial adenosine 5'-triphosphatase and the release of ATPase inhibitor protein during and following the reenergization of mitochondria from ischemic cardiac muscle. Arch Biochem Biophys. 1989 Dec;275(2):385–394. doi: 10.1016/0003-9861(89)90386-x. [DOI] [PubMed] [Google Scholar]
- Rouslin W., Broge C. W. Regulation of mitochondrial matrix pH and adenosine 5'-triphosphatase activity during ischemia in slow heart-rate hearts. Role of Pi/H+ symport. J Biol Chem. 1989 Sep 15;264(26):15224–15229. [PubMed] [Google Scholar]
- Rouslin W., Broge C. W. Regulation of the mitochondrial adenosine 5'-triphosphatase in situ during ischemia and in vitro in intact and sonicated mitochondria from slow and fast heart-rate hearts. Arch Biochem Biophys. 1990 Jul;280(1):103–111. doi: 10.1016/0003-9861(90)90524-3. [DOI] [PubMed] [Google Scholar]
- Rouslin W., Erickson J. L., Solaro R. J. Effects of oligomycin and acidosis on rates of ATP depletion in ischemic heart muscle. Am J Physiol. 1986 Mar;250(3 Pt 2):H503–H508. doi: 10.1152/ajpheart.1986.250.3.H503. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Factors affecting the reactivation of the oligomycin-sensitive adenosine 5'-triphosphatase and the release of ATPase inhibitor protein during the re-energization of intact mitochondria from ischemic cardiac muscle. J Biol Chem. 1987 Mar 15;262(8):3472–3476. [PubMed] [Google Scholar]
- Rouslin W. Persistence of mitochondrial competence during myocardial autolysis. Am J Physiol. 1987 May;252(5 Pt 2):H985–H989. doi: 10.1152/ajpheart.1987.252.5.H985. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Protonic inhibition of the mitochondrial oligomycin-sensitive adenosine 5'-triphosphatase in ischemic and autolyzing cardiac muscle. Possible mechanism for the mitigation of ATP hydrolysis under nonenergizing conditions. J Biol Chem. 1983 Aug 25;258(16):9657–9661. [PubMed] [Google Scholar]
- Rouslin W., Pullman M. E. Protonic inhibition of the mitochondrial adenosine 5'-triphosphatase in ischemic cardiac muscle. Reversible binding of the ATPase inhibitor protein to the mitochondrial ATPase during ischemia. J Mol Cell Cardiol. 1987 Jul;19(7):661–668. doi: 10.1016/s0022-2828(87)80374-7. [DOI] [PubMed] [Google Scholar]
- Rouslin W. The mitochondrial adenosine 5'-triphosphatase in slow and fast heart rate hearts. Am J Physiol. 1987 Mar;252(3 Pt 2):H622–H627. doi: 10.1152/ajpheart.1987.252.3.H622. [DOI] [PubMed] [Google Scholar]
- Runswick M. J., Walker J. E., Gibson B. W., Williams D. H. The frayed N-terminal of the inhibitor protein of bovine mitochondrial F1-ATPase. Biochem J. 1986 Apr 15;235(2):515–519. doi: 10.1042/bj2350515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako E. Y., Kingsley-Hickman P. B., From A. H., Foker J. E., Ugurbil K. ATP synthesis kinetics and mitochondrial function in the postischemic myocardium as studied by 31P NMR. J Biol Chem. 1988 Aug 5;263(22):10600–10607. [PubMed] [Google Scholar]
- Satre M., de Jerphanion M. B., Huet J., Vignais P. V. ATPase inhibitor from yeast mitochondria. Purification and properties. Biochim Biophys Acta. 1975 May 15;387(2):241–255. doi: 10.1016/0005-2728(75)90107-3. [DOI] [PubMed] [Google Scholar]
- Schwerzmann K., Müller M., Carafoli E. The inhibitor peptide of the mitochondrial F1.F0-ATPase interacts with calmodulin and stimulates the calmodulin-dependent Ca2+-ATPase of erythrocytes. Biochim Biophys Acta. 1985 Jun 11;816(1):63–67. doi: 10.1016/0005-2736(85)90393-1. [DOI] [PubMed] [Google Scholar]
- Schwerzmann K., Pedersen P. L. Proton--adenosinetriphosphatase complex of rat liver mitochondria: effect of energy state on its interaction with the adenosinetriphosphatase inhibitory peptide. Biochemistry. 1981 Oct 27;20(22):6305–6311. doi: 10.1021/bi00525a004. [DOI] [PubMed] [Google Scholar]
- Schwerzmann K., Pedersen P. L. Regulation of the mitochondrial ATP synthase/ATPase complex. Arch Biochem Biophys. 1986 Oct;250(1):1–18. doi: 10.1016/0003-9861(86)90695-8. [DOI] [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The mechanism of the conservation of energy of biological oxidations. Eur J Biochem. 1987 Aug 3;166(3):489–504. doi: 10.1111/j.1432-1033.1987.tb13542.x. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Purification of membrane attachment and inhibitory subunits of the proton translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1977 Jan 25;16(2):306–311. doi: 10.1021/bi00621a023. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Branca D., Ferguson S. J. The rate of ATP synthesis by submitochondrial particles can be independent of the magnitude of the protonmotive force. Biochem J. 1980 Jun 15;188(3):945–948. doi: 10.1042/bj1880945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Synthesis of adenosine triphosphate by an artificially imposed electrochemical proton gradient in bovine heart submitochondrial particles. J Biol Chem. 1975 Jul 25;250(14):5330–5335. [PubMed] [Google Scholar]
- Tuena Gómez-Puyou M. T., Martins O. B., Gómez-Puyou A. Synthesis and hydrolysis of ATP by the mitochondrial ATP synthase. Biochem Cell Biol. 1988 Jul;66(7):677–682. doi: 10.1139/o88-077. [DOI] [PubMed] [Google Scholar]
- Unitt J. F., McCormack J. G., Reid D., MacLachlan L. K., England P. J. Direct evidence for a role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in the stimulated rat heart. Studies using 31P n.m.r. and ruthenium red. Biochem J. 1989 Aug 15;262(1):293–301. doi: 10.1042/bj2620293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Powell S. J., Kostina M., Dyer M. R. ATP synthase from bovine mitochondria: sequences of imported precursors of oligomycin sensitivity conferral protein, factor 6, and adenosinetriphosphatase inhibitor protein. Biochemistry. 1987 Dec 29;26(26):8613–8619. doi: 10.1021/bi00400a018. [DOI] [PubMed] [Google Scholar]
- Wan B., LaNoue K. F., Cheung J. Y., Scaduto R. C., Jr Regulation of citric acid cycle by calcium. J Biol Chem. 1989 Aug 15;264(23):13430–13439. [PubMed] [Google Scholar]
- Westerhoff H. V., Melandri B. A., Venturoli G., Azzone G. F., Kell D. B. A minimal hypothesis for membrane-linked free-energy transduction. The role of independent, small coupling units. Biochim Biophys Acta. 1984 Dec 17;768(3-4):257–292. doi: 10.1016/0304-4173(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Wong S. Y., Galante Y. M., Hatefi Y. Equilibrium binding of 125I-labeled adenosinetriphosphatase inhibitor protein to complex V of the mitochondrial oxidative phosphorylation system. Biochemistry. 1982 Nov 9;21(23):5781–5787. doi: 10.1021/bi00266a009. [DOI] [PubMed] [Google Scholar]
- Yagi T., Matsuno-Yagi A., Vik S. B., Hatefi Y. Modulation of the kinetics and the steady-state level of intermediates of mitochondrial coupled reactions by inhibitors and uncouplers. Biochemistry. 1984 Feb 28;23(5):1029–1036. doi: 10.1021/bi00300a035. [DOI] [PubMed] [Google Scholar]
- Yamada E. W., Huzel N. J. Calcium-binding ATPase inhibitor protein of bovine heart mitochondria. Role in ATP synthesis and effect of Ca2+. Biochemistry. 1989 Dec 12;28(25):9714–9718. doi: 10.1021/bi00451a026. [DOI] [PubMed] [Google Scholar]
- Yamada E. W., Huzel N. J., Dickison J. C. Reversal by uncouplers of oxidative phosphorylation and by Ca2+ of the inhibition of mitochondrial ATPase activity by the ATPase inhibitor protein of rat skeletal muscle. J Biol Chem. 1981 Oct 10;256(19):10203–10207. [PubMed] [Google Scholar]
- Yamada E. W., Huzel N. J. Isolation of two ATPase inhibitor proteins from mitochondria of rat skeletal muscle. Biosci Rep. 1983 Oct;3(10):947–954. doi: 10.1007/BF01140664. [DOI] [PubMed] [Google Scholar]
- Yamada E. W., Huzel N. J. The calcium-binding ATPase inhibitor protein from bovine heart mitochondria. Purification and properties. J Biol Chem. 1988 Aug 15;263(23):11498–11503. [PubMed] [Google Scholar]
- Yamada E. W., Shiffman F. H., Huzel N. J. Ca2+-regulated release of an ATPase inhibitor protein from submitochondrial particles derived from skeletal muscles of the rat. J Biol Chem. 1980 Jan 10;255(1):267–273. [PubMed] [Google Scholar]
- Zoratti M., Petronilli V., Azzone G. F. ATP synthase-mediated proton fluxes and phosphorylation in rat liver mitochondria: dependence on delta mu H. Biochim Biophys Acta. 1986 Aug 13;851(1):123–135. doi: 10.1016/0005-2728(86)90255-0. [DOI] [PubMed] [Google Scholar]
- Zoratti M., Petronilli V. Multiple relationships between rate of oxidative phosphorylation and delta microH in rat liver mitochondria. FEBS Lett. 1985 Dec 2;193(2):276–282. doi: 10.1016/0014-5793(85)80168-x. [DOI] [PubMed] [Google Scholar]