Abstract
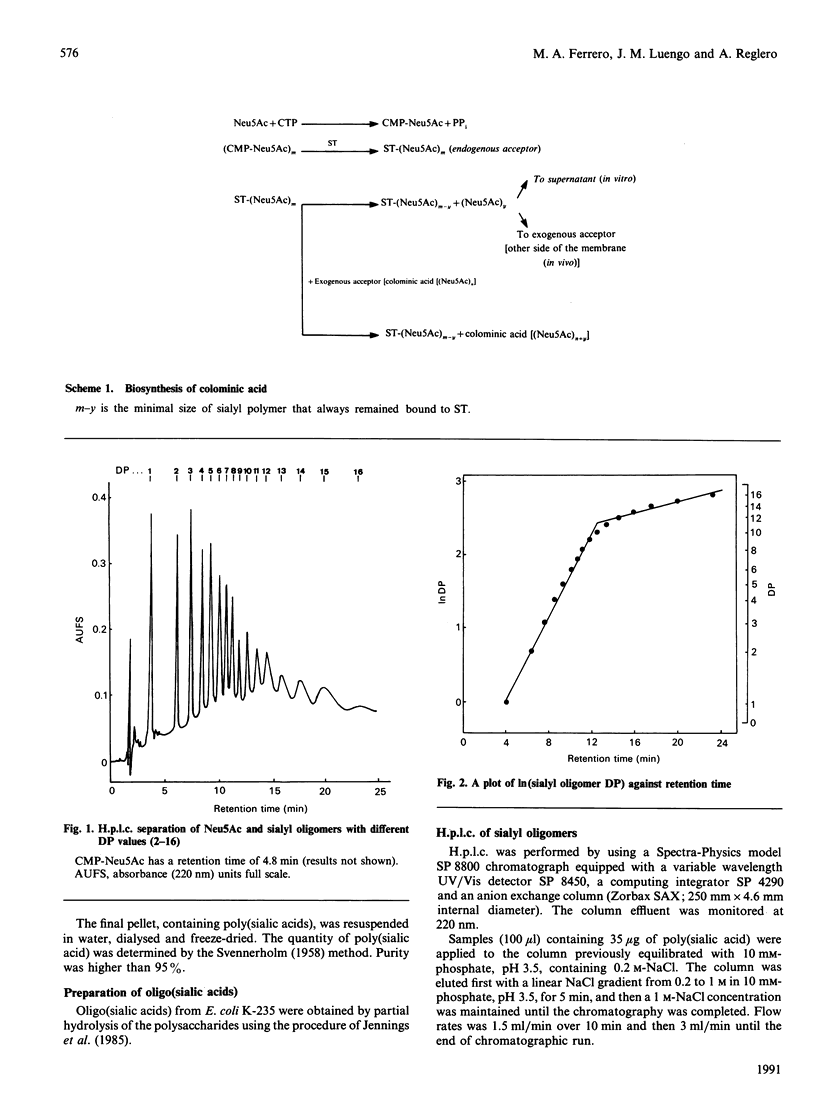
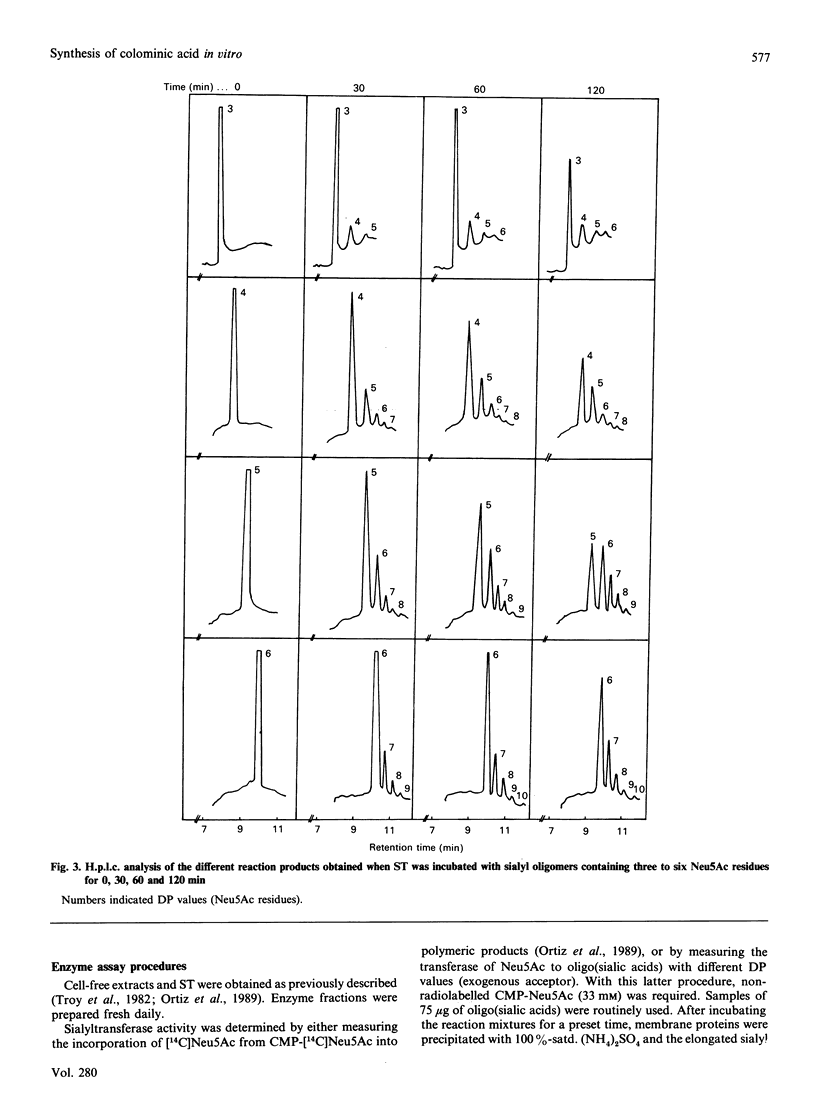
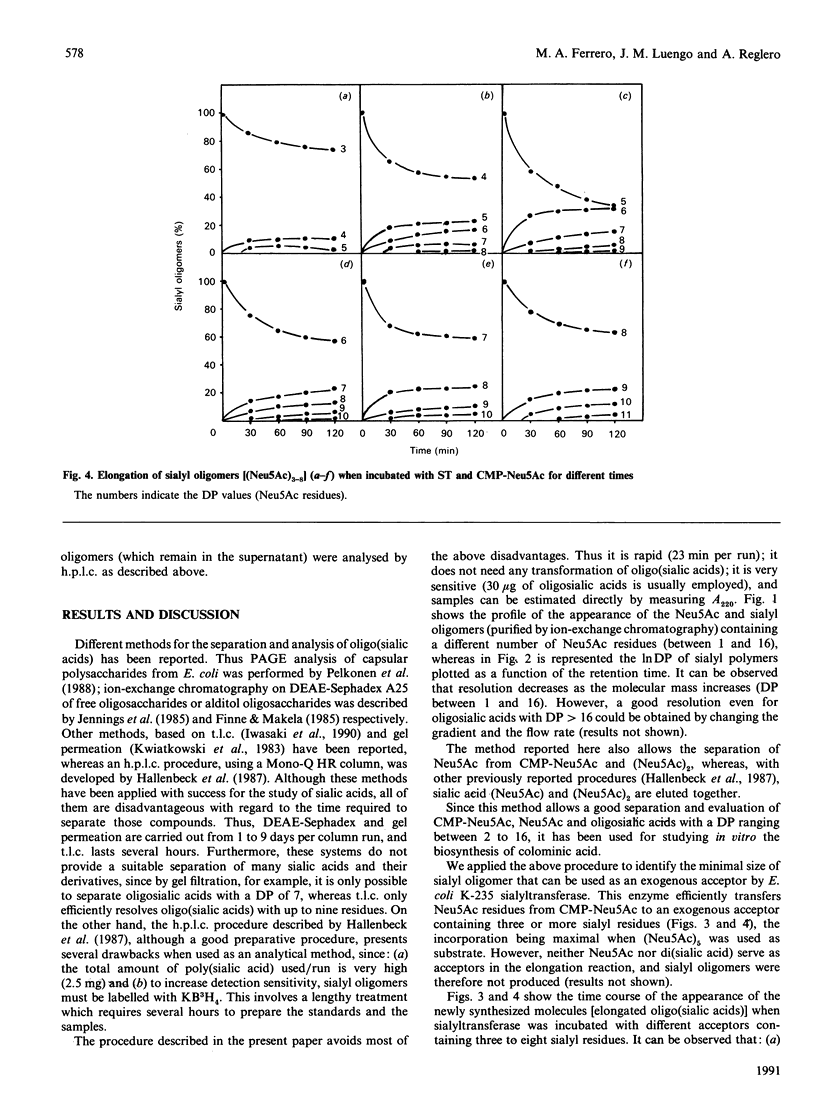
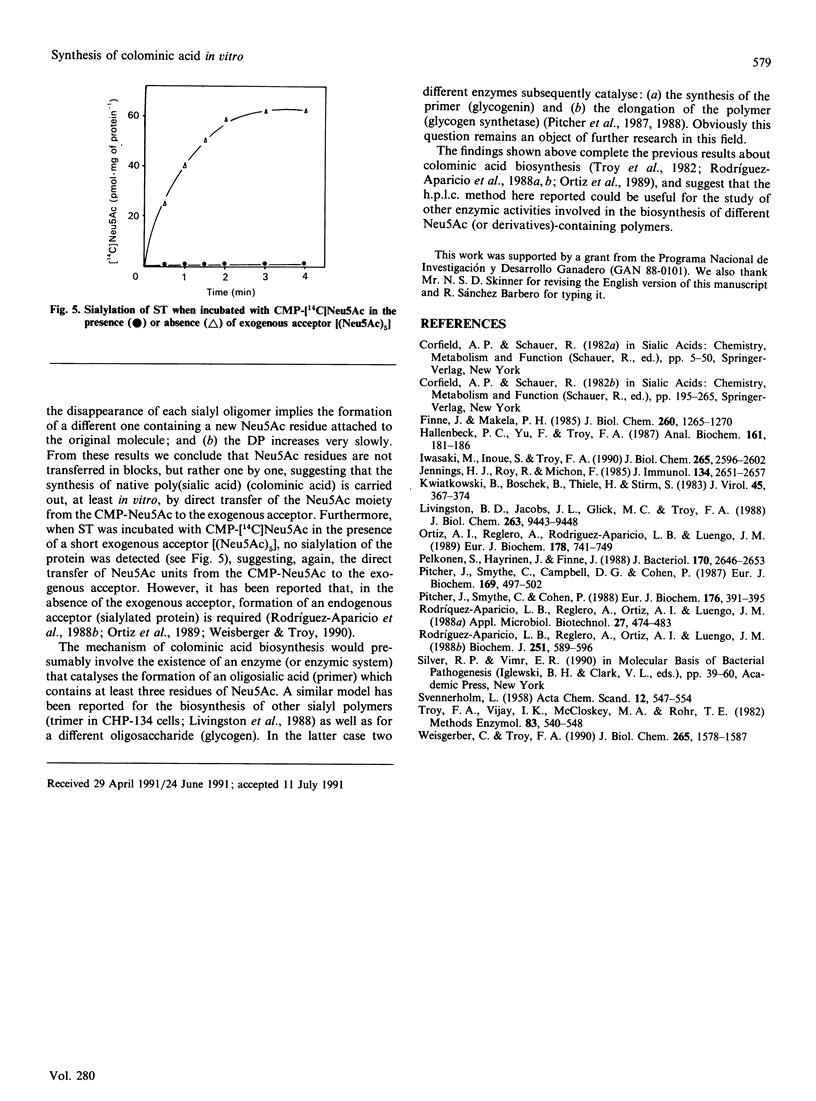
A rapid, sensitive and easy h.p.l.c. method was developed for the quantitative analysis of oligosialic acids. This procedure which permits the complete separation (in 23 min) of several sialyloligomers with a degree of polymerization of between 1 and 16, has been employed to establish the minimal chain length of oligomer accepted, as an exogenous acceptor, by Escherichia coli K-235 sialytransferase complex (ST) leading to the synthesis in vitro of colominic acid. We showed that this membrane-bound enzyme catalyses the direct transfer of Neu5Ac residues (one by one) from CMP-Neu5Ac to an exogenous acceptor molecule which contains at least three Neu5Ac residues. Free Neu5Ac or (Neu5Ac)2 were not recognized as substrates, whereas the maximal rate of polymer elongation was achieved when (Neu5Ac)5 was used as substrate.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Hallenbeck P. C., Yu F., Troy F. A. Rapid separation of oligomers of polysialic acid by high-performance liquid chromatography. Anal Biochem. 1987 Feb 15;161(1):181–186. doi: 10.1016/0003-2697(87)90670-1. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Inoue S., Troy F. A. A new sialic acid analogue, 9-O-acetyl-deaminated neuraminic acid, and alpha -2,8-linked O-acetylated poly(N-glycolylneuraminyl) chains in a novel polysialoglycoprotein from salmon eggs. J Biol Chem. 1990 Feb 15;265(5):2596–2602. [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985 Apr;134(4):2651–2657. [PubMed] [Google Scholar]
- Kwiatkowski B., Boschek B., Thiele H., Stirm S. Substrate specificity of two bacteriophage-associated endo-N-acetylneuraminidases. J Virol. 1983 Jan;45(1):367–374. doi: 10.1128/jvi.45.1.367-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston B. D., Jacobs J. L., Glick M. C., Troy F. A. Extended polysialic acid chains (n greater than 55) in glycoproteins from human neuroblastoma cells. J Biol Chem. 1988 Jul 5;263(19):9443–9448. [PubMed] [Google Scholar]
- Ortiz A. I., Reglero A., Rodríguez-Aparicio L. B., Luengo J. M. In vitro synthesis of colominic acid by membrane-bound sialyltransferase of Escherichia coli K-235. Kinetic properties of this enzyme and inhibition by CMP and other cytidine nucleotides. Eur J Biochem. 1989 Jan 2;178(3):741–749. doi: 10.1111/j.1432-1033.1989.tb14505.x. [DOI] [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J., Smythe C., Campbell D. G., Cohen P. Identification of the 38-kDa subunit of rabbit skeletal muscle glycogen synthase as glycogenin. Eur J Biochem. 1987 Dec 15;169(3):497–502. doi: 10.1111/j.1432-1033.1987.tb13637.x. [DOI] [PubMed] [Google Scholar]
- Pitcher J., Smythe C., Cohen P. Glycogenin is the priming glucosyltransferase required for the initiation of glycogen biogenesis in rabbit skeletal muscle. Eur J Biochem. 1988 Sep 15;176(2):391–395. doi: 10.1111/j.1432-1033.1988.tb14294.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Aparicio L. B., Reglero A., Ortiz A. I., Luengo J. M. A protein-sialyl polymer complex involved in colominic acid biosynthesis. Effect of tunicamycin. Biochem J. 1988 Apr 15;251(2):589–596. doi: 10.1042/bj2510589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy F. A., Vijay I. K., McCloskey M. A., Rohr T. E. Synthesis of capsular polymers containing polysialic acid in Escherichia coli 07-K1. Methods Enzymol. 1982;83:540–548. doi: 10.1016/0076-6879(82)83050-4. [DOI] [PubMed] [Google Scholar]
- Weisgerber C., Troy F. A. Biosynthesis of the polysialic acid capsule in Escherichia coli K1. The endogenous acceptor of polysialic acid is a membrane protein of 20 kDa. J Biol Chem. 1990 Jan 25;265(3):1578–1587. [PubMed] [Google Scholar]


