Abstract
To instruct the production of millet porridge, the effect of cooking methods on flavor and texture of millet porridge was investigated. A total of 91 volatiles were detected and most volatile compounds decreased with cooking time, e.g. alcohols. The esters as major volatiles had a high content in electric rice cooker (IC). Multiple chemometric results indicated that volatiles from different cooking methods were distinguished respectively. Texture analysis indicated that the hardness of millet porridge prepared in IC had a more dominant decrease trend than electromagnetic oven and the electric pressure cooker before 40 min. In conclusion, different cooking methods had a more significant influence on the volatiles than cooking time, while the texture is opposite. The comprehensive sensory score reached its peak in IC-30 min. The comprehensive sensory scores of IC and EC decreased with the prolongation of cooking time. This study helps to improve the sensory attributes of millet porridge.
Keywords: Millet porridge, Volatile compounds, Texture, Cooking methods
Highlights
-
•
The esters as major volatiles have a relatively high content in electric rice cooker (IC).
-
•
The hardness of IC had a more significant decrease trend before 40 min.
-
•
IC-30 min attained the highest sensory comprehensive score.
-
•
Different cooking methods had more influence on the flavor than cooking time.
-
•
Different cooking time had more influence on the texture than cooking methods.
1. Introduction
Millet as a Poaceae Setaria herb is originally produced in China. Its output accounts for >90% of the world's total output (Li, Hu, Zhang, Jiang, & Han, 2019; Yang et al., 2017). Millet is a warm, drought-tolerant, short-day crop, with low soil requirements and strong adaptability. Millet has high nutritional value, rich in protein, vitamins, niacin, calcium, etc., suitable for people to supplement nutrition. It also has functions such as strengthening the spleen, nourishing the stomach, tonifying the kidneys, clearing heat and detoxifying, and diuresis (Qiao & Wang, 2015). That is why millet has always been one of the important major food crops worldwide. The peeled grain is called “millet”, which is the raw material for maltose making and wine making, and its main way of eating is to cook porridge. Millet porridge is popular among consumers because of its rich nutrition and unique flavor.
The variety of millet will affect the volatile compounds and texture attributes that produce the unique aroma of millet porridge. It has been found that the viscosity of different millet cooked into porridge is positively correlated with amylose content, and negatively correlated with protein content; the aldehydes, ketones, and acid compounds are negatively correlated with protein content (Zhou et al., 2023). Compared with millet varieties, cooking methods have a greater impact on the flavor of millet porridge (Bi et al., 2019; Liu, Liu, Zhao, Li, & Zhang, 2011; Zhang et al., 2019). Odor is one of the important sensory qualities of food, mainly produced by volatile components that stimulate olfactory cells and directly affect consumers' liking. Previous study evaluated the effects of different cooking methods (roasting, boiling, freeze-drying after boiling) on volatile compounds in millet (Bi et al., 2019). However, the object of this experiment was millet, not cooked millet porridge. For millet porridge, the difference of volatile components in millet porridge cooked in different electric cookers was studied based on GC-IMS technology (Zhang et al., 2021). In addition, some studies believed that high-pressure cooking could improve the sensory characteristics of cereals (Deng et al., 2013; Yu, Turner, Fitzgerald, Stokes, & Witt, 2017). However, current research on porridge was still limited to commercial research, lacking guiding research for residents. It is difficult to improve the eating quality of porridge through these methods in daily life. Therefore, the effect of both cooking methods and cooking time on millet porridge were taken into account in our study. This is the most effective way to improve the quality of millet porridge in homemade.
In this study, Shanxi yellow millet was selected as the experimental material. The volatile compounds and texture attributes were taken as the main evaluation objects to study the effects of different cookers and different cooking time (30 min, 40 min, 50 min, 60 min) on the flavor and texture of porridge. The volatile compounds in millet porridge were abstracted by headspace solid phase microextraction (HS-SPME) and analyzed by gas chromatography–mass spectrometry (GC–MS). Texture analysis used texture instruments to imitate people's chewing process, quantify the texture attributes of porridge. The sensory evaluation was added to better understand consumer preferences. The study can fill in the lack of influence of different cooking methods and time on the flavor and texture attributes of millet porridge in scientific research literature. It also improves the sensory quality of millet porridge, helping to provide guidance for residents to cook high quality millet porridge.
2. Materials and methods
2.1. Materials
Shanxi yellow millet (protein content 9.7 g/100 g, fat content 2.5 g/100 g, carbohydrate 74.9 g/100 g) was preliminarily reaped in Shanxi province in China.
2.2. Porridge cooking and sample preparation
Shanxi yellow millet was washed repeatedly with distilled water for at least 3 times. Then 100 g of millet were taken out each and placed them in an electric rice cooker (IC), (Triangle Inc., Guangdong, China), electric pressure cooker (EPC) (Supor Inc., Zhejiang, China), electromagnetic oven (EC) (Supor Inc., Zhejiang, China), respectively. The water was added to different cookware in a certain proportion (millet: water = 1:20) to ensure that the water completely submerges millet. The cooker was closed and set the power to 900 W and started cooking. The millet porridge samples were made at different cooking time (30 min, 40 min, 50 min, 60 min). To avoid any effect of retrogradation, the volatile compounds and textural analysis procedures were started immediately after cooking.
2.3. Determination of volatile compounds
The volatile compounds of millet porridge were abstracted by the means of headspace-solid phase microextraction (HS-SPME). The SPME method was referred to Xu et al. (2022) with slight changes. The millet porridge sample was weight 8 g and put into a 20 mL vial. Then 10 μL of standard solution (1,2-dichlorobenzene, 5.155 μg/mL) was added to each sample. Subsequently, the sample bottle was stirred at 60 °C for 30 min at 200 rpm and heated in a magnetic water bath according to our previous study (Wang et al., 2023, Wang et al., 2024). The aged extraction head was inserted into the headspace of the sample bottle and extracted at 60 °C for 30 min. The adsorbed extraction head was taken out and quickly inserted into the GC injection port, analyzed at 280 °C for 5 min. The Clarus690-SQ8T (PerkinElmer, USA) was used to analyze the volatile compounds on GC–MS apparatus based on the protocol described by previous studies (Jiang et al., 2023; Xu et al., 2022).
The headspace sampling used the 50 μm/30 μm DVB/CAR/PDMS fiber (Supelco Inc., Bellefonte, PA, USA). The capillary chromatography column was Elite-5MS column (30 m × 0.25 mm × 0.5 μm). Program heating: The starting temperature was 40 °C and kept for 3 min, then it raise from 10 °C/min to 230 °C and kept for 3 min. The carrier gas was Helium, and the flow rate was 1 mL /min.
For mass spectrometry analysis, it was running in electron ionization mode (EI, 70 eV). The data collection is installed to a mass fragment from 30 m/z to 500 m/z. The volatile compounds were assigned by contrasting their retention indices (RI), and real standards for mass spectrometry data. The internal standard method was used to calculate the quantification of each compound. The calculation formula for retention index (RI) is as follows: RI = 100Z + 100 [logt'R (x) - logt'R (z)]/[logt'R (z + 1) - logt'R (z)], where t'R is the corrected retention time; Z and Z + 1 represent the number of carbon atoms present in the n-alkanes of the target compound (X) before and after efflux, respectively; Here: t'R (z) < t'R (x) < t'R (z + 1), the number of carbon atoms Z in normal alkanes is generally >4.
2.4. Texture profile analysis (TPA)
The texture profile analysis method was executed according to Wang et al. (2023) with slight modifications. After the cooked millet porridge was filtered, it was pressed with the bottom of the spoon and laid evenly on the bottom of the small beaker to prepare the sample to be tested. The textural analysis of millet porridge samples was analyzed by a texture analyzer (TA-XT plus, Stable Micro Systems Ltd., UK). The porridge samples were arranged on the platform and conducted testing. Texture analysis used dual loop compression program (TPA), and TPA parameters were as follows: 60% strain, the pre-test speed, test speed, and post-test speed were set at 1.0, 30, and 1 mm/s respectively, and the pattern of cylindrical probe is P/36. Each sample was repeated 3 times. After each parallel experiment, the plane of the sample to be tested must be flattened again to avoid experimental errors caused by uneven thickness.
2.5. Sensory evaluation
The sensory evaluation method was executed according to Mestres, Briffaz, and Valentin (2019) with minor modifications. Briefly, 20 professional members (10 women and 10 men, aged between 23 and 30) were selected to constitute a sensory evaluation team. All the participants gave their consent to take part in the sensory study and use their information and the ethical permission of this study was given by Qingdao University Ethics Committee (No. QDU-HEC-2023159). The team members engaged in a long-term training course to recognize, categorize, and strengthen the proportion of millet porridge quality characteristics to generate appropriate terminology to describe the general millet porridge quality. Finally, 6 descriptors were formed to describe and distinguish the different porridge samples. They described the appearance, texture, fragrance and taste of the porridges. In order to understand the overall situation, a comprehensive rating was also compiled in the end. The strength of these 6 descriptors was scored in a ratio of 0–9 (from 0, invisible, to 9, strong). The samples (30 mL) were placed in 50 mL plastic cups, encoded with numbers, presented to the team members randomly. The temperature of porridge samples was controlled at 35 °C, and then evaluated by each volunteers. Each sample was analyzed in triplicate.
2.6. Data analysis
All analyses were repeated in triplicate. The relational results were indicated as mean ± standard deviation (SD). The one-factor analysis of variance (ANOVA) was conducted by SPSS version 20 (SPSS Inc., Chicago, IL, USA). The P-values (95% confidence intervals) were evaluated by the ANOVA test and Duncan's multiple comparisons test. The principal component analysis (PCA) was conducted by SIMCA-P (version 14.1, Umea, Sweden). The heatmap for unsupervised clustering (Chen et al., 2020) was performed using the TBtools (version 1.120, China). The OriginPro 2021 (OriginLab Corporation, Northampton, MA, USA.) was applied to generate the figures.
3. Results and discussion
3.1. Influence of cooking methods on volatile compounds of millet porridge
3.1.1. PCA and Heatmap, hierarchical cluster analysis
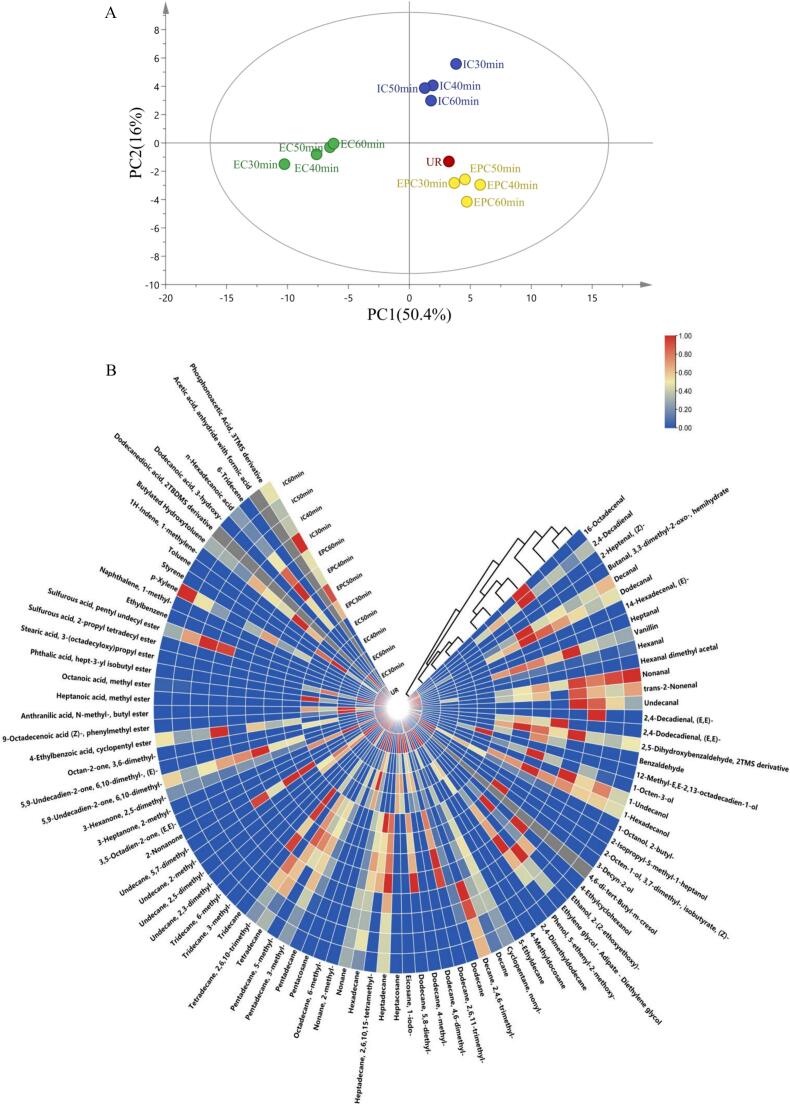
The influence of cooking methods and time on the volatile compounds and texture of rice porridge was described by our previous study (Wang et al., 2023). However, limited information is available to evaluate the cooking quality of millet porridge. The principal component analysis is a well-known method to remove redundant information in multidimensional data sets by means of mode reduction (Martinez & Kak, 2001). Therefore, a PCA chart (Fig. 1A) was suitable to evaluate the changing trend in volatile compounds under different cooking methods and time in millet porridge. In general, the PCA model was regarded as the separation model when the contribution rate achieved 60% (Chen et al., 2020). The PCA showed that PC1 and PC2 amounted to 66.4% of the total variance (50.4% and 16.0%, separately), indicating that the PC1 and PC2 can explain most of the volatile compound information. In general, a total of 13 kinds of millet porridge samples were distributed respectively, indicating that the millet porridge was affected significantly by different cooking methods.
Fig. 1.
PCA analysis (A) and heatmap analysis (B) of aroma components in millet porridge prepared in 3 kinds of cookers with different cooking time, EPC: Millet porridge prepared in the electric pressure cooker; EC: Millet porridge prepared in the electromagnetic oven; IC: Millet porridge prepared in the electric rice cooker.
On the basis of the results of PCA, the millet porridge samples under different cooking methods were divided into 4 groups according to the volatile compounds. The first group included EC-30 min, EC-40 min, EC-50 min, and EC-60 min, almost all located in the lower left quadrant throughout the PCA score plot. The second group embodied EPC-30 min, EPC-40 min, EPC-50 min, and EPC-60 min, which were collected in the lower right quadrant. The third group involved IC-30 min, IC-40 min, IC-50 min, and IC-60 min, gathered in the upper right quadrant. The fourth group is the Uncooked millet (UR) group, located in the lower right quadrant. However there is still a difference in the distribution position from the EPC.
The heatmap (Fig. 1B) can further explain why the volatile compounds of millet porridge under different cooking methods can be divided into four groups. The volatile compounds involving aldehydes, alcohols, hydrocarbons, aromatic compounds, esters, ketones, and acids, were determined and presented in the heatmap. As shown in Fig. 1B, each average concentration of volatile compound was presented (from blue to red) by rectangular colored legends on the heatmap. The first group contained EC-30 min, EC-40 min, EC-50 min, and EC-60 min, owing to their relatively low aldehydes content, which was opposite to the fourth group. The second group contained EPC-30 min, EPC-40 min, EPC-50 min, and EPC-60 min, which might be due to a low content of esters. The third group embraced IC-30 min, IC-40 min, IC-50 min, and IC-60 min, as a result of a generally low content of most substances except for some aldehydes. The aldehyde content of the UR sample in the fourth batch was higher than that of other samples.
3.1.2. Change of key volatile compounds in millet porridge during cooking
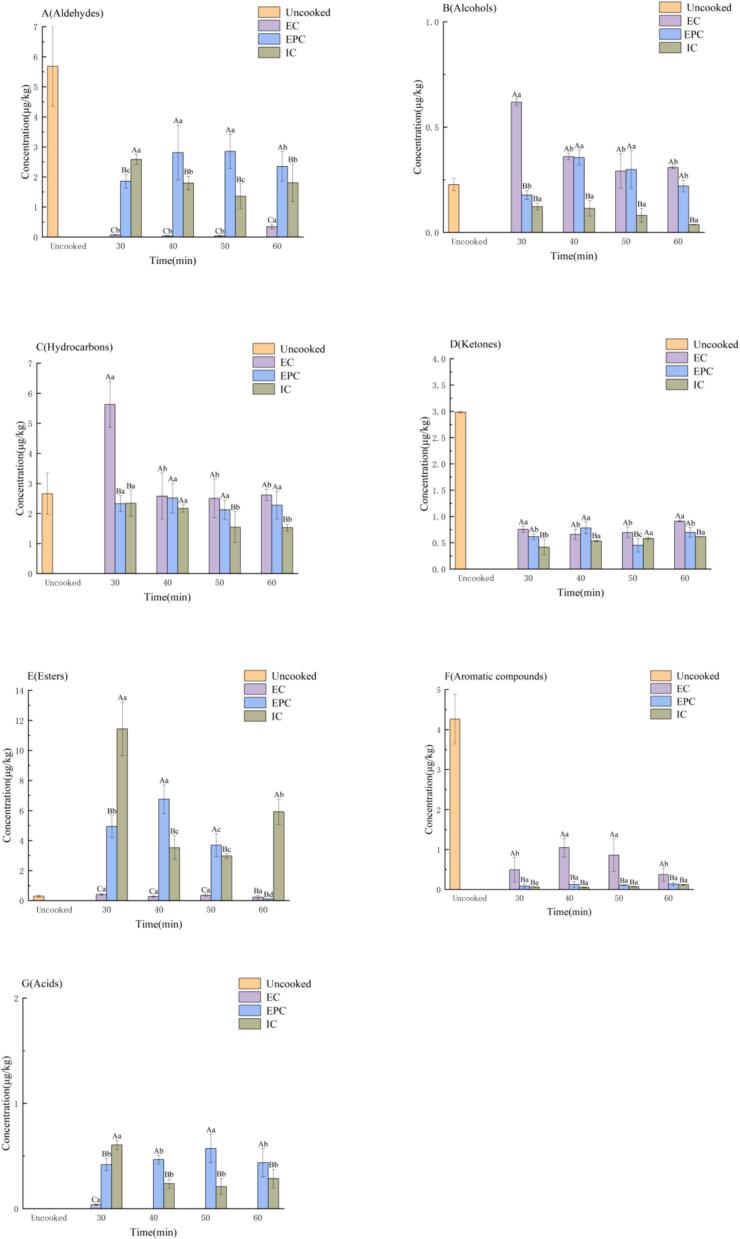
As shown in Fig. 2, 91 volatile compounds in millet porridge samples were classified and showed. Fig. 2 (A) presented the total concentration of aldehydes in EC, EPC, IC and uncooked millet. Aldehydes had a low threshold and played an important role in aroma contribution, mainly associated with oil, fat, fruity, and nutty flavors (Zhang et al., 2024). Meanwhile, aldehydes were regarded as crucial volatile compounds in rice porridge recently (Wang et al., 2023). Specifically, Fig. 2 (A) revealed that the total concentration of aldehydes is the highest in uncooked millet porridge compared with three different cooking methods. This is may be explained that the high steam and heat had negative influence on volatile compounds (Zhang et al., 2019). Similar to the properties of low boiling point volatile compounds like aldehydes, the Fig. 2 (B) also showed that the total alcohol concentration caused by the three cooking methods decreased over heating time due to high steam. The highest alcohols concentration occurred in EC-30 min, and the lowest concentration was in IC-60 min.
Fig. 2.
The concentration of different kinds of aroma compounds in millet porridge samples, Aldehydes (A); Alcohols (B); Hydrocarbons (C); Ketones (D); Esters (E); Aromatic compounds (F); Acids (G); EPC: Millet porridge prepared in the electric pressure cooker; EC: Millet porridge prepared in the electromagnetic oven; IC: Millet porridge prepared in the electric rice cooker; UR: Uncooked millet.
Hydrocarbons could be observed in Fig. 2 (C) with the relatively high content of all the volatile compounds. The n-hydrocarbons identified ranged mainly from C8 to C19 and may be due to decarboxylation and decomposition of carbon chains of longer fatty acids and most had little contribution to flavor characteristics (Zhang et al., 2019). As time went on, the content of hydrocarbons in EC and IC gradually decreased. Hydrocarbon had the most content in EC, followed by EPC and IC. Fig. 2 (D) indicated that the content of ketones was the highest in uncooked millet; the EC trend decreased first and then increased at 40 min. However, at different time periods, the ketones content did not differ significantly among three different cooking methods.
Fig. 2 (E) displayed the changes of esters. Compared with other volatile compounds, esters are associated with fruit, flower, sweetness odor, and have low odor threshold values, resulting in contributing significantly to the sense of smell (Xu, Zhao, et al., 2022). IC had the highest total esters content compared with EC and EPC. The reason was that EPC generated more heat and EC generated more steam during cooking, which made the esters in millet porridge hydrolyze into fatty acids, losing a large amount of esters. Therefore, IC reserved more ester content comparatively (Tananuwong & Lertsiri, 2010). Fig. 2(F) showed the changes of aromatic compounds in EC, EPC and IC. Aromatic compounds were abundant in uncooked millet. The aromatic compounds content in EC increased first, reached its peak in EC-40 min and then decreased. The changing tendency of aromatic compounds in EPC and IC is similar and their contents are very low. As Fig. 2 (G), a large amount of acid was detected in EPC and IC, what is more, only partial acid can be observed at EC-30 min due to excessive steam evaporating. After 30 min, due to different heating method, the change in acid content in IC was more obvious than that in EPC. When the millet porridge boiled, the acids evaporated from millet samples with abundant steam due to its volatility. Similar to the aforementioned principle, the acid content in EPC was higher than that in IC after 30 min.
The content of hydrocarbons gradually decreased with the cooking time. The changing trend in IC and EC were more significant. This result may be due to the large amount of steam generated by IC and EC during the cooking process, which affected negatively on volatile compounds. Due to its relatively closed state, EPC reduced the wastage of volatile compounds and stabilized their content. However, the negative influence of temperature led to relatively low but steady changes in EPC.
3.1.3. Analysis of volatile compounds in millet porridge
According to GC–MS analysis, 91 volatiles were detected, containing 18 aldehydes, 9 alcohols, 31 hydrocarbons, 6 ketones, 14 esters, 9 aromatic compounds, 4 acids (Table 1). In these compounds, 20 kinds of volatiles were unique to uncooked millet, and 51 were detected in other different cooking methods. The volatiles discovered in uncooked millet were mainly aldehydes, ketones, and aromatic compounds. The new volatiles of millet porridge in other different cooking methods were mainly alcohols, hydrocarbons and acids. Moreover, most esters in millet porridge increased rapidly compared with uncooked millet after cooking.
Table 1.
Changes of key aroma compounds of millet porridges prepared in 3 kinds of cookers with different cooking time.
|
Volatile compounds(μg/kg) |
Cooking time/min |
||||||
|---|---|---|---|---|---|---|---|
| RI | Cooker | Uncooked millet | 30 | 40 | 50 | 60 | |
| Aldehydes | |||||||
| Vanillin | 949 | EC | ND | ND | ND | ND | ND |
| EPC | 0.565 ± 0.065Bb | 0.618 ± 0.2Aa | 0.673 ± 0.17Aa | 0.465 ± 0.062Ac | |||
| IC | 0.8 ± 0.032Aa | 0.35 ± 0.023Bb | 0.272 ± 0.095Bc | 0.225 ± 0.012Bc | |||
| Undecanal | 1699 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.038 ± 0.0001a | 0.033 ± 0a | 0.021 ± 0.001b | 0.011 ± 0.011c | |||
| 16-Octadecenal | 2097 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | 0.034 ± 0.008Aa | |||
| IC | ND | ND | ND | ND | |||
| Nonanal | 1099 | EC | 0.504 ± 0.004 | ND | ND | ND | 0.215 ± 0.064Ba |
| EPC | 0.183 ± 0.019Bc | 0.373 ± 0.141Ba | 0.273 ± 0Bb | 0.244 ± 0.088Bb | |||
| IC | 0.507 ± 0.045Aab | 0.423 ± 0.059Ab | 0.496 ± 0.147Aab | 0.534 ± 0.126Aa | |||
| Hexanal dimethyl acetal | 1088 | EC | 0.142 ± 0.049 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Hexanal | 787 | EC | 3.44 ± 0.835 | ND | ND | ND | 0.037 ± 0.008Ca |
| EPC | 0.093 ± 0.043Bc | 0.417 ± 0.112Aa | 0.467 ± 0.051Aa | 0.151 ± 0.014Bb | |||
| IC | 0.122 ± 0Ac | 0.342 ± 0.061Ba | 0.067 ± 0.025Bd | 0.293 ± 0.265Ab | |||
| Dodecanal | 1591 | EC | 0.037 ± 0.011 | ND | ND | ND | ND |
| EPC | 0.028 ± 0.001Bd | 0.052 ± 0.025Aa | 0.032 ± 0.0007Ac | 0.044 ± 0.024Ab | |||
| IC | 0.036 ± 0.0007Aa | 0.028 ± 0.002Bb | 0.018 ± 0.002Bc | 0.024 ± 0.005Bb | |||
| Decanal | 1200 | EC | 0.196 ± 0.064 | 0.07 ± 0.009Bb | 0.038 ± 0.008Cc | 0.038 ± 0.011Cc | 0.091 ± 0.001Ca |
| EPC | 0.07 ± 0.001Bc | 0.235 ± 0.161Aa | 0.098 ± 0.025Bc | 0.125 ± 0.059Bb | |||
| IC | 0.14 ± 0.024Aa | 0.101 ± 0.012Bb | 0.122 ± 0.021Aab | 0.162 ± 0.019Aa | |||
| Butanal, 3,3-dimethyl-2-oxo-, hemihydrate | 1487 | EC | 0.033 ± 0.013 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Heptanal | 897 | EC | 0.691 ± 0.22 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| trans-2-Nonenal | 1154 | EC | 0.058 ± 0.0005 | ND | ND | ND | ND |
| EPC | 0.021 ± 0.002Bb | 0.035 ± 0.007Aa | 0.028 ± 0.007Ab | ND | |||
| IC | 0.052 ± 0.002Aa | 0.037 ± 0.001Ab | 0.024 ± 0.002Ac | 0.04 ± 0.014Ab | |||
| 2-Heptenal, (Z)- | 955 | EC | 0.107 ± 0.003 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| E-14-Hexadecenal | 1144 | EC | 0.028 ± 0.003 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 2,5-Dihydroxybenzaldehyde, 2TMS derivative | 1096 | EC | 0.33 ± 0.091 |
ND |
ND |
ND |
ND |
| EPC | 0.499 ± 0.065Bc | 0.618 ± 0.273Ab | 0.743 ± 0.28Aa | 0.403 ± 0.066Ac | |||
| IC | 0.746 ± 0.041Aa | 0.306 ± 0.046Bb | 0.259 ± 0.091Bc | 0.368 ± 0.118Ab | |||
| 2,4-Decadienal | 1413 | EC | ND | ND | ND | ND | ND |
| EPC | 0.176 ± 0.004Ab | 0.188 ± 0.017Ab | 0.266 ± 0.016Ab | 0.42 ± 0.078Aa | |||
| IC | 0.134 ± 0.02Ba | 0.017 ± 0.001Bc | 0.074 ± 0.021Bb | 0.146 ± 0.046Ba | |||
| Benzaldehyde | 1296 | EC | 0.11 ± 0.019 | ND | ND | ND | ND |
| EPC | 0.042 ± 0.011b | 0.082 ± 0.057a | ND | 0.038 ± 0.007b | |||
| IC | ND | ND | ND | ND | |||
| 2,4-Decadienal, (E,E)- | 1413 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | 0.017 ± 0.001Aa | ND | ND | |||
| 2,4-Dodecadienal, (E,E)- | 1315 | EC | ND | ND | ND | ND | ND |
| EPC | 0.176 ± 0.004b | 0.188 ± 0.017b | 0.266 ± 0.016b | 0.42 ± 0.078a | |||
| IC | ND | ND | ND | ND | |||
| Alcohols | |||||||
| 1-Undecanol | 1666 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.043 ± 0.008a | 0.024 ± 0.0007c | 0.033 ± 0.015b | 0.018 ± 0.0004d | |||
| 1-Octen-3-ol | 978 | EC | 0.134 ± 0.019 | ND | ND | ND | ND |
| EPC | 0.041 ± 0.001Ac | 0.205 ± 0.134Aa | 0.067 ± 0.002Ab | 0.074 ± 0.04Ab | |||
| IC | ND | 0.066 ± 0.025Ba | 0.008 ± 0.008Bb | ND | |||
| 1-Octanol, 2-butyl- | 1726 | EC | 0.057 ± 0.006 | 0.578 ± 0.012Aa | 0.36 ± 0.016Ab | 0.291 ± 0.082Ab | 0.308 ± 0.005Ab |
| EPC | 0.091 ± 0.002Ba | 0.095 ± 0.037Ba | 0.101 ± 0.002Ba | 0.05 ± 0.05Bb | |||
| IC | ND | ND | ND | ND | |||
| 2-Octen-1-ol, 3,7-dimethyl-, isobutyrate, (Z)- | 1056 | EC | ND | 0.039 ± 0.002Aa |
ND |
ND |
ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 3-Decyn-2-ol | 1388 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | 0.023 ± 0.003Aa | ND | |||
| IC | ND | ND | ND | ND | |||
| 1-Hexadecanol | 1842 | EC | 0.035 ± 0.003 | ND | ND | ND | ND |
| EPC | 0.028 ± 0.0004Ab | 0.04 ± 0.01Aa | 0.029 ± 0.003Ab | 0.029 ± 0.003Ab | |||
| IC | 0.029 ± 0.002Aa | 0.023 ± 0.01Ba | 0.023 ± 0.007Aa | 0.018 ± 0.0006Bab | |||
| 1-Heptanol, 2-propyl- | 1054 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | 0.015 ± 0.001Aa | ND | |||
| 4-Ethylcyclohexanol | 1389 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | ND | ND | ND | 0.048 ± 0.006Aa | |||
| IC | ND | ND | ND | ND | |||
| Ethanol, 2-(2-ethoxyethoxy)- | 1345 | EC | ND | ND | ND | ND | ND |
| EPC | 0.016 ± 0.016Bb | 0.012 ± 0.012Ab | 0.077 ± 0.077Aa | 0.016 ± 0.016Ab | |||
| IC | 0.049 ± 0.004Aa | ND | ND | ND | |||
| Hydrocarbons | |||||||
| Undecane, 5,7-dimethyl- | 1012 | EC | ND | ND | ND | ND | ND |
| EPC | 0.039 ± 0.002a | 0.018 ± 0.018b | 0.04 ± 0.002a | 0.037 ± 0.009a | |||
| IC | ND | ND | ND | ND | |||
| Undecane, 2-methyl- | 1162 | EC | ND | 0.079 ± 0.012a | 0.062 ± 0.007b | 0.039 ± 0.016d | 0.045 ± 0.0001c |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Undecane, 2,3-dimethyl- | 1546 | EC | 0.104 ± 0.076 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Undecane, 2,5-dimethyl- | 1241 | EC | ND | 0.136 ± 0.012a | 0.089 ± 0.009c | 0.08 ± 0.017c | 0.103 ± 0.004b |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Tridecane | 1198 | EC | ND | ND | ND | ND | ND |
| EPC | 0.053 ± 0.002a | 0.03 ± 0b | 0.051 ± 0.006a | 0.051 ± 0.016a | |||
| IC | ND | ND | ND | ND | |||
| Tetradecane, 2,6,10-trimethyl- | 2063 | EC | ND |
0.08 ± 0.025Aa |
0.033 ± 0.0005Bb |
0.032 ± 0.006Ab |
0.008 ± 0.008Bc |
| EPC | ND | ND | ND | ND | |||
| IC | 0.064 ± 0.005Ba | 0.048 ± 0.001Ab | 0.023 ± 0.011Bc | 0.016 ± 0.0002Ad | |||
| Tetradecane | 1271 | EC | 0.444 ± 0.012 | 0.207 ± 0.008Ba | 0.078 ± 0.004Bb | 0.067 ± 0.007Bb | 0.04 ± 0.003Cc |
| EPC | 0.304 ± 0.021Aab | 0.391 ± 0.031Aa | 0.333 ± 0.004Aa | 0.296 ± 0.035Ab | |||
| IC | 0.283 ± 0.001Aab | 0.304 ± 0.026Aa | 0.088 ± 0.088Bc | 0.171 ± 0.018Bb | |||
| Tridecane, 3-methyl- | 1368 | EC | 0.11 ± 0.002 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Tridecane, 6-methyl- | 1500 | EC | 0.032 ± 0.004 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Pentadecane, 3-methyl- | 1748 | EC | 0.066 ± 0.01 | ND | ND | ND | ND |
| EPC | 0.036 ± 0.0003Aa | 0.042 ± 0.005Aa | 0.033 ± 0.01Aa | 0.037 ± 0.005Aa | |||
| IC | 0.041 ± 0.006Aa | 0.027 ± 0.003Bb | 0.014 ± 0.003Bb | 0.015 ± 0.001Bb | |||
| Pentadecane | 1689 | EC | 0.202 ± 0.112 | 0.81 ± 0.221Aa | 0.411 ± 0.048Ab | 0.372 ± 0.025Abc | 0.214 ± 0.021Bc |
| EPC | 0.278 ± 0.05Bb | 0.207 ± 0.032Bbc | 0.278 ± 0.052Bb | 0.314 ± 0.163Aa | |||
| IC | 0.236 ± 0.038Ba | 0.13 ± 0.02Cb | 0.115 ± 0.025Cb | 0.124 ± 0.009Cb | |||
| Pentadecane, 5-methyl- | 2055 | EC | 0.082 ± 0.041 | ND | ND | ND | ND |
| EPC | 0.034 ± 0.005b | 0.062 ± 0.02a | 0.03 ± 0.003b | 0.037 ± 0.01b | |||
| IC | ND | ND | ND | ND | |||
| Octadecane, 6-methyl- | 1413 | EC | ND | 0.121 ± 0.013a | 0.07 ± 0.004b | 0.035 ± 0.012c | 0.018 ± 0.018d |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Nonane, 2-methyl- | 961 | EC | ND | ND | 0.073 ± 0.012a | 0.049 ± 0.049b | 0.047 ± 0.006b |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Nonane | 895 | EC | ND | 0.914 ± 0.081Aa | 0.263 ± 0.02Ac | 0.264 ± 0.056Ac | 0.502 ± 0.023Ab |
| EPC | 0.341 ± 0.086Ba | 0.161 ± 0.161Bb | 0.173 ± 0.173Bb | 0.166 ± 0.057Bb | |||
| IC | 0.341 ± 0.224Ba | 0.275 ± 0.012Ab | 0.274 ± 0.165Ab | 0.271 ± 0.054Bb | |||
| Hexadecane | 1715 | EC | 0.516 ± 0.322 | ND | ND | ND | ND |
| EPC | 0.242 ± 0.015Ab | 0.357 ± 0.076Aa | 0.245 ± 0.004Ab | 0.289 ± 0.063Ab | |||
| IC | 0.2 ± 0.01Aa | 0.168 ± 0.026Bb | 0.247 ± 0.153Aa | 0.1 ± 0.004Bb | |||
| Heptacosane | 2075 | EC | ND | 0.191 ± 0.072a | 0.128 ± 0.016a | 0.125 ± 0.012a | 0.053 ± 0.009b |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Heptadecane, 2,6,10,15-tetramethyl- | 1828 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | 0.054 ± 0.027a | 0.029 ± 0.003b | 0.028 ± 0.002b | 0.034 ± 0.009b | |||
| IC | ND | ND | ND | ND | |||
| Heptadecane | 1877 | EC | ND | ND | ND | ND | ND |
| EPC | 0.086 ± 0.01Ab | 0.085 ± 0.018Ab | 0.077 ± 0.004Ac | 0.104 ± 0.031Aa | |||
| IC | 0.069 ± 0.007Ba | 0.047 ± 0.007Bb | 0.044 ± 0.0005Bb | 0.045 ± 0.008Bb | |||
| Eicosane, 1-iodo- | 2278 | EC | ND | 0.145 ± 0.055a | 0.1 ± 0.0003a | 0.061 ± 0.006b | 0.056 ± 0.009b |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Dodecane, 4-methyl- | 1540 | EC | ND | 0.139 ± 0.016a | 0.08 ± 0.008b | 0.025 ± 0.025d | 0.061 ± 0.002c |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Dodecane, 4,6-dimethyl- | 1252 | EC | ND | 0.381 ± 0.002a | 0.236 ± 0.016b | 0.099 ± 0.078d | 0.16 ± 0.005c |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Dodecane | 1197 | EC | 0.44 ± 0.003 | 0.325 ± 0.051Aa | 0.189 ± 0.006Ab | 0.158 ± 0.024Ac | 0.138 ± 0.009Ad |
| EPC | 0.118 ± 0.001Bbc | 0.154 ± 0.014Ba | 0.124 ± 0.001Bb | 0.114 ± 0.01Bc | |||
| IC | 0.135 ± 0.015Bab | 0.142 ± 0.004Ca | 0.092 ± 0.013Cb | 0.089 ± 0.001Cbc | |||
| Dodecane, 5,8-diethyl- | 2121 | EC | ND | ND | ND | ND | ND |
| EPC | 0.033 ± 0.01b | 0.03 ± 0.01b | 0.026 ± 0.004bc | 0.047 ± 0.003a | |||
| IC | ND | ND | ND | ND | |||
| Dodecane, 2,6,11-trimethyl- | 1734 | EC | 0.034 ± 0.0006 | ND | ND | ND | ND |
| EPC | 0.051 ± 0b | 0.064 ± 0.006a | 0.043 ± 0.001c | 0.059 ± 0.012ab | |||
| IC | ND | ND | ND | ND | |||
| Decane, 2,4,6-trimethyl- | 1260 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.036 ± 0.001a | 0.032 ± 0.002a | 0.022 ± 0.003b | 0.023 ± 0.0004b | |||
| Decane | 1349 | EC | 0.486 ± 0.068 | 1.826 ± 0.146Aa | 0.608 ± 0.608Cc | 0.976 ± 0.28Abc | 1.085 ± 0.062Ab |
| EPC | 0.609 ± 0.033Cc | 0.831 ± 0.08Ba | 0.593 ± 0.064Bc | 0.64 ± 0.021Bb | |||
| IC | 0.894 ± 0.13Bb | 0.956 ± 0.013Aa | 0.599 ± 0.04Bd | 0.648 ± 0.027Bc | |||
| Cyclopentane, nonyl- | 1626 | EC | 0.096 ± 0.021 | ND | ND | ND | ND |
| EPC | 0.037 ± 0.001Ac | 0.05 ± 0.005Aa | 0.042 ± 0.0001Ab | 0.044 ± 0.01Ab | |||
| IC | 0.038 ± 0.001Aa | 0.034 ± 0.001Ba | 0.023 ± 0.006Bb | 0.022 ± 0.001Bb | |||
| 4-Methyldocosane | 1960 | EC | ND | 0.04 ± 0.012a | 0.018 ± 0.0004b | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 5-Ethyldecane | 1517 | EC | 0.036 ± 0.011 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 2,4-Dimethyldodecane | 1292 | EC | ND | 0.23 ± 0.038a | 0.133 ± 0.007b | 0.113 ± 0.023b | 0.078 ± 0.007c |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Aromatic compounds | |||||||
| Toluene | 758 | EC | ND | 0.494 ± 0.307c | 1.047 ± 0.235a | 0.86 ± 0.413b | 0.372 ± 0.164d |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| p-Xylene | 1183 | EC | ND | ND | ND | ND | ND |
| EPC | 0.042 ± 0.007Ab | 0.071 ± 0.037Aa | 0.026 ± 0.001Bcd | 0.032 ± 0.001Bc | |||
| IC | ND | 0.024 ± 0.0005Bc | 0.053 ± 0.005Ab | 0.103 ± 0.009Aa | |||
| Phenol, 5-ethenyl-2-methoxy- | 1701 | EC | ND | ND | ND | ND | ND |
| EPC | 0.012 ± 0.012Bc | 0.031 ± 0.008Ab | 0.029 ± 0.004Abc | 0.045 ± 0.01Aa | |||
| IC | 0.029 ± 0.0009Aa | 0.02 ± 0.001Ba | ND | ND | |||
| Butylated Hydroxytoluene | 1694 | EC | ND | ND | ND | ND | ND |
| EPC | 0.033 ± 0.033Ab | 0.024 ± 0.024Ac | 0.053 ± 0.003Aa | 0.027 ± 0.027Ac | |||
| IC | 0.034 ± 0.004Aa | 0.01 ± 0.01Bb | 0.016 ± 0.002Bb | 0.016 ± 0.0005Bb | |||
| Furan, 2-propyl- | 1357 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | ND | ND | ND | 0.035 ± 0.009Aa | |||
| IC | ND | ND | ND | ND | |||
| Naphthalene, 1-methyl- | 1687 | EC | 0.024 ± 0.004 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Ethylbenzene | 854 | EC | 0.593 ± 0.087 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 1H-Indene, 1-methylene- | 1182 | EC | 0.066 ± 0.004 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Benzene, 1,3-dimethyl- | 1183 | EC | 3.579 ± 0.524 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Esters | |||||||
| Sulfurous acid, pentyl undecyl ester | 2009 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.027 ± 0.003a | 0.029 ± 0.001a | 0.02 ± 0.002ab | 0.008 ± 0.008b | |||
| Sulfurous acid, pentadecyl 2-propyl ester | 2093 | EC | ND | ND | ND | ND | ND |
| EPC | 0.036 ± 0.001Aa | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Sulfurous acid, hexyl pentadecyl ester | 1657 | EC | 0.062 ± 0.023 |
ND |
ND |
ND |
ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Sulfurous acid, 2-propyl tetradecyl ester | 2064 | EC | 0.064 ± 0.028 |
ND |
ND |
ND |
ND |
| EPC |
ND |
ND |
ND |
ND |
|||
| IC | ND | ND | ND | ND | |||
| Sulfurous acid, dodecyl pentyl ester | 2010 | EC |
ND |
ND |
ND |
ND |
ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | 0.008 ± 0.008Aa | |||
| Stearic acid, 3-(octadecyloxy)propyl ester | 1658 | EC |
ND |
0.131 ± 0.007a | 0.104 ± 0.004a | 0.041 ± 0.025b | 0.037 ± 0.006b |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester | 1763 | EC | 0.073 ± 0.002 |
ND |
0.036 ± 0.006Aa |
ND |
ND |
| EPC | ND | 0.036 ± 0.017Aa | ND | ND | |||
| IC | ND | 0.034 ± 0.0007Aa | 0.02 ± 0.005Ab | 0.032 ± 0.012Aa | |||
| Phthalic acid, hept-3-yl isobutyl ester | 2259 | EC | 0.026 ± 0.005 |
ND |
ND |
ND |
ND |
| EPC |
ND |
ND |
ND |
ND |
|||
| IC | ND | ND | ND | ND | |||
| Octanoic acid, methyl ester | 1122 | EC | 0.066 ± 0.006 | 0.044 ± 0.044Ab | 0.071 ± 0.012Ab | 0.242 ± 0.025Aa |
ND |
| EPC |
ND |
ND |
ND |
ND |
|||
| IC |
ND |
ND |
0.009 ± 0.00Ba | 0.009 ± 0.00Aa | |||
| Oxalic acid, decyl propyl ester | 1414 | EC | ND |
ND |
ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | 0.111 ± 0.003Aa | ND | ND | |||
| Hydratropic acid, isohexyl ester | 1327 | EC | ND | 0.153 ± 0.007Aa | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Anthranilic acid, N-methyl-, butyl ester | 1233 | EC | ND | 0.046 ± 0.005bc | 0.03 ± 0.003c | 0.053 ± 0.004b | 0.076 ± 0.009a |
| EPC |
ND |
ND |
ND |
ND |
|||
| IC | ND | ND | ND | ND | |||
| 9-Octadecenoic acid (Z)-, phenylmethyl ester | 1470 | EC | ND | 0.04 ± 0.006b | 0.029 ± 0.029c | 0.015 ± 0.015d | 0.128 ± 0.102a |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 4-Ethylbenzoic acid, cyclopentyl ester | 1234 | EC | ND | ND | ND | ND | ND |
| EPC | 4.897 ± 0.725Bb | 6.717 ± 1.634Aa | 3.69 ± 1.17Ac | 0.103 ± 0.011Bd | |||
| IC | 11.408 ± 2.785Aa | 3.35 ± 0.795Bc | 2.922 ± 2.922Bd | 5.86 ± 1.403Ab | |||
| Acids | |||||||
| Phosphonoacetic Acid, 3TMS derivative | 1230 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | 0.304 ± 0.047Bb | 0.219 ± 0.219Ac | 0.437 ± 0.13Aa | 0.226 ± 0.026Ac | |||
| IC | 0.482 ± 0.018Aa | 0.182 ± 0.029Bc | 0.17 ± 0.067Bc | 0.228 ± 0.077Ab | |||
| n-Hexadecanoic acid | 1940 | EC | ND | ND | ND | ND | ND |
| EPC | 0.114 ± 0.011Ab | 0.248 ± 0.119Aa | 0.133 ± 0.005Ab | 0.21 ± 0.108Aa | |||
| IC | 0.123 ± 0.024Aa | 0.055 ± 0.014Bb | 0.04 ± 0.009Bbc | 0.058 ± 0.031Bb | |||
| Dodecanedioic acid, 2TBDMS derivative | 1473 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Dodecanoic acid, 3-hydroxy- | 1750 | EC | ND |
ND |
ND |
ND |
ND |
| EPC | 0.038 ± 0.006Aa | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Ketones | |||||||
| 2-Nonanone | 1457 | EC | 0.026 ± 0.002 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 3,5-Octadien-2-one, (E,E)- | 1461 | EC | 0.078 ± 0.012 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 5,9-Undecadien-2-one, 6,10-dimethyl- | 1622 | EC |
ND |
ND |
ND |
ND |
ND |
| EPC | 0.056 ± 0.007Bc | 0.108 ± 0.049Aa | 0.052 ± 0.016Ac | 0.081 ± 0.042Ab | |||
| IC | 0.074 ± 0.003Aa | 0.054 ± 0.008Bc | 0.03 ± 0.001Bd | 0.06 ± 0Bb | |||
| 3-Hexanone, 2,5-dimethyl- | 933 | EC | 2.88 ± 0 | ND | ND | ND | ND |
| EPC | 0.559 ± 0.046Ab | 0.674 ± 0.06Aa | 0.4 ± 0.109Bc | 0.617 ± 0.046Aa | |||
| IC | 0.343 ± 0.143Bc | 0.48 ± 0.004Bb | 0.551 ± 0.025Aa | 0.553 ± 0.003Ba | |||
| Octan-2-one, 3,6-dimethyl- | 1740 | EC | ND | 0.046 ± 0.008a | 0.025 ± 0.004b | 0.022 ± 0.004b |
ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 3-Heptanone, 2-methyl- | 1290 | EC | ND |
0.71 ± 0.046b |
0.634 ± 0.097c |
0.672 ± 0.099c |
0.912 ± 0.015a |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
Values represent the means ± SD; Different superscript lowercase letters indicate significant differences (P < 0.05) within a row; different superscript uppercase letters indicate significant differences (P < 0.05) within a column. RI: retention indices; EPC: Rice porridge prepared in the electric pressure cooker; EC: Rice porridge prepared in the electromagnetic oven; IC: Rice porridge prepared in the electric rice cooker.
In Table 1, the first kind is aldehydes. In this study, aldehydes were mainly observed in uncooked millet. Aldehydes had a strong aroma, generally with light fragrance, fruit fragrance, etc., and even an important part of the aroma of steamed bread (Zhang et al., 2023). In addition, previous research has identified aldehydes as having green, fruity, and malty aromas, which exerted a significant effect on the flavor of cereals (Zheng, Wang, Xiong, & Zhang, 2023). A large number of hydrocarbons had been detected, and their content and types were relatively high. The threshold value of ordinary hydrocarbons was relatively high, which may not contribute much to the smell of millet porridge, but the high content of hydrocarbons hd an important contribution to the overall harmony of smell (Liu, Liu, Zhao, Zuo, & Zhang, 2010). The alcohols likely made little contribution to flavor and mainly played a synergistic role (Zhang et al., 2019). Moreover, aldehydes are the most abundant in millet porridge. They generally have creamy, fatty, herbaceous, and light scents. The low threshold value may be closely related to the flavor characteristics of millet porridge (Liu et al., 2010). Therefore, the focus of the study was on aldehydes. These aldehydesd include saturated aldehydes and unsaturated aldehydes.
Vanillin (Vanilla-like) was a naturally occurring plant spice and a kind of important volatile compound and flavor in natural herbs, mainly found in vanilla beans, with a strong milky aroma (Martău, Călinoiu, & Vodnar, 2021; Wu, Liang, Luo, Wang, & Chen, 2024). It is the second most popular flavoring agent after saffron and is extensively used in various applications, e.g., as a food additive in food and beverages (Banerjee & Chattopadhyay, 2019). The concentration in millet porridge samples of EPC and IC was high, and the highest concentration reached in IC-30 min and EPC-50 min respectively. The undecanal led to a fresh and lemon-like aroma (Hu, Lu, Guo, & Zhu, 2020), which was only discovered in the IC. The highest concentration was observed in IC-30 min (0.038 μg/kg). The content of undecanal gradually decreased during the cooking process. The nonanal had a volatile flavor of beef fat and green grass, was produced by oxidation (Huang et al., 2022). Nonanal had the maximum content in IC-60 min (0.534 μg/kg). That may be due to its formation was related to lipid oxidation and its content significantly increased with temperature within a certain temperature range (Gao et al., 2023). Hexanal (Green, grass-like) had green, oil and fat flavor, which may contribute to the flavor characteristics of millet porridge, and had pleasant grass flavor when the content was low (Liu et al., 2010). Hexanal was reported to be abundant in wheat starch, maize and potato (Pico, Martínez, Bernal, & Gómez, 2017). In fact, hexanal had high content in IC and EPC, and a small amount in EC, with the highest concentration in uncooked millet. The phenomenon might be may be caused by the level of enzymatic reaction and autoxidation in various cooking methods (Deng et al., 2013). Decanal (Green, soapy) was generated by amino acid degradation and fatty oxidation (Chang, Wu, Zhang, Jin, & Wang, 2020). Decanal had the most content at EPC-40 min (0.235 μg/kg) in different cooking methods.
In the unsaturated aldehyde, (E,E)-2,4-Decadienal may be formed by the self oxidation of arachidonic acid and linoleic acid (Andaleeb, Zhang, Jiang, Zhang, & Liu, 2023). It was only found in IC-40 min (0.017 μg/kg), which had a very low threshold. The low aroma threshold can regard this component as important flavorants for millet porridge flavor. Therefore, it also may be the most important flavor component in millet porridge. 2,4-Decadienal (Fatty, waxy) was a volatile compound produced by lipid oxidation that can be continuously consumed by more chemical reactions such as Maillard reaction, oxidation and degradation (Xu et al., 2023). It could be detected except EC. There was a upward tendency with the heating time in EPC, while the concentration of 2,4-Decadienal in IC was lower than that in EPC. Dodecanal (Citrus, Fat, Floral) was generated from oil oxidation, and observed in other cooking methods except for EC. Such flavor substances like (Z)-2-heptenal, E-14-hexadecenal, hexanal dimethyl acetal, and 3,3-dimethyl-2-oxo-butanal were only detected in uncooked millet.
The saturated alcohol compounds have high thresholds, so they may not contribute much to the flavor characteristics of millet porridge. While unsaturated alcohol compounds have low threshold values, such as 1-octen-3-ol have a low odor threshold with the scent of mushrooms and herbs contributing to the flavor characteristics of millet porridge (Gao et al., 2023; Liu et al., 2010). The 1-octen-3-ol was not found in EC in this research with the maximum concentration in uncooked millet. The key reason may be that the water vapor took away the small molecular alcohols with lower boiling points (Zhang, Gao, Zhang, Feng, & Zhuang, 2022). The nonanal, a specific volatile compound in millet porridge, had high content in different cooking methods. The highest concentration appeared IC-60 min (0.534 μg/kg).
The hydrocarbons were the largest group of compounds with the most diverse types detected from the millet porridge samples. In general, the high threshold of hydrocarbon may contribute little to the odor of millet porridge, but the high content of hydrocarbon like hexadecane has an important contribution to the overall coordination of odor, so their influence on the flavor characteristics of millet porridge cannot be ignored. Six kinds of ketones were detected in millet porridge and ketones generally contribute to the aroma of milk and fruits and vegetables (Liu et al., 2010). Their threshold is higher than the aldehydes, and their contribution to flavor is relatively small. 2-Nonanone and (E,E)-3,5-Octadien-2-one could only be detected in the uncooked millet porridge. The 2,5-dimethyl-3-Hexanone had the highest content among all kinds of ketones and it could be detected in different cooking methods except EC.
As for aromatic compounds, one substance deserved to be discussed. The 2-propyl-furan presented the floral scent, therefore, which had an impact on the flavor characteristics of millet porridge (Liu et al., 2010). It was only discovered in EPC-60 min (0.035 μg/kg). Dodecanoic acid is present in various natural products and is abundant in coconut oil, palm kernels, and coffee seeds (Xu et al., 2023). It was also detected, which may not contribute much to the flavor of millet porridge due to their high threshold.
3.2. Influence of cooking methods on texture properties of millet porridge
Before texture analysis, In texture analysis, the millet porridge was analyzed by a two-cycle compression program, and 7 indicators were selected to to assess its texture characteristics (hardness, adhesion, cohesiveness, elasticity, stickiness, chewiness, resilience). Table 2 presented the results of a one-way analysis of variance (ANOVA) conducted for the cooking method and time variables, as well as a repeated measurements analysis employing a double-factor variance. The P-value is used to describe the degree of difference between the observed results and the expected results, with a range of values between 0 and 1, excluding 0 or 1. The smaller P value indicated that there was a more significant difference between the observed results and the expected results. Therefore, a lower P value meant that the cooking time and methods have a greater impact on the texture. As shown in Table 2, both cooking methods and time significantly affected the texture of maize porridge, with a P value <0.05 in at least one case in one-factor and two-factor conditions. More specifically, among indicators, the effect on hardness and elasticity was apparent. However, the final result showed that cooking time had a notable effect on the texture properties in millet porridge.
Table 2.
Effect of time and cooker on texture properties of millet porridge samples.
|
Cooking time/min |
P |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Units | Cooker | 30 | 40 | 50 | 60 | M | T | M × T | |
| Hardness | N | EC | 8.25 ± 0.69Ba | 5.12 ± 0.59Ab | 2.25 ± 0.80Ac | 1.11 ± 0.23Bd | *** | *** | *** |
| EPC | 8.67 ± 0.49Ba | 4.62 ± 0.35Bb | 2.97 ± 0.00Ac | 1.50 ± 0.02Ad | |||||
| IC | 10.90 ± 1.25Aa | 5.66 ± 0.27Ab | 2.79 ± 0.88Ac | 0.85 ± 0.10Cd | |||||
| Adhesion | N.mm | EC | 2.13 ± 0.04Aa | 1.14 ± 0.88Ab | 0.32 ± 0.02Bc | 0.35 ± 0.01Bc | NS | *** | NS |
| EPC | 1.43 ± 0.81Aa | 0.73 ± 0.87Ab | 0.86 ± 0.75Ab | 0.37 ± 0.008Bc | |||||
| IC | 2.21 ± 0.25Aa | 1.07 ± 0.80Ab | 1.34 ± 0.60Aab | 0.44 ± 0.05Ab | |||||
| Cohesiveness | Ratio | EC | 0.42 ± 0.02Ac | 0.49 ± 0.04Bc | 0.68 ± 0.05Ab | 0.76 ± 0.05Ba | NS | *** | NS |
| EPC | 0.40 ± 0.01Ac | 0.54 ± 0.10Ab | 0.57 ± 0.04Bb | 0.73 ± 0.03Ba | |||||
| IC | 0.39 ± 0.01Ad | 0.49 ± 0.05Bc | 0.62 ± 0.12Ab | 0.85 ± 0.03Aa | |||||
| Elasticity | mm | EC | 13.64 ± 0.37Ba | 13.59 ± 0.66Ba | 13.72 ± 0.22Ba | 13.85 ± 0.26Ba | *** | NS | NS |
| EPC | 14.48 ± 0.46Ab | 16.04 ± 1.76Aa | 15.06 ± 0.26Aab | 14.95 ± 0.08Aab | |||||
| IC | 13.40 ± 0.28Bb | 13.45 ± 0.35Bb | 14.15 ± 0.18Ba | 14.28 ± 0.44Ba | |||||
| Stickiness | N | EC | 3.48 ± 0.32Ba | 2.35 ± 0.30Ab | 1.49 ± 0.41Ac | 0.84 ± 0.14Bd | * | *** | * |
| EPC | 3.46 ± 0.21Ba | 2.46 ± 0.34Ab | 1.93 ± 0.24Ab | 1.10 ± 0.85Ac | |||||
| IC | 4.28 ± 0.32Aa | 2.77 ± 0.21Ab | 1.63 ± 0.38Ac | 0.73 ± 0.08Bd | |||||
| Chewiness | mj | EC | 47.43 ± 4.00Ba | 32.17 ± 5.46Ab | 20.43 ± 5.43Bc | 11.70 ± 2.16Bd | * | *** | NS |
| EPC | 50.26 ± 3.86Ba | 40.01 ± 9.79Ab | 32.04 ± 3.91Ac | 16.48 ± 0.93Ad | |||||
| IC | 57.30 ± 3.47Aa | 37.34 ± 3.66Ab | 23.08 ± 5.31Bc | 10.41 ± 1.09Bd | |||||
| Resilience | EC | 0.03 ± 0.005Bb | 0.11 ± 0.07Ab | 0.28 ± 0.07Aa | 0.35 ± 0.06Aa | * | *** | * | |
| EPC | 0.06 ± 0.02Ab | 0.18 ± 0.12Aab | 0.13 ± 0.07Ab | 0.29 ± 0.01Aa | |||||
| IC | 0.02 ± 0.008Bb | 0.09 ± 0.06Aab | 0.12 ± 0.14Aab | 0.26 ± 0.09Aa | |||||
Values represent the means ± SD; Different superscript lowercase letters indicate significant differences (P < 0.05) within a row; different superscript uppercase letters indicate significant differences (P < 0.05) within a column. NS, P > 0.05; *, P < 0.05, **, P < 0.01, ***, P < 0.001. Abbreviations are: M, cooking method; T, time; EPC: Millet porridge prepared in the electric pressure cooker; EC: Millet porridge prepared in the electromagnetic oven; IC: Millet porridge prepared in the electric rice cooker.
Hardness is the most direct indicator that reflects taste. In texture profile analysis, it directly affects chewiness and cohesivity. If the hardness is high, it is not easy to chew and therefore not easily accepted by consumers. In the TPA diagram, the maximum force value within the first. From Table 2, it could be seen that the hardness under different cooking methods all reached its peak at 30 min, and then the hardness showed a downward trend. In addition, the downward tendency was more pronounced when the cooking time increased from 30 min to 40 min. Moreover, the hardness of millet porridge prepared by IC decreased significantly more than that of EC and EPC before 40 min. However, the IC showed a higher value of hardness at 40 min (5.66 N) than EC (4.81 N) and EPC (4.62 N). The content of amylose is negatively correlated with the palatability of porridge (Zhang & Shen, 2021). The starch content of millet porridge was different under different pressure conditions, thus affecting the hardness. This could be due to the rearrangement of starch granules caused by pressure cooking, which may lead to the formation of micropores. Micropores could absorb and retain more water through capillary action, and the increase of water content made the granules gel more fully (Cappa, Lucisano, Barbosa-Cánovas, & Mariotti, 2016). Ultimately, it resulted in a decrease in hardness and elasticity (Bhattacharya, Narasimha, & Bhattacharya, 2006).
Compared with IC, the millet porridge prepared by EC showed smaller value of hardness in general. However, the lowest peak of hardness value occurred in IC-60 min (0.85 N). The hardness of IC and EC at 50 min was lower than that of EPC, which may be due to the evaporation of abundant water in EC and IC at 50 min of cooking, and the heat transfer rate in EC and IC is higher than that in EPC. This resulted in millet containing more moisture in IC and EC. This led to a softer texture in EC-50 min and IC-50 min.
Elasticity referred to the ability of food to deform when exposed to force and then recover its original shape when the force is removed. Table 2 showed that the elasticity of millet porridge in IC, EPC, EC tend to increase. Meanwhile, the minimum elasticity was observed at IC-30 min (13.40 mm).
The chewiness is defined as adhesiveness multiplied by elasticity. It can be explained as the energy required to chew solid food and is difficult to measure accurately. It presented a similar tendency with hardness. The maximum chewiness also appeared at 30 min, and the minimum chewiness occurred at IC-60 min (10.41 N), positively correlated with hardness.
3.3. Sensory evaluation of different millet porridge samples
The volatile compounds and texture analysis provided limited information to judge millet porridge characteristics accurately. Therefore, the experiment added sensory analysis on 12 millet porridge samples to assess their sensory quality. The assessors chose six descriptors for evaluation after discussion. The results were presented in Table 3. The aroma score of millet porridge presented a downward trend with the increase of heating time, while the cohesiveness on the contrary. In 30 min, IC had the strongest cereal aroma. Compared with IC and EC, the EPC aroma was weaker before 30 min. However, with the heating time, the trend of fading flavor of IC and EC had increased compared with EPC. Additionally, on the basis of cohesiveness and stiffness, there was no obvious difference among them, and cooking time had a more notable impact than methods. With the heating time, the sweet scores of different cooking methods increased and the color scores decreased. The cooking methods had little effect on the color with no obvious difference. In summary, the comprehensive sensory reached its peak in IC-30 min due to its good texture, flavor and taste rating. The comprehensive sensory scores of IC and EC decreased with the prolongation of cooking time. It is recommended to use IC and EC during short-term cooking. On the contrary, if longer cooking time is required, it is advised to use EPC.
Table 3.
Effect of time and cooker on sensory evaluation of millet porridge samples.
|
Cooking time/min |
|||||
|---|---|---|---|---|---|
| Cooker | 30 | 40 | 50 | 60 | |
| Appearance | |||||
| Color | EPC | 8.75 ± 0.42Aa | 8.45 ± 0.49Aa | 7.85 ± 0.56Ab | 6.65 ± 0.56Ac |
| EC | 8.20 ± 0.85Aa | 7.55 ± 0.90Bab | 6.60 ± 0.48Bb | 5.15 ± 0.83Bc | |
| IC | 8.20 ± 0.59Aa | 7.85 ± 0.89ABa | 7.45 ± 0.84Aa | 5.10 ± 0.68Bb | |
| Texture | |||||
| Cohesiveness | EPC | 3.00 ± 0.98Ac | 5.00 ± 0.12Bb | 7.05 ± 0.79Aa | 7.75 ± 0.92Ba |
| EC | 3.10 ± 0.92Ad | 5.20 ± 0.79Bc | 7.15 ± 0.89Ab | 7.85 ± 0.89Ba | |
| IC | 3.20 ± 0.91Ac | 6.00 ± 0.87Ab | 6.05 ± 0.79Bb | 8.20 ± 0.73Aa | |
| Stiffness | EPC | 8.15 ± 0.71Aa | 6.65 ± 0.71Bb | 5.25 ± 0.68ABc | 3.10 ± 0.75Ad |
| EC | 8.25 ± 0.61Aa | 7.05 ± 0.72Ab | 4.80 ± 0.39Bc | 2.60 ± 0.57Ad | |
| IC | 8.50 ± 0.49Aa | 6.82 ± 1.14Ab | 5.95 ± 0.79Ab | 2.25 ± 0.97Bc | |
| Aroma | |||||
| Cereal | EPC | 6.85 ± 0.64Ba | 7.45 ± 0.95Aa | 7.10 ± 0.92Aa | 6.60 ± 0.65Aa |
| EC | 6.90 ± 0.43Ba | 4.35 ± 0.56Bb | 2.90 ± 1.15Bc | 2.45 ± 0.65Cc | |
| IC | 8.60 ± 0.48Aa | 6.85 ± 0.77Ab | 6.30 ± 0.76Ab | 5.70 ± 0.82Bc | |
| Taste | |||||
| Sweet | EPC | 7.15 ± 0.83Abc | 7.20 ± 1.29Abc | 7.80 ± 0.66Ab | 8.55 ± 0.65Aa |
| EC | 6.45 ± 0.49Bc | 7.60 ± 0.89ABb | 8.35 ± 0.71Aa | 8.33 ± 0.64Aa | |
| IC | 7.30 ± 0.76Ab | 7.10 ± 1.23Ab | 7.80 ± 0.66Ab | 8.60 ± 0.78Aa | |
| Comprehensive score | EPC | 6.95 ± 0.49Ca | 7.35 ± 0.83Aa | 7.70 ± 0.45Aa | 7.50 ± 0.49Aa |
| EC | 7.40 ± 0.84Ba | 6.75 ± 0.75Ba | 6.30 ± 0.88Aa | 4.60 ± 1.36Bb | |
| IC | 8.50 ± 0.58Aa | 7.80 ± 0.85Ab | 7.05 ± 0.84Ab | 4.45 ± 1.00Bc | |
Values represent the means ± SD; Different superscript lowercase letters indicate significant differences (P < 0.05) within a row; different superscript uppercase letters indicate significant differences (P < 0.05) within a column. EPC: Millet porridge prepared in the electric pressure cooker; EC: Millet porridge prepared in the electromagnetic oven; IC: Millet porridge prepared in the electric rice cooker.
4. Conclusions
This study explored the effect of three different cooking methods and different cooking time on volatile compounds and texture of millet porridge by a multifaceted method, including HS-SPME combined with GC–MS, multiple chemometrics analysis, and texture analysis. The results indicated that different cooking methods produced different volatile compounds. Furthermore, 91 volatiles were detected, containing 18 aldehydes, 9 alcohols, 31 hydrocarbons, 6 ketones, 14 esters, 9 aromatic compounds, 4 acids. Moreover, aldehydes, alcohols and esters were considered to be the important volatile compounds in millet porridge. Texture analysis indicated that cooking methods and time had remarkable effects, with different cooking time having a greater impact on texture characteristics than cooking methods. When the cooking time prolonged from 30 min to 40 min, the decrease trend in hardness become more pronounced. On the contrary, different cooking methods had a more significant influence on the volatiles than cooking time. In summary, in view of the result of sensory evaluation, 30 min was a critical transition point. The comprehensive sensory score reached its peak in IC-30 min. The comprehensive sensory scores of IC and EC decreased with the prolongation of cooking time. IC and EC were more suitable for short-term cooking. The study results may offer new insights into the sensory improvement of millet porridge and provide theoretical and technical references for industrial production.
CRediT authorship contribution statement
Xinyang Liu: Writing – review & editing, Writing – original draft, Project administration, Investigation, Formal analysis, Data curation, Conceptualization. Shihao Wang: Writing – review & editing, Data curation, Conceptualization. Meifan Pan: Methodology, Investigation, Data curation. Ailing Tian: Writing – original draft, Formal analysis, Data curation. Kaixuan Chen: Resources, Methodology, Data curation. Wenwen Qu: Software, Resources. Wenkai Zhou: Writing – review & editing. Yarui Zhou: Visualization, Writing – review & editing. Lijjiao Fan: Writing – review & editing. Cong Zhao: Validation, Writing – review & editing. Lingyun Qu: Data curation. Qiangwei Liu: Validation, Supervision. Saihan Wang: Software, Data curation. Chuanxu Zheng: Software, Data curation. Lili Zheng: Writing – review & editing. Feng Zhong: Formal analysis, Data curation. Lirong Xu: Funding acquisition, Conceptualization. Aiguo Ma: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financed by the National Natural Science Foundation of China (Project No.32202083), China Postdoctoral Science Foundation (Project No.2023 M731849), Qingdao Postdoctoral Innovation Project (Project No. QDBSH20220202216).
Conflict of interest: The authors declare that they have no conflict of interest.
In this project, the rights and interests of the subjects are fully protected and meet the ethical requirements, and was approved by the Qingdao University Ethics Committee (No. QDU-HEC-2023159).
Data availability
Data will be made available on request.
References
- Andaleeb R., Zhang D., Jiang S., Zhang Y., Liu Y. Volatile profile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala. Food science and human wellness, 2023. 2023;12(1):57–68. (SCI, 8.022/Q1) [Google Scholar]
- Banerjee G., Chattopadhyay P. Vanillin biotechnology: The perspectives and future. Journal of the Science of Food and Agriculture. 2019;99(2):499–506. doi: 10.1002/jsfa.9303. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Narasimha H.V., Bhattacharya S. Rheology of corn dough with gum arabic: Stress relaxation and two-cycle compression testing and their relationship with sensory attributes. Journal of Food Engineering. 2006;74(1):89–95. [Google Scholar]
- Bi S., Wang A., Wang Y., Xu X., Luo D., Shen Q., Wu J. Effect of cooking on aroma profiles of Chinese foxtail millet (Setaria italica) and correlation with sensory quality. Food Chemistry. 2019;289:680–692. doi: 10.1016/j.foodchem.2019.03.108. [DOI] [PubMed] [Google Scholar]
- Cappa C., Lucisano M., Barbosa-Cánovas G.V., Mariotti M. Physical and structural changes induced by high pressure on corn starch, rice flour and waxy rice flour. Food Research International. 2016;85:95–103. doi: 10.1016/j.foodres.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Chang C., Wu G., Zhang H., Jin Q., Wang X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Critical Reviews in Food Science and Nutrition. 2020;60(9):1496–1514. doi: 10.1080/10408398.2019.1575792. [DOI] [PubMed] [Google Scholar]
- Chen X., Chen H., Xiao J., Liu J., Tang N., Zhou A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Research International. 2020;138 doi: 10.1016/j.foodres.2020.109717. [DOI] [PubMed] [Google Scholar]
- Deng Y., Zhong Y., Yu W., Yue J., Liu Z., Zheng Y., Zhao Y. Effect of hydrostatic high pressure pretreatment on flavor volatile profile of cooked rice. Journal of Cereal Science. 2013;58(3):479–487. [Google Scholar]
- Gao H., Liu M., Zheng L., Zhang T., Chang X., Liu H., Sun J. Comparative Analysis of Key Odorants and Aroma Characteristics in Hot-Pressed Yellow Horn (Xanthoceras sorbifolia bunge) Seed Oil Via Gas Chromatography–Ion Mobility Spectrometry and Gas Chromatography–Olfactory-Mass Spectrometry. Foods. 2023;12(17):3174. doi: 10.3390/foods12173174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Lu L., Guo Z., Zhu Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends in Food Science & Technology. 2020;97:136–146. [Google Scholar]
- Huang Q., Dong K., Wang Q., Huang X., Wang G., An F., Luo P. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131103. [DOI] [PubMed] [Google Scholar]
- Jiang S., Zhu Y., Peng J., Zhang Y., Zhang W., Liu Y. Characterization of stewed beef by sensory evaluation and multiple intelligent sensory technologies combined with chemometrics methods. Food Chemistry. 2023;408 doi: 10.1016/j.foodchem.2022.135193. [DOI] [PubMed] [Google Scholar]
- Li X., Hu M., Zhang Y., Jiang J., Han Y. Taste difference of millet food was explored based on beige and transcriptional analysis. Journal of Shanxi Agricultural University (Natural Science Edition) 2019;05:1–9. [Google Scholar]
- Liu J., Liu S., Zhao W., Li Y., Zhang Y. Effect of water dosage on the volatile flavor ingredients of millet porridge. Food Research and Development. 2011;32(9):5–8. [Google Scholar]
- Liu J., Liu S., Zhao W., Zuo X., Zhang Y. Analysis and study on volatile flavor compounds in millet congee. Food and Feed Industry. 2010;11:31–33. [Google Scholar]
- Martău G.A., Călinoiu L.F., Vodnar D.C. Bio-vanillin: Towards a sustainable industrial production. Trends in Food Science & Technology. 2021;109:579–592. [Google Scholar]
- Martinez A.M., Kak A.C. Pca versus lda. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2001;23(2):228–233. doi: 10.1109/34.908962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres C., Briffaz A., Valentin D. 2019. Rice cooking and sensory quality. [Google Scholar]
- Pico J., Martínez M.M., Bernal J., Gómez M. Evolution of volatile compounds in gluten-free bread: From dough to crumb. Food Chemistry. 2017;227:179–186. doi: 10.1016/j.foodchem.2017.01.098. [DOI] [PubMed] [Google Scholar]
- Qiao L., Wang X. Nutrition, health care and medicinal characteristics of millet. Agricultural Science and Technology and Equipment. 2015;11:41–42. [Google Scholar]
- Tananuwong K., Lertsiri S. Changes in volatile aroma compounds of organic fragrant rice during storage under different conditions. Journal of the Science of Food and Agriculture. 2010;90(10):1590–1596. doi: 10.1002/jsfa.3976. [DOI] [PubMed] [Google Scholar]
- Wang S., Tian A., Zhao K., Zhang R., Lei Z., Qin X., Ma A. Effect of cooking methods on volatile compounds and texture properties in rice porridge. LWT- Food Science and Technology. 2023 [Google Scholar]
- Wang S., Chen K., Tian A., Pan M., Liu X., Qu L.…Ma A. Effect of cooking methods on volatile compounds and texture properties in maize porridge. Food Chemistry. 2024;101515 doi: 10.1016/j.fochx.2024.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Liang C., Luo R., Wang Z., Chen R. Determination of natural vanillin content in rice and rice using ultra-high performance liquid chromatography tandem mass spectrometry. Laboratory detection. 2024;01:46–51. [Google Scholar]
- Xu L., Mei X., Wu G., Karrar E., Jin Q., Wang X. Inhibitory effect of antioxidants on key off-odors in French fries and oils and prolong the optimum frying stage. LWT- Food Science and Technology. 2022;162 [Google Scholar]
- Xu L., Wu G., Huang J., Zhang H., Jin Q., Wang X. Sensory-directed flavor analysis of key odorants compounds development of french fries and oils in the break-in, optimum and degrading frying stage. Food Science and Human Wellness. 2023;12(1):140–150. [Google Scholar]
- Xu Y., Zhao J., Liu X., Zhang C., Zhao Z., Li X., Sun B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130920. [DOI] [PubMed] [Google Scholar]
- Xu Z., Liu J., Sun Y., Shi Y., Wu Y., Zhang R. Effects of lauric acid on slaughter performance, muscle quality, and antioxidant function of broiler chickens. Journal of Animal Husbandry and Veterinary Medicine. 2023;11:4691–4701. [Google Scholar]
- Yang Y.B., Jia G.Q., Deng L.G., Ling Q.I.N., Chen E.Y., Cong X.J., Yin Y.P. Genetic variation of yellow pigment and its components in foxtail millet (Setaria italica (L.) P. Beauv.) from different eco-regions in China. Journal of Integrative Agriculture. 2017;16(11):2459–2469. [Google Scholar]
- Yu L., Turner M.S., Fitzgerald M., Stokes J.R., Witt T. Review of the effects of different processing technologies on cooked and convenience rice quality. Trends in Food Science & Technology. 2017;59:124–138. [Google Scholar]
- Zhang C., Fang X., Chen Z., Luo F., Zhong H., Du M. Effects of different processing techniques on the composition of volatile substances in Chinese torreya seed oil. Chinese Journal of Oils and Fats. 2024:1–13. [Google Scholar]
- Zhang F., Shen Q. The impact of endogenous proteins on hydration, pasting, thermal and rheology attributes of foxtail millet based on GC-IMS. Journal of Cereal Science. 2021;100 [Google Scholar]
- Zhang J., Li P., Zhang A., Zhao W., Li S., Wang Y., Sun L. Analysis of volatile components of millet porridge cooked by different electric cooker. Journal of the Chinese Cereals and Oils Association. 2021;36:145–160. [Google Scholar]
- Zhang J., Li P., Zhao W., Zhang A., Wang Y., Liu J. Analysis of the difference of volatile components in different beans Mantou based on electronic nose and GC-IMS technology. Journal of the Chinese Cereals and Oils Association. 2023;6:156–163. [Google Scholar]
- Zhang K., Gao L., Zhang C., Feng T., Zhuang H. Analysis of volatile flavor compounds of corn under different treatments by GC-MS and GC-IMS. Frontiers in Chemistry. 2022;10 doi: 10.3389/fchem.2022.725208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang S., Fan W., Duan M., Han Y., Li H. Identification of volatile compounds and odour activity values in quinoa porridge by gas chromatography–mass spectrometry. Journal of the Science of Food and Agriculture. 2019;99(8):3957–3966. doi: 10.1002/jsfa.9621. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Wang Z., Xiong F., Zhang G. Enzyme inactivation induced by thermal stabilization in highland barley and impact on lipid oxidation and aroma profiles. Frontiers in Nutrition. 2023;10:1097775. doi: 10.3389/fnut.2023.1097775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Xu S., Wang M., Yao Y., Zhou C., Li H. Correlation analysis between nutritional components of millet and quality and flavor characteristics of millet porridge. Journal of the Chinese Cereals and Oils Association. 2023;05:58–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.