Abstract
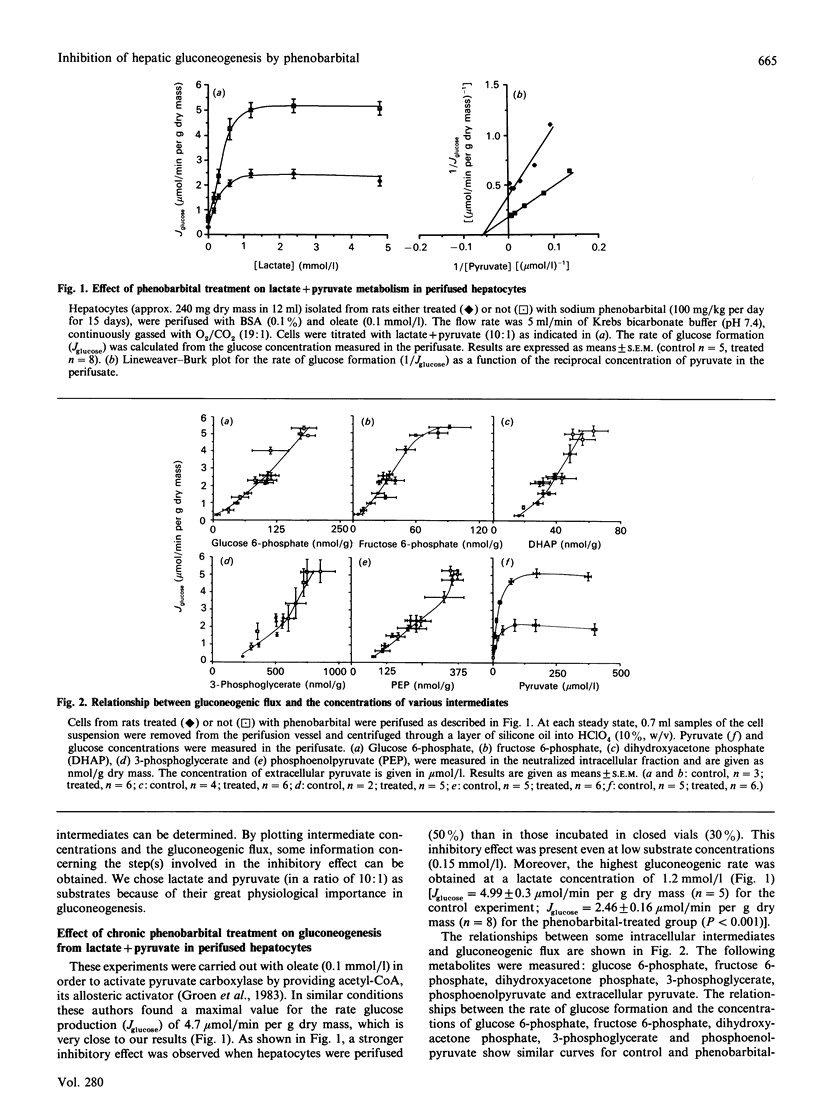
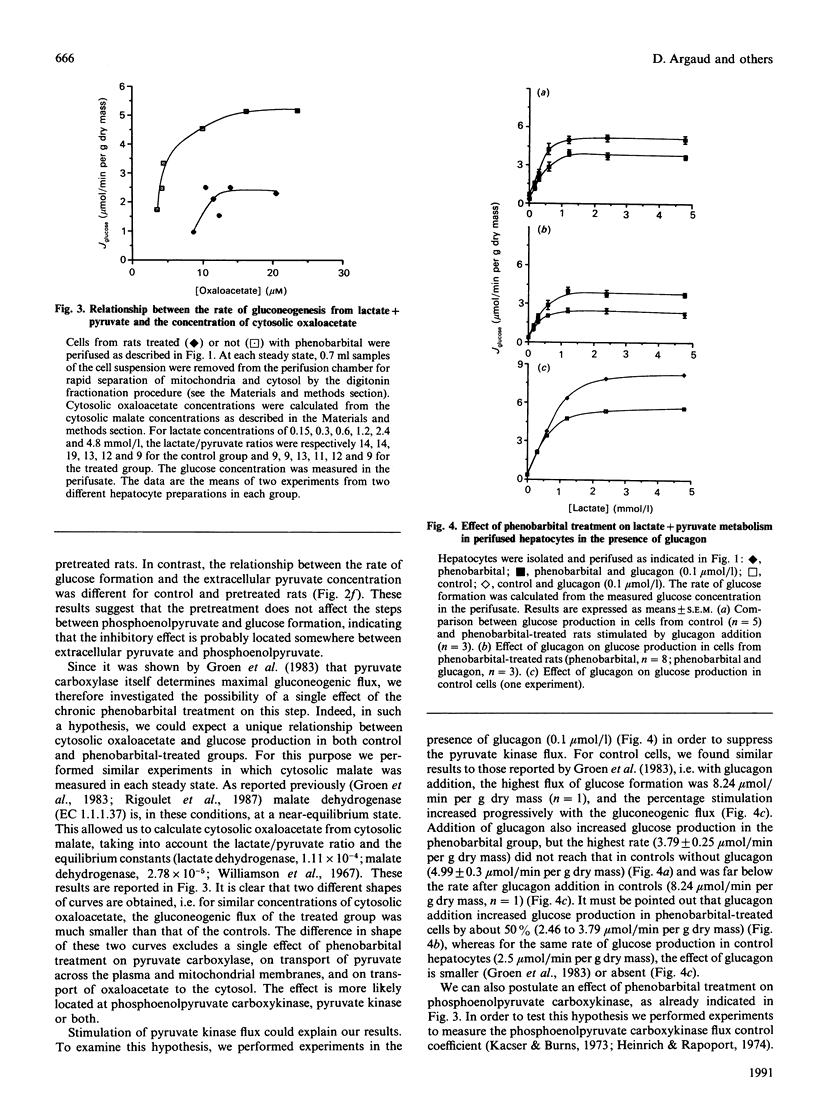
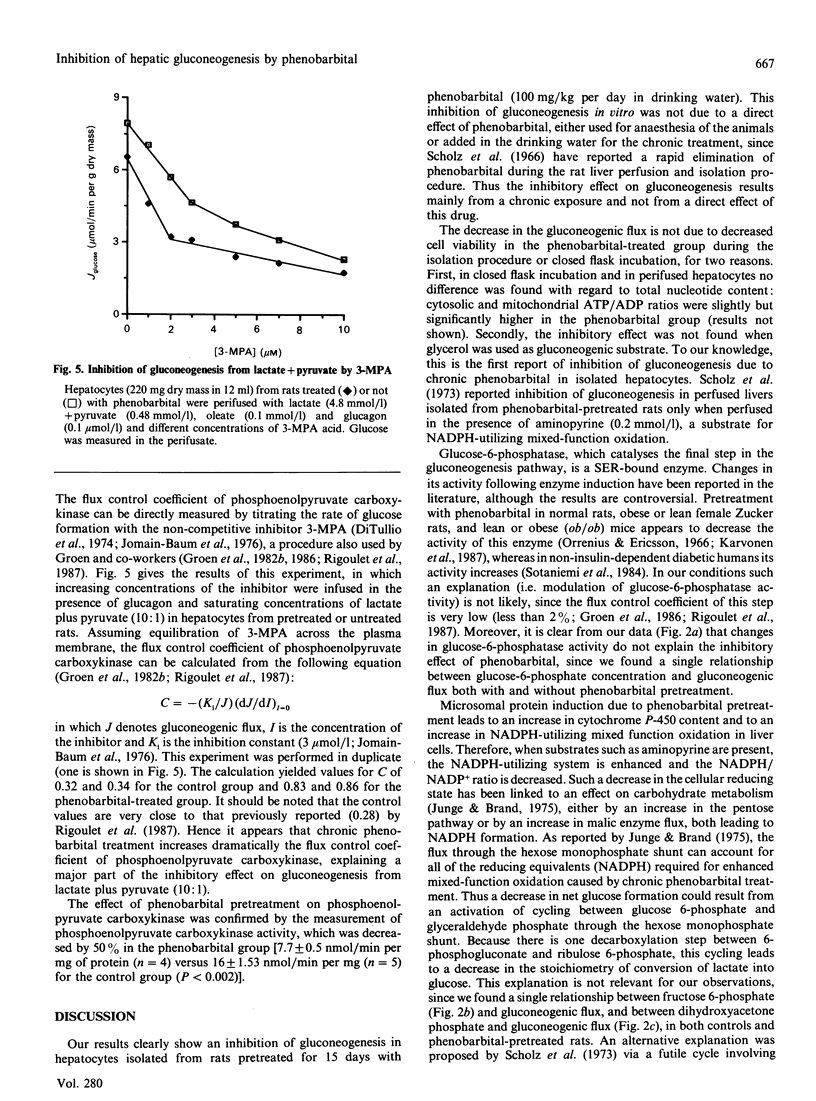
Gluconeogenesis was studied in hepatocytes isolated from phenobarbital-pretreated rats fasted for 24 h. In closed vial incubations, glucose production from lactate (20 mmol/l) and pyruvate (2 mmol/l), alanine (20 mmol/l) or glutamine (20 mmol/l) was suppressed by about 30-45%, although glycerol metabolism was not affected. In hepatocytes perifused with lactate and pyruvate (ratio 10:1), glucose production was inhibited by 50%, even at low gluconeogenic flux. From the determination of gluconeogenic intermediates at several steady states of gluconeogenic flux, we have found a single relationship between phosphoenolpyruvate and the rate of glucose production (Jglucose), and two different curves between cytosolic oxaloacetate and Jglucose in controls and in phenobarbital-pretreated hepatocytes. By using 3-mercaptopicolinate to determine the flux control coefficient of phosphoenolpyruvate carboxykinase we found that phenobarbital pretreatment led to an increase in this coefficient from 0.3 (controls) to 0.8 (phenobarbital group). These observations were confirmed by the finding that the activity of phosphoenolpyruvate carboxykinase was decreased by 50% after phenobarbital treatment. Hence we conclude that the inhibitory effect of phenobarbital on gluconeogenesis is due, at least partly, to a decrease in the flux through phosphoenolpyruvate carboxykinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale E. G., Hartley J. L., Granner D. K. N6,O2'-dibutyryl cycle AMP and glucose regulate the amount of messenger RNA coding for hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1982 Feb 25;257(4):2022–2028. [PubMed] [Google Scholar]
- Beale E., Andreone T., Koch S., Granner M., Granner D. Insulin and glucagon regulate cytosolic phosphoenolpyruvate carboxykinase (GTP) mRNA in rat liver. Diabetes. 1984 Apr;33(4):328–332. doi: 10.2337/diab.33.4.328. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Kun E., Werner H. V. Regulatory role of reducing-equivalent transfer from substrate to oxygen in the hepatic metabolism of glycerol and sorbitol. Eur J Biochem. 1973 Mar 15;33(3):407–417. doi: 10.1111/j.1432-1033.1973.tb02697.x. [DOI] [PubMed] [Google Scholar]
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Cimbala M. A., Lamers W. H., Nelson K., Monahan J. E., Yoo-Warren H., Hanson R. W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J Biol Chem. 1982 Jul 10;257(13):7629–7636. [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Denton R. M., Halestrap A. P. Regulation of pyruvate metabolism in mammalian tissues. Essays Biochem. 1979;15:37–77. [PubMed] [Google Scholar]
- DiTullio N. W., Berkoff C. E., Blank B., Kostos V., Stack E. J., Saunders H. L. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974 Mar;138(3):387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granner D. K., Andreone T. L. Insulin modulation of gene expression. Diabetes Metab Rev. 1985;1(1-2):139–170. doi: 10.1002/dmr.5610010108. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Sips H. J., Vervoorn R. C., Tager J. M. Intracellular compartmentation and control of alanine metabolism in rat liver parenchymal cells. Eur J Biochem. 1982 Feb;122(1):87–93. doi: 10.1111/j.1432-1033.1982.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Vervoorn R. C., Van der Meer R., Tager J. M. Control of gluconeogenesis in rat liver cells. I. Kinetics of the individual enzymes and the effect of glucagon. J Biol Chem. 1983 Dec 10;258(23):14346–14353. [PubMed] [Google Scholar]
- Groen A. K., van Roermund C. W., Vervoorn R. C., Tager J. M. Control of gluconeogenesis in rat liver cells. Flux control coefficients of the enzymes in the gluconeogenic pathway in the absence and presence of glucagon. Biochem J. 1986 Jul 15;237(2):379–389. doi: 10.1042/bj2370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Jomain-Baum M., Schramm V. L., Hanson R. W. Mechanism of 3-mercaptopicolinic acid inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1976 Jan 10;251(1):37–44. [PubMed] [Google Scholar]
- Junge O., Brand K. Mixed function oxidation of hexobarbital and generation of NADPH by the hexose monophosphate shunt in isolated rat liver cells. Arch Biochem Biophys. 1975 Dec;171(2):398–406. doi: 10.1016/0003-9861(75)90048-x. [DOI] [PubMed] [Google Scholar]
- Karvonen I., Stengård J. H., Huupponen R., Stenbäck F. G., Sotaniemi E. A. Effects of enzyme induction therapy on glucose and drug metabolism in obese mice model of non-insulin dependent diabetes mellitus. Diabetes Res. 1989 Feb;10(2):85–92. [PubMed] [Google Scholar]
- Karvonen I., Stengård J. H., Saarni H. U., Stenbäck F., Sotaniemi E. A. Hepatic mixed function oxidase system and enzymatic glucose metabolism in rats. Diabetes Res. 1987 Apr;4(4):195–200. [PubMed] [Google Scholar]
- Kida K., Nishio T., Yokozawa T., Nagai K., Matsuda H., Nakagawa H. The circadian change of gluconeogenesis in the liver in vivo in fed rats. J Biochem. 1980 Oct;88(4):1009–1013. doi: 10.1093/oxfordjournals.jbchem.a133051. [DOI] [PubMed] [Google Scholar]
- Lahtela J. T., Arranto A. J., Sotaniemi E. A. Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes. 1985 Sep;34(9):911–916. doi: 10.2337/diab.34.9.911. [DOI] [PubMed] [Google Scholar]
- Lahtela J. T., Arranto A. J., Stenbäck F., Sotaniemi E. A. Insulin-mediated glucose metabolism is related to liver structure and microsomal function. Scand J Gastroenterol. 1986 Aug;21(6):737–743. doi: 10.3109/00365528609011110. [DOI] [PubMed] [Google Scholar]
- Lahtela J. T., Särkkä P., Sotaniemi E. A. Phenobarbital treatment enhances insulin mediated glucose metabolism in man. Res Commun Chem Pathol Pharmacol. 1984 May;44(2):215–226. [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverve X. M., Groen A. K., Verhoeven A. J., Tager J. M. Kinetic analysis of short-term effects of alpha-agonists on gluconeogenesis in isolated rat hepatocytes. FEBS Lett. 1985 Feb 11;181(1):43–46. doi: 10.1016/0014-5793(85)81110-8. [DOI] [PubMed] [Google Scholar]
- Leverve X. M., Verhoeven A. J., Groen A. K., Meijer A. J., Tager J. M. The malate/aspartate shuttle and pyruvate kinase as targets involved in the stimulation of gluconeogenesis by phenylephrine. Eur J Biochem. 1986 Mar 17;155(3):551–556. doi: 10.1111/j.1432-1033.1986.tb09523.x. [DOI] [PubMed] [Google Scholar]
- Lyonnet S., Coupé C., Girard J., Kahn A., Munnich A. In vivo regulation of glycolytic and gluconeogenic enzyme gene expression in newborn rat liver. J Clin Invest. 1988 Jun;81(6):1682–1689. doi: 10.1172/JCI113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. J., McLean A. E. The effect of oral phenobarbitone on hepatic microsomal cytochrome P-450 and demethylation activity in rats fed normal and low protein diets. Biochem Pharmacol. 1969 Jan;18(1):153–157. doi: 10.1016/0006-2952(69)90020-3. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoulet M., Leverve X. M., Plomp P. J., Meijer A. J. Stimulation by glucose of gluconeogenesis in hepatocytes isolated from starved rats. Biochem J. 1987 Aug 1;245(3):661–668. doi: 10.1042/bj2450661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R. Induction of liver growth by xenobiotic compounds and other stimuli. CRC Crit Rev Toxicol. 1974 Sep;3(1):97–158. doi: 10.3109/10408447409079856. [DOI] [PubMed] [Google Scholar]
- Sotaniemi E. A., Stengård J. H., Saarni H. U., Arranto A. J., Keinänen K., Kerola T., Sutinen S. Hepatic glucose-6-phosphatase activity in non-insulin dependent diabetics. Effect of enzyme-inducing drugs. Acta Med Scand. 1984;215(4):323–331. doi: 10.1111/j.0954-6820.1984.tb05014.x. [DOI] [PubMed] [Google Scholar]
- Van Der Meer R., Tager J. M. A simple method for the perfusion of isolated liver cells. FEBS Lett. 1976 Aug 1;67(1):36–40. doi: 10.1016/0014-5793(76)80865-4. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Jakob A., Refino C. Control of the removal of reducing equivalents from the cytosol in perfused rat liver. J Biol Chem. 1971 Dec 25;246(24):7632–7641. [PubMed] [Google Scholar]


