Abstract
Background
Simethicone can improve bowel preparation quality, but the optimal timing of oral simethicone before colonoscopy has not been determined. This study aimed to explore the effect of the time interval between oral simethicone and the start of colonoscopy (S-C) on bowel preparation quality.
Material/Methods
A total of 364 patients undergoing colonoscopy at our department from August 1, 2021 to November 30, 2021 were included in the training cohort, and 420 consecutive patients from December 15, 2021 to January 31, 2022 comprised the validation cohort. They were classified into short and long S-C groups according to the median S-C. Bowel preparation quality evaluated by the Boston Bowel Preparation Scale was compared between the 2 groups. Logistic regression analyses were performed to explore the correlation between S-C and bowel preparation quality, and we explored the effect of run-way time and time of starting colonoscopy on bowel preparation quality.
Results
In the training cohort, 182 and 182 patients were classified into the short and long S-C groups, respectively; in the validation cohort, 210 and 210 patients were classified into the 2 groups, respectively. In the 2 cohorts, the short S-C group had a significantly higher rate of adequate/excellent bowel preparation than the long S-C group. Logistic regression analyses showed that shorter S-C, shorter run-way time, and colonoscopy in the morning were all correlated with adequate/excellent bowel preparation.
Conclusions
Bowel preparation quality may be affected by S-C, run-way time, and time of starting colonoscopy. S-C shortening should be given equal importance as run-way time shortening.
Keywords: Endoscopy, Colonoscopy, Simethicone
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide, with more than 945 000 new cases each year [1]. Early diagnosis and resection of colorectal precancerous lesions is fundamental for the prevention of CRC [2,3]. Colonoscopy is the criterion standard for detection of colorectal precancerous lesions [4], and high-quality bowel preparation during colonoscopy will improve their detection rate [5,6].
Bowel preparation quality can be influenced by several factors, including age, sex, body mass index (BMI), abnormal defecation, run-way time (the time interval between the last bowel preparation and the start of colonoscopy), and time of starting colonoscopy [7–14]. Both the European Society of Gastrointestinal Endoscopy and the Chinese bowel preparation guidelines recommend the addition of oral simethicone for improvement of mucosal visibility [11,15]. Our recent meta-analysis has also confirmed that oral simethicone can improve bowel preparation quality [16]. However, the optimal time to use simethicone before colonoscopy has not been previously determined.
Several previous studies compared the effects of oral simethicone at different time points before colonoscopy on bowel preparation quality, but their findings were controversial [17–21] due to differences in definitions of the time interval between oral simethicone and the start of colonoscopy (S-C), and regimens of laxatives given. First, in some studies, the time when oral simethicone was given was the day before colonoscopy or the day of colonoscopy; by comparison, in others, the time when oral simethicone was given was 2 hours, 1 hour, or half an hour before colonoscopy [17–21]. Second, single-dose laxative regimens were employed in some studies, but split-dose laxative regimens were used in others [17–22].
In this retrospective study we explored the optimal S-C for bowel preparation before colonoscopy by adjusting the factors potentially affecting bowel preparation quality. Furthermore, considering that S-C closely correlates with run-way time and time of starting colonoscopy, we further separately determined the effect of run-way time and the time of colonoscopy on bowel preparation quality by performing logistic regression analyses.
Material and Methods
Study Design
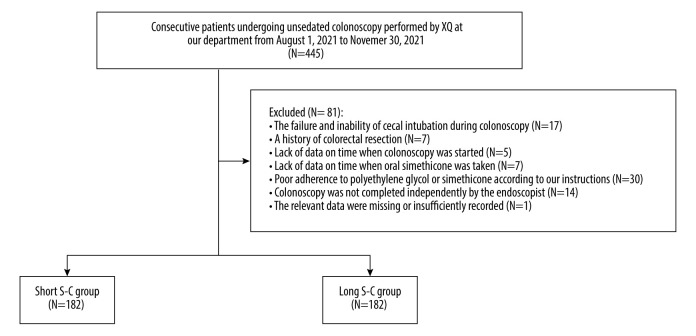
In the training cohort, we retrospectively screened all consecutive patients who underwent unsedated colonoscopy by the same endoscopist (XQ) at the Department of Gastroenterology of the General Hospital of Northern Theater Command between August 1, 2021 and November 30, 2021. Exclusion criteria were: (1) the failure and inability of cecal intubation during colonoscopy due to various reasons (colonic lumen stenosis caused by colorectal occupation; only polypectomy is performed, but cecal intubation is unnecessary; and the patient’s poor compliance compromises cecal intubation); (2) a history of colorectal resection; (3) lack of data on time when colonoscopy was started; (4) lack of data on time when oral simethicone was taken; (5) poor adherence to PEG or simethicone according to our instructions; (6) colonoscopy was not completed independently by the endoscopist; and (7) the relevant data were missing or insufficiently recorded.
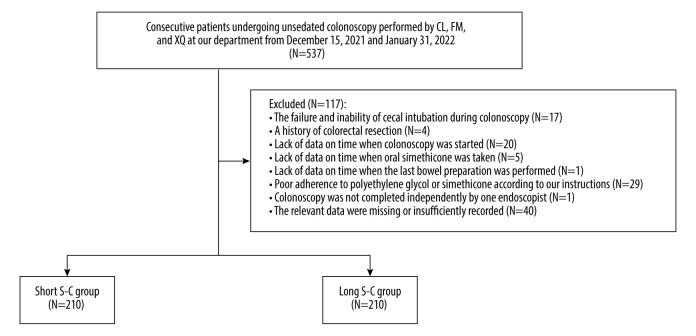
In the validation cohort, we retrospectively analyzed all consecutive patients who underwent unsedated colonoscopy performed by 3 endoscopists (XQ, FM, and CL) at our department between December 15, 2021 and January 31, 2022. We also excluded patients with missing data on the time of the last bowel preparation.
This study was performed following the Declaration of Helsinki guidelines.
The study protocol was approved by the medical ethics committee of our hospital, with an approval number Y (2022) 089 on August 2, 2022. The requirement for informed consent was waived, and all the data were de-identified.
Data Collection
The following data were collected: age, sex, height, weight, history of colonoscopy and abdominal surgery, indications for colonoscopy (eg, abdominal pain/discomfort, bloating, abnormal defecation, polyp, health checkup), time of starting colonoscopy, and time and dose of PEG and oral simethicone. BMI, run-way time, and S-C were calculated.
Groups and Definitions
Patients were divided into the short and long S-C groups according to the median S-C.
Bowel preparation quality was evaluated using the Boston bowel preparation scale (BBPS) [23]. The BBPS score for each colon segment, including right-side, transverse, and left-side colon, was rated from 0 to 3. The right-side colon included the cecum and ascending colon; the transverse colon included the hepatic flexure, transverse colon, and splenic flexure; and the left-side colon included the descending colon, sigmoid colon, and rectum. The total BBPS score was the sum of the BBPS scores for the 3 colon segments, ranging from 0 to 9. Adequate bowel preparation was defined as a total BBPS score of ≥6 with a BBPS score of ≥2 for each colon segment. Excellent bowel preparation was defined as a total BBPS score of ≥8. BBPS scores were recorded in endoscopic reports by endoscopists who were unaware of the S-C.
The presence of significant bubbles would be recorded according to the findings of colonoscopy. Adverse events were defined as the occurrence of gastrointestinal symptoms after the use of oral simethicone, including nausea, vomiting, abdominal pain, and bloating.
Bowel Preparation Procedure
A split-dose laxative regimen has been regularly recommended for bowel preparation at our department. Patients were instructed to drink 1000 mL water with 1 bag of PEG (68.56 g, Wanhe Pharmaceutical Co. Ltd., China) the night before the colonoscopy, and then to drink another 2000 mL water with 2 bags of PEG on the morning of the colonoscopy. A bottle of oral simethicone (40 mg/mL, 30 mL, Berlin-Chemie AG, Berlin, Germany) with 100 mL water was recommended to be taken before colonoscopy, but the time point was not specified.
Statistical Analyses
All statistical analyses were performed by using IBM SPSS version 22.0 statistical software (IBM Corp., Armonk, NY). Continuous variables were expressed in terms of median (range) and compared by the non-parametric Mann-Whitney U test. Categorical variables were expressed in terms of frequency (percentage) and compared by the chi-square test or Fisher’s exact test. In the training cohort, logistic regression analyses were performed to explore the impact of S-C and time of starting colonoscopy on bowel preparation quality by adjusting for age, sex, BMI, and abnormal defecation. In the validation cohort, an additional factor (run-way time) was further explored. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A two-tailed P<0.05 was considered statistically significant.
Results
Training Cohort
Patients
Overall, 364 patients were included. Their median age was 53 (range 17–85) years and 47.3% (172/364) were female. Of them, 94.0% (342/364) and 40.7% (148/364) had adequate and excellent bowel preparation, respectively; 1.6% (6/364) had significant bubbles in the colon; 6.3% (23/364), 1.4% (5/364), 0.3% (1/364), and 0.8% (3/364) had nausea, vomiting, abdominal pain, and bloating, respectively. The time of oral simethicone ranged from 0: 00 am to 1: 00 pm on the day of colonoscopy.
Groups
The median S-C was 6.20 hours. Thus, 182 and 182 patients were divided into the short (≤6.20 hours) and long (>6.20 hours) S-C groups, respectively (Figure 1). There was no statistically significant difference between the 2 groups in terms of age, weight, height, BMI, history of abdominal surgery, or most indications for colonoscopy. The characteristics of included patients are described in Table 1.
Figure 1.
Flowchart of patients’ enrollment in the training cohort. (The figure was created by Microsoft Office PowerPoint 2019).
Table 1.
Baseline characteristics of included patients in the training cohort.
| Variables | Overall (N=364) | Short S-C group (N=182) | Long S-C group (N=182) | P value |
|---|---|---|---|---|
| Median (range) or Frequency (percentage) | Median (range) or Frequency (percentage) | Median (range) or Frequency (percentage) | ||
| Age (years) | 53.00 (17.00–85.00) | 54.00 (18.00–85.00) | 53.00 (17.00–77.00) | 0.777 |
| Female | 172 (47.3%) | 76 (41.8%) | 96 (52.7%) | 0.036 |
| BMI (kg/m2) | 24.20 (14.90–47.80) | 24.20 (16.70–33.50) | 23.90 (14.90–47.80) | 0.628 |
| Weight (kg) | 67.50 (40.00–130.00) | 68.00 (46.50–110.00) | 67.25 (40.00–130.00) | 0.216 |
| Height (m) | 1.67 (1.46–1.88) | 1.68 (1.50–1.85) | 1.66 (1.46–1.88) | 0.141 |
| History of colonoscopy | 189 (51.9%) | 108 (59.3%) | 81 (44.5%) | 0.005 |
| History of abdominal surgery | 102 (28.0%) | 50 (27.5%) | 52 (28.6%) | 0.815 |
| Indications for colonoscopy | ||||
| Abdominal pain/discomfort | 116 (31.9%) | 53 (29.1%) | 63 (34.6%) | 0.261 |
| Bloating | 22 (6.0%) | 8 (4.4%) | 14 (7.7%) | 0.187 |
| Abnormal defecation | 261 (71.7%) | 121 (66.5%) | 140 (76.9%) | 0.027 |
| Polyp | 59 (16.2%) | 39 (21.4%) | 20 (11.0%) | 0.007 |
| Health checkup | 50 (13.7%) | 31 (17.0%) | 19 (10.4%) | 0.068 |
| Others | 12 (3.3%) | 6 (3.3%) | 6 (3.3%) | 1.000 |
| Colonoscopy started in the morning | 199 (54.7%) | 156 (85.7%) | 43 (23.6%) | <0.001 |
| Significant bubbles | 6 (1.6%) | 1 (0.5%) | 5 (2.7%) | 0.217 |
| Adverse event | ||||
| Nausea | 23 (6.3%) | 11 (6.0%) | 12 (6.6%) | 0.829 |
| Vomiting | 5 (1.4%) | 2 (1.1%) | 3 (1.6%) | 1.000 |
| Abdominal pain | 1 (0.3%) | 1 (0.3%) | 0 (0%) | 1.000 |
| Bloating | 3 (0.8%) | 2 (1.1%) | 1 (0.5%) | 1.000 |
S-C – time interval between oral simethicone and the start of colonoscopy; BMI – body mass index.
Bowel Preparation Quality
Regarding the BBPS score, the short S-C group had a significantly higher total BBPS score than the long S-C group [8.00 (4.00–9.00) vs 7.00 (3.00–9.00); P<0.001]. The right-side colon, transverse colon, and left-side colon BBPS scores were also significantly higher in the short S-C group than in the long S-C group (Table 2). As for the grade of bowel preparation quality, the short S-C group had significantly higher bowel preparation quality than the long S-C group [Adequate: 178/182 (97.8%) vs 164/182 (90.1%), P=0.002; Excellent: 94/182 (51.6%) vs 54/182 (29.7%), P<0.001] (Figure 2).
Table 2.
Comparison of bowel preparation quality between short and long S-C groups in the training cohort.
| Outcomes | Short S-C group (N=182) | Long S-C group (N=182) | P value |
|---|---|---|---|
| Median (range) | Median (range) | ||
| BBPS score | |||
| Total | 8.00 (4.00–9.00) | 7.00 (3.00–9.00) | <0.001 |
| Right-side colon | 2.00 (1.00–3.00) | 2.00 (0.00–3.00) | <0.001 |
| Transverse colon | 3.00 (2.00–3.00) | 3.00 (1.00–3.00) | 0.001 |
| Left-side colon | 3.00 (1.00–3.00) | 2.00 (1.00–3.00) | <0.001 |
S-C – time interval between oral simethicone and the start of colonoscopy; BBPS – Boston Bowel Preparation Scale.
Figure 2.
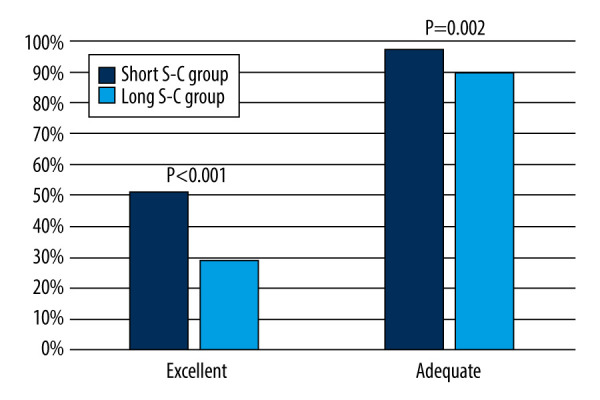
Comparison of bowel preparation quality between the short and long S-C groups in the training cohort. (The figure was created by Microsoft Office PowerPoint 2019). S-C – time interval between oral simethicone and the start of colonoscopy.
Logistic Regression Analyses
Univariate logistic regression analyses showed that both shorter S-C and colonoscopy in the morning were significantly associated with adequate/excellent bowel preparation. Multivariate logistic regression analyses showed that both shorter S-C and colonoscopy in the morning were independent risk factors of adequate/excellent bowel preparation (Table 3).
Table 3.
Logistic regression analyses regarding the effect of shorter S-C and colonoscopy in the morning on bowel preparation quality in the training cohort.
| Outcomes | OR | 95% CI | P value |
|---|---|---|---|
| Effect of shorter S-C on adequate bowel preparation | |||
| Univariate logistic regression | 4.884 | (1.619, 14.731) | 0.005 |
| Multivariate logistic regression | 5.112 | (1.676, 15.586) | 0.004 |
| Effect of shorter S-C on excellent bowel preparation | |||
| Univariate logistic regression | 2.532 | (1.646, 3.896) | <0.001 |
| Multivariate logistic regression | 2.888 | (1.843, 4.527) | <0.001 |
| Effect of colonoscopy in the morning on adequate bowel preparation | |||
| Univariate logistic regression | 5.969 | (1.978, 18.011) | 0.002 |
| Multivariate logistic regression | 5.723 | (1.877, 17.448) | 0.002 |
| Effect of colonoscopy in the morning on excellent bowel preparation | |||
| Univariate logistic regression | 2.344 | (1.518, 3.619) | <0.001 |
| Multivariate logistic regression | 2.487 | (1.589, 3.893) | <0.001 |
S-C – time interval between oral simethicone and the start of colonoscopy; OR – odd ratio; CI – confidence interval; BBPS – Boston Bowel Preparation Scale. Multivariate logistic regression analyses were performed by adjusting for age, gender, BMI, and abnormal defecation for exploring the effect of shorter S-C and colonoscopy in the morning on bowel preparation quality, respectively.
Validation Cohort
Patients
Overall, 420 patients were included. Their median age was 52 (range 16–82) years and 42.9% (180/420) were female. Of them, 91.2% (383/420) and 48.6% (204/408) had adequate and excellent bowel preparation, respectively; 0.5% (2/420) had significant bubbles in the colon; 3.8% (16/420), 0.7% (3/420), 0% (0/420), and 0% (0/420) had nausea, vomiting, abdominal pain, and bloating, respectively. The time of oral simethicone ranged from 9: 00 pm the night before colonoscopy to 11: 00 am on the day of colonoscopy, and the time of the last bowel preparation ranged from 1: 00 am to 9: 30 am on the day of colonoscopy.
Groups
The median S-C was 5.50 hours. Thus, 210 and 210 patients were divided into the short (≤5.50 hours) and long (>5.50 hours) S-C groups, respectively (Figure 3). There was no statistically significant difference between the 2 groups in terms of age, weight, height, BMI, and most indications for colonoscopy. The characteristics of included patients are described in Table 4.
Figure 3.
Flowchart of patients’ enrollment in the validation cohort. (The figure was created by Microsoft Office PowerPoint 2019).
Table 4.
Baseline characteristics of included patients in the validation cohort.
| Variables | Overall (N=420) | Short S-C group (N=210) | Long S-C group (N=210) | P value |
|---|---|---|---|---|
| Median (range) or Frequency (percentage) | Median (range) or Frequency (percentage) | Median (range) or Frequency (percentage) | ||
| Age (years) | 52.00 (16.00–82.00) | 53.50 (16.00–81.00) | 52.00 (18.00–82.00) | 0.140 |
| Female | 180 (42.9%) | 90 (42.9%) | 90 (42.9%) | 1.000 |
| BMI (kg/m2) | 24.25 (16.00–51.40) | 24.20 (16.00–33.00) | 24.70 (16.10–51.40) | 0.223 |
| Weight (kg) | 67.50 (40.00–143.00) | 67.75 (40.00–101.00) | 67.50 (40.00–143.00) | 0.499 |
| Height (m) | 1.70 (1.44–1.90) | 1.70 (1.50–1.90) | 1.70 (1.44–1.90) | 0.772 |
| History of colonoscopy | 187 (44.5%) | 103 (49.0%) | 84 (40.0%) | 0.062 |
| History of abdominal surgery | 121 (28.8%) | 50 (23.8%) | 71 (33.8%) | 0.024 |
| Indications for colonoscopy | ||||
| Abdominal pain/discomfort | 127 (30.2%) | 55 (26.2%) | 72 (34.3%) | 0.071 |
| Bloating | 19 (4.5%) | 10 (4.8%) | 9 (4.3%) | 0.814 |
| Abnormal defecation | 258 (61.4%) | 121 (57.6%) | 137 (65.2%) | 0.109 |
| Polyp | 32 (7.6%) | 27 (12.9%) | 5 (2.4%) | <0.001 |
| Health checkup | 68 (16.2%) | 39 (18.6%) | 29 (13.8%) | 0.185 |
| Others | 8 (1.9%) | 4 (1.9%) | 4 (1.9%) | 1.000 |
| Run-way time | 6.68 (3.15–12.27) | 5.58 (3.15–10.29) | 8.55 (6.10–12.27) | <0.001 |
| Colonoscopy started in the morning | 295 (70.2%) | 203 (96.7%) | 92 (43.8%) | <0.001 |
| Significant bubbles | 2 (0.5%) | 1 (0.5%) | 1 (0.5%) | 1.000 |
| Adverse event | ||||
| Nausea | 16 (3.8%) | 10 (4.8%) | 6 (2.9%) | 0.308 |
| Vomiting | 3 (0.7%) | 3 (1.4%) | 0 (0%) | 0.247 |
| Abdominal pain | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Bloating | 0 (0%) | 0 (0%) | 0 (0%) | – |
S-C – time interval between oral simethicone and the start of colonoscopy; BMI – body mass index.
Bowel Preparation Quality
As for the BBPS score, the short S-C group had a significantly higher total BBPS score than the long S-C group [8.00 (4.00–9.00) vs 7.00 (1.00–9.00); P<0.001]. The right-side colon, transverse colon, and left-side colon BBPS scores were also significantly higher in the short S-C group than in the long S-C group (Table 5). As for the grade of bowel preparation quality, the short S-C group had significantly higher bowel preparation quality than the long S-C group [Adequate: 199/210 (94.8%) vs 184/210 (87.6%), P=0.010; Excellent: 125/210 (59.5%) vs 79/210 (37.6%), P<0.001] (Figure 4).
Table 5.
Comparison of bowel preparation quality between short and long S-C groups in the validation cohort.
| Outcomes | Short S-C group (N=210) | Long S-C group (N=210) | P value |
|---|---|---|---|
| Median (range) | Median (range) | ||
| BBPS score | |||
| Total | 8.00 (4.00–9.00) | 7.00 (1.00–9.00) | <0.001 |
| Right-side colon | 2.00 (1.00–3.00) | 2.00 (0.00–3.00) | <0.001 |
| Transverse colon | 3.00 (2.00–3.00) | 3.00 (1.00–3.00) | 0.006 |
| Left-side colon | 3.00 (1.00–3.00) | 3.00 (0.00–3.00) | 0.001 |
S-C – time interval between oral simethicone and the start of colonoscopy; BBPS – Boston Bowel Preparation Scale.
Figure 4.
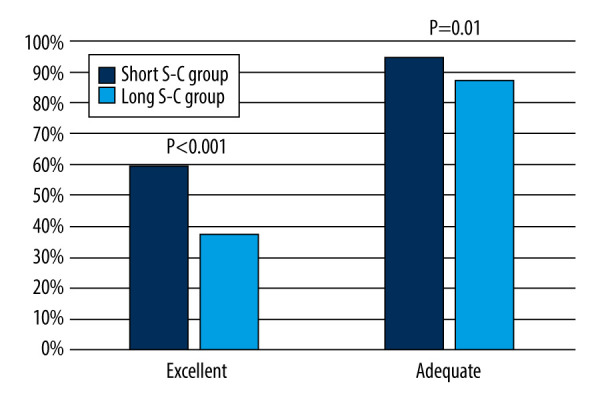
Comparison of bowel preparation quality between the short and long S-C groups in the validation cohort. (The figure was created by Microsoft Office PowerPoint 2019). S-C – time interval between oral simethicone and the start of colonoscopy.
Logistic regression analyses
Univariate logistic regression analyses showed that shorter S-C, shorter run-way time, and colonoscopy in the morning were all significantly associated with adequate/excellent bowel preparation. Multivariate logistic regression analyses showed that shorter S-C, shorter run-way time, and colonoscopy in the morning were all independent risk factors of adequate/excellent bowel preparation (Table 6).
Table 6.
Logistic regression analyses regarding the effect of shorter S-C, shorter run-way time, and colonoscopy in the morning on bowel preparation quality in the validation cohort.
| Outcomes | OR | 95% CI | P value |
|---|---|---|---|
| Effect of shorter S-C on adequate bowel preparation | |||
| Univariate logistic regression | 2.556 | (1.228, 5.320) | 0.012 |
| Multivariate logistic regression | 2.973 | (1.399, 6.319) | <0.001 |
| Effect of shorter S-C on excellent bowel preparation | |||
| Univariate logistic regression | 2.439 | (1.647, 3.610) | <0.001 |
| Multivariate logistic regression | 2.558 | (1.715, 3.817) | <0.001 |
| Effect of shorter run-way time on adequate bowel preparation | |||
| Univariate logistic regression | 2.951 | (1.390, 6.264) | 0.005 |
| Multivariate logistic regression | 3.344 | (1.546, 7.233) | 0.002 |
| Effect of shorter run-way time on excellent bowel preparation | |||
| Univariate logistic regression | 3.112 | (2.090, 4.635) | <0.001 |
| Multivariate logistic regression | 3.229 | (2.152, 4.845) | <0.001 |
| Effect of colonoscopy in the morning on adequate bowel preparation | |||
| Univariate logistic regression | 3.115 | (1.571, 6.176) | 0.001 |
| Multivariate logistic regression | 3.874 | (1.870, 8.029) | <0.001 |
| Effect of colonoscopy in the morning on excellent bowel preparation | |||
| Univariate logistic regression | 2.799 | (1.798, 4.358) | <0.001 |
| Multivariate logistic regression | 3.025 | (1.916, 4.775) | <0.001 |
S-C – time interval between oral simethicone and the start of colonoscopy; OR – odd ratio; CI – confidence interval; BBPS – Boston Bowel Preparation Scale. Multivariate logistic regression analyses were performed by adjusting for age, gender, BMI, and abnormal defecation for exploring the effect of shorter S-C, shorter run-way time, and colonoscopy in the morning on bowel preparation quality, respectively.
Discussion
Based on the current study, bowel preparation quality may be improved by shortening S-C. There are some potential explanations for this finding, as follows. First, during bowel preparation period, the intake of oral simethicone eliminates most of the bubbles in the colon [24]. Then, the gas in bubbles are released, further accelerating the transit of colonic gas [25], which stimulates colonic peristalsis and distal translocation of remaining stool or mucus [26]. In this case, if colonoscopy was performed immediately, bowel preparation quality might be acceptable. By contrast, if colonoscopy was performed after a long S-C, bowel preparation quality might be poor, probably because mucus and bile from the small intestine flows continuously to the colon, further generating new bubbles and cloudy fluid that affect the exposure of the mucosa [27,28]. Therefore, oral simethicone can not only eliminate the bubbles, but also further promotes the excretion of small intestinal fluid. Notably, in the current study, there was no significant difference in term of bubbles between short and long S-C groups. In this case, the benefit of shortening S-C on bowel preparation quality might be mainly attributed to the reduced volume of small intestinal fluid. Second, the time of oral simethicone is often close to that of the last bowel preparation according to the bowel preparation instructions provided by our department. Thus, patients in the short S-C group had shorter run-way time than those in the long S-C group (5.58 hours vs 8.55 hours). Previous studies have confirmed that shorter run-way time was associated with higher bowel preparation quality [29,30] because the duration of secretion of intestinal fluid may be shortened before colonoscopy [7]. Third, the last bowel preparation is often performed in the morning of colonoscopy according to the bowel preparation instructions provided at our department. As a result, patients who underwent colonoscopy in the morning typically had shorter run-way time and thus higher bowel preparation quality than those who had colonoscopy in the afternoon. Moreover, previous studies have demonstrated the benefits of colonoscopy scheduled in the morning on the improvement of bowel preparation quality [9,31]. In the current study, most patients in the short S-C group underwent colonoscopy in the morning (the training cohort: 85.7%; the validation cohort: 96.7%) and thus had higher bowel preparation quality.
As previously reported, some factors, including age, sex, BMI, and abnormal defecation can influence bowel preparation quality [10,13]. Thus, they were selected as adjusted factors in multivariate logistic regression analyses, and then we found that S-C, run-way time, and colonoscopy in the morning all independently improved bowel preparation quality. Notably, among the 3 factors mentioned above, S-C and run-way time can be controlled simply by determining when colonoscopy is performed. In addition, if we only focus on shortening run-way time, newly generated bubbles might continue to influence the visibility of the colonic mucosa. In that situation, endoscopists can repeatedly irrigate bubbles to ensure visibility during colonoscopy, but the bubbles would only be washed to the surface of nearby mucosa rather than burst directly. Therefore, while we emphasize shortening run-way time, we should also pay attention to shortening S-C.
Our study had several strengths that make our findings more reliable. First, there were 2 cohorts of patients. Considering the effect of run-way time and the time of colonoscopy on bowel preparation quality, the data from the validation cohort were used to validate the results of the training cohort. Second, the participants had the same bowel preparation regimen and the same group of investigators evaluated the bowel preparation quality to minimize the risk of bias in assessing the outcomes of interest. Third, linear and logistic regression analyses were performed to explore the association between S-C and bowel preparation quality.
Our study also had several limitations. First, it was performed at a single center. Second, this was a retrospective study, and some patients were excluded due to lack of relevant data. Third, the median S-C interval was subjectively selected for grouping to balance the number of patients assigned between the 2 groups. Fourth, all 3 endoscopists were trained in evaluation of bowel preparation quality, but they may have performed the evaluation differently.
Conclusions
Bowel preparation quality may be affected by S-C, run-way time, and the time of colonoscopy performed. S-C shortening should be given equal importance as run-way time shortening. In addition, at the time of the colonoscopy appointment, the time of colonoscopy should be determined as much as possible to facilitate the control of S-C and run-way time. The current findings need to be verified by large-scale prospective studies in the future.
Acknowledgements
We would like to express gratitude to our study team for establishing and updating the database, including Hongxin Chen, Haijuan Yao, Cong Gao, Yingchao Li, and Yuhang Yin.
Abbreviations
- CRC
colorectal cancer
- S-C
time interval between oral simethicone and the start of colonoscopy
- BMI
body mass index
- BBPS
Boston Bowel Preparation Scale
- OR
odds ratio
- CI
confidence interval
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet (London, England) 2005;365(9454):153–65. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 3.Pan J, Xin L, Ma YF, et al. Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: A meta-analysis. Am J Gastroenterol. 2016;111(3):355–65. doi: 10.1038/ajg.2015.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pullens HJ, Siersema PD. Quality indicators for colonoscopy: Current insights and caveats. World J Gastrointest Endosc. 2014;6(12):571–83. doi: 10.4253/wjge.v6.i12.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papanikolaou IS, Sioulas AD, Magdalinos N, et al. Improved bowel preparation increases polyp detection and unmasks significant polyp miss rate. World J Clin Cases. 2015;3(10):880–86. doi: 10.12998/wjcc.v3.i10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman W, Marshall S. Optimising bowel preparation before colonoscopy. Br J Nurs. 2020;29(Suppl 13):S3–S12. doi: 10.12968/bjon.2020.29.Sup13.S3. [DOI] [PubMed] [Google Scholar]
- 7.Moolla M, Dang JT, Shaw A, et al. Simethicone decreases bloating and improves bowel preparation effectiveness: A systematic review and meta-analysis. Surg Endosc. 2019;33(12):3899–909. doi: 10.1007/s00464-019-07066-5. [DOI] [PubMed] [Google Scholar]
- 8.Abudeeb H, Khan K, Maung M, Malcomson L, Brown A. Quality optimisation in colonoscopy: A function of time of colonoscopy or bowel preparation. Pan Afr Med J. 2019;32:205. doi: 10.11604/pamj.2019.32.205.16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athreya PJ, Owen GN, Wong SW, et al. Achieving quality in colonoscopy: Bowel preparation timing and colon cleanliness. ANZ J Surg. 2011;81(4):261–65. doi: 10.1111/j.1445-2197.2010.05429.x. [DOI] [PubMed] [Google Scholar]
- 10.Fayad NF, Kahi CJ, Abd El-Jawad KH, et al. Association between body mass index and quality of split bowel preparation. Clin Gastroenterol Hepatol. 2013;11(11):1478–85. doi: 10.1016/j.cgh.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2019. Endoscopy. 2019;51(8):775–94. doi: 10.1055/a-0959-0505. [DOI] [PubMed] [Google Scholar]
- 12.Hwang YJ, Shin DW, Kim N, et al. Sex difference in bowel preparation quality and colonoscopy time. Korean J Intern Med. 2021;36(2):322–31. doi: 10.3904/kjim.2019.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DW, Koo JS, Kang S, et al. Association between bowel habits and quality of bowel preparation for colonoscopy. Medicine. 2017;96(29):e7319. doi: 10.1097/MD.0000000000007319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alghamry A, Ponnuswamy SK, Agarwal A, et al. Split-dose bowel preparation with polyethylene glycol for colonoscopy performed under propofol sedation. Is there an optimal timing? J Dig Dis. 2017;18(3):160–68. doi: 10.1111/1751-2980.12458. [DOI] [PubMed] [Google Scholar]
- 15.Digestive Endoscopy Special Committee of Endoscopic Physicians Branch of Chinese Medical Association; Cancer Endoscopy Committee of China Anti-Cancer Association. [Chinese guideline for bowel preparation for colonoscopy (2019, Shanghai)]. Zhonghua Nei Ke Za Zhi. 2019;58(7):485–95. doi: 10.3760/cma.j.issn.0578-1426.2019.07.002. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 16.Cao RR, Wang L, Gao C, et al. Effect of oral simethicone on the quality of colonoscopy: A systematic review and meta-analysis of randomized controlled trials. J Dig Dis. 2022;23(3):134–48. doi: 10.1111/1751-2980.13084. [DOI] [PubMed] [Google Scholar]
- 17.Ji C, Zhang W, Ma H, et al. [Effect of simethicone at different time points on bowel preparation before colonoscopy]. Zhonghua Xiao Hua Nei Jing Za Zhi. 2019;36(2):131–33. [in Chinese] [Google Scholar]
- 18.Liu F, li H, Zhang W. Effects of simethicone administration at three time points on bowel preparation and polyp detection rate in patients undergoing colonoscopy. J Math Med. 2020;33(6):929–31. [Google Scholar]
- 19.Cesaro P, Hassan C, Spada C, et al. A new low-volume isosmotic polyethylene glycol solution plus bisacodyl vs split-dose 4 L polyethylene glycol for bowel cleansing prior to colonoscopy: A randomised controlled trial. Dig Liver Dis. 2013;45(1):23–27. doi: 10.1016/j.dld.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Ko BM, Goong HJ, et al. Optimal timing of simethicone addition for bowel preparation using polyethylene glycol plus ascorbic acid. Dig Dis Sci. 2019;64(9):2607–13. doi: 10.1007/s10620-019-05599-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu ZW, Zhan SG, Yang MF, et al. Optimal timing of simethicone supplement for bowel preparation: A prospective randomized controlled trial. Can J Gastroenterol Hepatol. 2021;2021:4032285. doi: 10.1155/2021/4032285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Li YY, Luo XT, et al. Split-dose 4-L polyethylene glycol regimen for patients with previous colorectal surgery in bowel preparation before colonoscopy: A randomized, controlled, single-blind study. J Dig Dis. 2018;19(6):359–68. doi: 10.1111/1751-2980.12608. [DOI] [PubMed] [Google Scholar]
- 23.Calderwood AH, Schroy PC, 3rd, Lieberman DA, et al. Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness. Gastrointest Endosc. 2014;80(2):269–76. doi: 10.1016/j.gie.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Liu J, Ma SL, et al. Impact of simethicone on bowel cleansing during colonoscopy in Chinese patients. World J Clin Cases. 2021;9(10):2238–46. doi: 10.12998/wjcc.v9.i10.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brecević L, Bosan-Kilibarda I, Strajnar F. Mechanism of antifoaming action of simethicone. J Appl Toxicol. 1994;14(3):207–11. doi: 10.1002/jat.2550140311. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Yen ZS, Lee CC, et al. Nonsurgical management of partial adhesive small-bowel obstruction with oral therapy: A randomized controlled trial. CMAJ. 2005;173(10):1165–69. doi: 10.1503/cmaj.1041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Yuan M, Li Z, et al. The efficacy of simethicone with polyethylene glycol for bowel preparation: A systematic review and meta-analysis. J Clin Gastroenterol. 2021;55(6):e46–e55. doi: 10.1097/MCG.0000000000001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Zheng D, Wang J, et al. Simethicone improves bowel cleansing with low-volume polyethylene glycol: A multicenter randomized trial. Endoscopy. 2018;50(4):412–22. doi: 10.1055/s-0043-121337. [DOI] [PubMed] [Google Scholar]
- 29.Bucci C, Rotondano G, Hassan C, et al. Optimal bowel cleansing for colonoscopy: Split the dose! A series of meta-analyses of controlled studies. Gastrointest Endosc. 2014;80(4):566–76.e2. doi: 10.1016/j.gie.2014.05.320. [DOI] [PubMed] [Google Scholar]
- 30.Marmo R, Rotondano G, Riccio G, et al. Effective bowel cleansing before colonoscopy: A randomized study of split-dosage vs non-split dosage regimens of high-volume vs low-volume polyethylene glycol solutions. Gastrointest Endosc. 2010;72(2):313–20. doi: 10.1016/j.gie.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 31.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797–802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]