Abstract
Compared with other T-helper subsets, Th17 cell numbers are very low in human blood but become elevated in chronic inflammatory diseases. In this study, we investigated mechanisms that may explain the frequent involvement of Th17 cells in autoimmune diseases such as uveitis. We compared Th17 and Th1 subsets and found that Th17 cells expressed lower IL-2 levels during Ag-priming and this correlated with their decreased susceptibility to activation-induced cell death (AICD). However, complete depletion of IL-2 with IL-2 neutralizing antibodies rendered Th17 cells as susceptible to apoptosis as Th1 cells, suggesting that the low levels of IL-2 produced by Th17 cells conferred survival advantages to this subset. We describe here a Th17 subtype that constitutively produces very low levels of IL-2 (Th17-DP). The Th17-DP population increased dramatically in the blood and retina of mice during experimental autoimmune uveitis, indicating their potential involvement in the etiology of uveitis. We further show that the majority of the memory Th17 cells in human blood are Th17-DP and are targets of daclizumab, an IL-2R antibody used in treating recalcitrant uveitis. Thus, Th17 cells may persist in tissues and contribute to chronic inflammation by limiting IL-2 production to levels that cannot provoke IL-2-induced AICD yet are sufficient to promote Th17 homeostatic expansion.
Keywords: Animal models, Autoimmunity, T helper cells, Inflammation, Clinical immunology
Introduction
Uveitis is a group of potentially sight-threatening idiopathic intraocular inflammatory diseases that includes Behçet’s disease, birdshot retinochoroidopathy, sympathetic ophthalmia, Vogt–Koyanagi–Harada (VKH) and ocular sarcoidosis, and may be of infectious or autoimmune etiology [1, 2]. Blinding uveitis is characterized by repeated cycles of remission and recurrent intraocular inflammation [2]. Although steroids and other anti-inflammatory drugs are effective therapy, renal toxicity or other adverse effects preclude prolonged usage [2]. Identifying T cells that initiate acute uveitis or mechanisms that allow autoreactive T cells to persist in the retina and mediate recurrent uveitis is a challenge. Interleukin-17-producing T (Th17) cells produce TNF-α, IL-21, IL-22, granzyme B, contribute to host defense against microbial agents and have recently been implicated in the development of chronic inflammation in several mouse models [3-5], suggesting that the Th17 subset may also be involved in the pathogenesis of immune-mediated diseases in humans, including uveitis. Several studies have reported that IL-23 and IL-17 are elevated in Behçet’s and VKH patients with active uveitis [6-9], while elevated serum levels of IL-23 and Th17-induced cytokines were found to correlate with intraocular inflammation after cataract surgery in VKH patients [10]. In addition, effective anti-uveitic drugs such as cyclosporine A, daclizumab, rapamycin and dexamethasone mediate their effects in part, by inhibiting production of IL-17 [11, 12]. Although these studies suggest the possible involvement of Th17 cells in clinical uveitis, more direct and compelling evidence has come from studies in experimental autoimmune uveitis (EAU) [13]. In this well-characterized mouse model of human uveitis [14], dynamic changes in the levels of IL-17-expressing cells occur during the course of uveitis [15]. Before the inception of EAU, only trace numbers of IL-17-expressing T cells are detectable in the PBMCs, LNs or retina but their numbers in these tissues increase with disease progression, with the highest levels detected in the retina at the peak of disease [15]. On the other hand, recovery from the disease coincides with rapid diminution in the IL-17-expressing T cells [15]. These studies revealed a positive correlation between EAU pathology and increases in IL-17-expressing T cells in the retina. Further evidence for the involvement of IL-17-expressing T cells in EAU etiology was provided by studies showing that the treatment of mice afflicted from EAU with IL-17-specific antibodies reduced the severity of the ocular inflammation [15, 16]. These studies in mice are in line with the results in humans showing that the blood of patients with uveitis contain more IL-17-expressing T cells than blood of healthy individuals; furthermore IL-17-producing T cell numbers increased during active uveitis but decreased following treatment, further suggesting involvement of Th17 cells in the etiology of non-infectious uveitis [15]. It is, however, notable that Th17 cells in the blood of healthy individuals are expanded by IL-2 in in vitro cultures of human PBMCs, suggesting that increase in IL-2 that accompany infections may trigger expansion of Th17 cells that promote chronic inflammation [15].
The Th17 lineage is characterized by a unique transcriptional program induced by STAT3; mice conditionally deficient in STAT3 in the CD4+ T-cell compartment (STAT3KO) cannot generate Th17 and do not develop EAU or EAE, underscoring the requirement of STAT3 for developing Th17-mediated diseases [17-19]. In view of the pivotal role of IL-2 in the proliferation or survival of mouse Th1, Th2 and Treg cell subsets and in the expansion of human Th17, it is puzzling that IL-2 inhibits differentiation of mouse Th17 cells [20]. Understanding the exact role of IL-2 in mouse Th17 cell development is further confounded by the results of a recent study showing that differentiating Th17 cells constitutively express IL-2 (Th17-DP cells) at an early stage in their development but lose their capacity to produce IL-2 as they undergo terminal differentiation into mature Th17 cells [21]. These observations suggest that regulation of IL-2 production may play a unique role in the physiology of Th17 subset.
In this report, we have examined whether Th17-DP cells also exist in human blood, if they are involved in the pathogenesis of uveitis, and investigated mechanisms that may explain the frequent involvement of Th17 cells in the etiology of many organ-specific autoimmune diseases. Our finding that the majority of Th17 cells expanded by IL-2 or TCR-activation in human PBMCs are Th17-DP cells and that substantial numbers of Th17 cells that persist in the retina during uveitis constitutively produce IL-2, establishes for the first time the potential clinical relevance of IL-2-expressing Th17-DP memory cells. We also found that because of sustained production of low levels of IL-2, Th17 were less susceptible to activation-induced cell death (AICD) upon TCR restimulation than Th1 effectors and this may contribute to the relatively high pathogenic potential of Th17 cells in autoimmune diseases.
Results
Human Th17-DP cells are targets for homeostatic expansion by IL-2 and daclizumab therapy
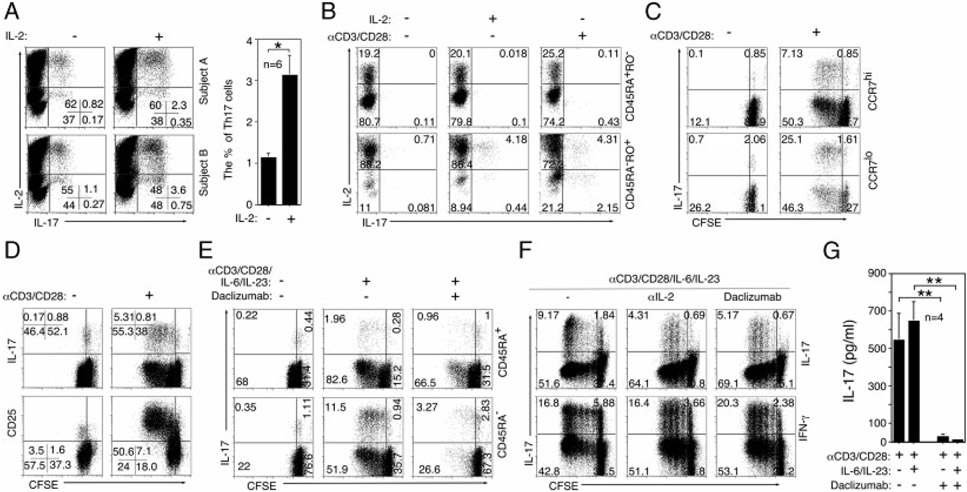
Previous work from our laboratory implicated Th17 cells in the etiology of human uveitis and revealed that IL-2 induces the expansion of Th17 memory cells [15]. Recently, two Th17 subtypes have been identified in vivo in the mouse; an early developmental stage (immature) that constitutively expresses IL-2 (Th17-DP) and a terminally differentiated mature Th17 phenotype (Th17-SP) that has lost the capacity to constitutively produce IL-2 [21]. We therefore examined whether Th17-DP cells also exist in human blood and whether their levels increase in response to cytokine or TCR stimulation. We show here that in blood of healthy subjects, as much as 80% of Th17 cells expanded by IL-2 were Th17-DP cells (Fig. 1A) and they derived from expansion of central (CD45RA−RO+CCR7Hi) and effector (CD45RA−RO+CCR7Lo) memory T cells (Fig. 1B and C). Moreover, 67% of Th17 cells expanded by TCR stimulation was Th17-DP, suggesting that substantial majority of Th17 cells expanded in healthy human blood are Th17-DP (Fig. 1B). It is of note that expansion of the Th17-DP cells by TCR activation was associated with rapidly proliferating Th17 cells (Fig. 1D) and elevated CD25 (IL-2Rα) expression (Fig. 1D). We further show that the Th17-DP memory cells are targets of daclizumab, a humanized anti-IL-2Rα antibody approved for treatment of recalcitrant uveitis [2]. The inhibitory effects of daclizumab and anti-IL-2 Abs on expansion of Th17 cells (Fig. 1E and F) were accompanied by marked reduction of IL-17 secretion (Fig. 1G). These results thus establish for the first time the potential clinical relevance of the Th17-DP subset.
Figure 1.
Human Th17-DP cells can be targeted for homeostatic expansion by IL-2 and daclizumab therapy. (A–F) CD4+ T cells isolated from the PBMCs of healthy human subjects were stimulated with cytokines and/or anti-CD3/anti-CD28 in the presence or absence of IL-2 as indicated. For analysis of intracellular cytokine expression, the cells were also stimulated with PMA and ionomycin as described in Materials and Methods. Plots were gated on CD4+ T cells and numbers in quadrants indicate percent T cells expressing IL-2 and/or IL-17, (A, left) representative results in two subjects are shown. (A, right) The percentages of Th17 cells detected in PBMCs of 20 healthy human subjects stimulated in the presence or absence of IL-2 are shown. (C–F) CFSE dilution and cell surface expression of (B) CD45RO, (C) CCR7, (D) CD25 or (E) CD45RA CD45RO were analyzed by flow cytometry. (E–G) Some cultures were stimulated in medium containing daclizumab or (F) anti-IL-2 Ab. Plots were gated on CD4+ T cells and numbers in quadrants indicate percent T cells expressing cytokines or cell surface marker. (G) IL-17 secretion was quantified by Multiplex ELISA. (A, G) The results are expressed as mean+SD. The results are representative of at least 10 independent experiments or subjects. *p<0.05; **p<0.01 as determined by the Student’s t-test.
Th17-DP cells persist in peripheral tissues after recovery from EAU
We further sought to understand the role of constitutive IL-2 expression at early stages of Th17 development and to determine whether the immature Th17-DP cell contributes to development of pathogenic autoimmune diseases. Specifically, we investigated whether Th17-DP is present in blood, LNs or retina of mice with EAU, a model of uveitis [14].
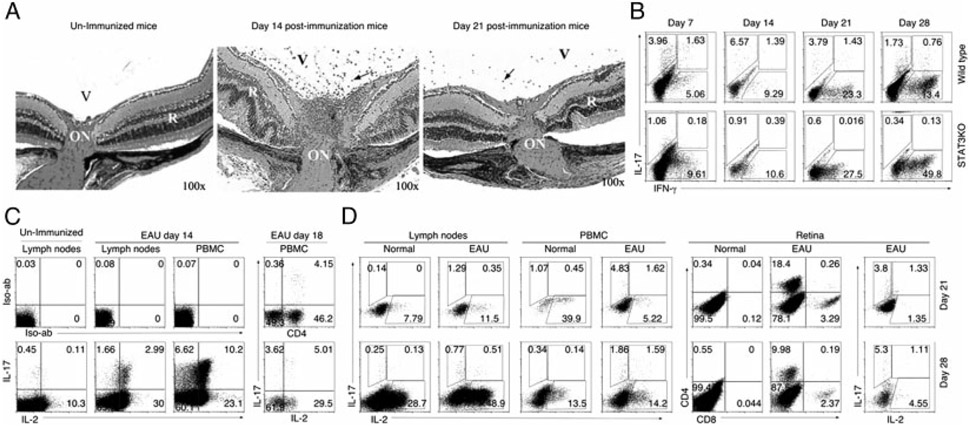
STAT3KO mice and WT mice were immunized with interphotoreceptor-retinoid-binding-protein (IRBP) in CFA and EAU was assessed by histology [19]. In line with the previous reports, initial signs of EAU appeared 7–12 d after immunization, with full-blown disease developing by day 14–16 post-immunization (Fig. 2A). By day 21–28, there was a substantial reduction in inflammatory cells in the retina with diminution of clinical symptoms of disease occurring thereafter. Consistent with the requirement of STAT3 for Th17 development, STAT3KO mice did not develop EAU [19] and pathologic lesions in WT mice retina correlated temporally with increase in Th17 in LNs (Fig. 2B). In line with the published reports [15, 19], EAU pathology in this model correlates with the Th17 and not Th1 subset (Fig. 2B). Analysis of freshly isolated T cells (day 14 post-immunization) revealed that Th17-DP cells comprised 64 and 61% of uveitogenic Th17 cells in LNs and PBMCs of mice with EAU, respectively (Fig. 2C). In contrast to the progressive decline of Th17 cells in LNs from 4.7% at day 14 (Fig. 2C) to <1.65% at the later time points of the disease, Th17 and Th17-DP cells continued to persist in the blood and retina (Fig. 2D). Persistence of these cells in blood and retina suggests that Th17 and Th17-DP cells in these peripheral tissues may be the source of new waves of uveitogenic T cells that perpetuate cycles of recurrent uveitis.
Figure 2.
Th17-DP cells persist in peripheral tissues after recovery from EAU. (A) Histological sections of 6-wk-old mouse retina at indicated time points after immunization with IRBP/CFA. Arrows indicate inflammatory cells. V, vitreous; R, retina; ON, optic nerve. (B–D) Detection of IL-2-, IL-17- or IFN-γ-expressing CD4+ T cells by intracellular cytokine staining assay. Analysis was performed on (B) cells from draining LNs of WT or STAT3KO mice at day 7, 14, 21 or 28 post-immunization, (C) freshly isolated PBMCs or LN cells from WT mice 14 days or 18 days post-immunization without further stimulation, (D) freshly isolated PBMCs, LNs or retina at day 21 or day 28 post-immunization. Numbers in quadrants indicate percent of T cells expressing IL-17, IFN-γ and/or IL-2. The results are representative of three independent experiments.
Th17-DP cells constitutively express IL-2 mRNA and secrete low levels of IL-2
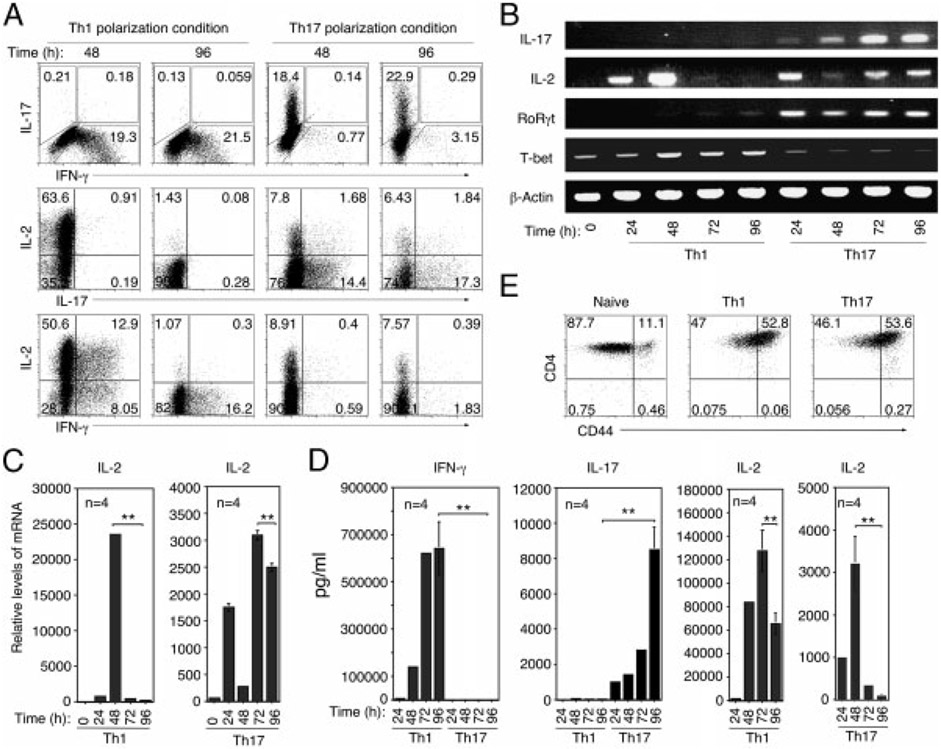
To further characterize IL-2 expression by Th17-DP cells, we cultured naïve CD4+ T cells for 4 days under Th1- or Th17-polarization conditions and compared the level and kinetics of IL-2 production by Th17 and Th1 subsets. We show that approximately 64% of T cells in 48 h Th1 cultures expressed IL-2 and by 96 h the percentage dropped to <1.5% (Fig. 3A). In contrast, 9.5% of T cells in Th17 cultures expressed IL-2 after 48 h in culture and the percentage of IL-2-expressing Th17 cells remained relatively constant (8.3%) through 96 h in culture (Fig. 3A). These results suggest that the kinetics of IL-2 expression in response to TCR stimulation is different between Th1 and Th17 cells. Although Th17-DP cells were also generated in vitro, Th17-DP comprised 9.6% of Th17 in the 96 h Th17 cultures (Fig. 3A; right panels) compared with 64% among IRBP-specific Th17 cells (Fig. 2C), suggesting that the Th17-DP phenotype may be more stable in vivo or preferentially recruited under pathological conditions. Similar to the Th17 subset, Th1 cells that produce IL-2 and IFN-γ (Th1-DP) were generated in vitro. However, Th1-DP rapidly lost their capacity for IL-2 production (Fig. 3A). As indicated in Fig. 3A, 62% of Th1 cells in the 48 h cultures were Th1-DP but by the 96 h time point their numbers declined precipitously to <2%. In contrast, Th17-DP cell levels remained relatively constant through 96 h of in vitro activation (Fig. 3A). RNA analysis performed on T cells that were not subjected to PMA/ionomycin treatment confirmed the transient nature of IL-2 production by Th1. Unlike 24 and 48 h cultures, expression of IL-2 mRNA transcripts was undetectable in 72 or 96 h Th1 cultures (Fig. 3B) even though the Th1 cells were viable, as indicated by similar levels of T-bet mLNA expression in 48, 72 and 96 h cultures. Similar to intracellular cytokine expression, Th17 cells in 72 and 96 h cultures maintain relatively constant, albeit lower levels of IL-2 transcription (Fig. 3B and C). We confirmed the relatively low levels of IL-2 secretion in Th17 cultures by ELISA (Fig. 3D). It is, however, important to emphasize that the production of lower amounts of IL-2 by Th17 cells was not due to their partial activation since the Th17 cells expressed similar levels of CD44 as Th1 cells (data not shown). It is also notable that low undetectable IL-2 mRNA expression in 72 and 96 h Th1 cultures coincided with relatively high IL-2 secretion. In contrast, IL-2 secreted in Th17 cultures at 72 or 96 h was very low and did not affect the rate of IL-2 transcription by Th17 cells. These results suggest that IL-2 expression is robust but transient in Th1, while Th17 continuously express IL-2 but at very low levels. It is, however, important to note that although Th1 and Th17 cells differ in the amount of IL-2 produced, they were activated to the same extent in response to anti-CD3/CD28 stimulation (Fig. 2E). Thus, the amount and kinetics of IL-2 production may be differentially regulated in Th1 and Th17 and may be of functional relevance to their development and survival.
Figure 3.
Th17 cells constitutively express IL-2 mRNA and secrete low levels of IL-2. (A) CD4+ T cells were propagated under Th1- or Th17-polarizing conditions for 4 days and then analyzed for intracellular cytokine expression. Numbers in quadrants indicate percent of T cells expressing IL-17, IFN-γ and/or IL-2. (B) RT-PCR and (C) qPCR analysis of IL-2 expression by differentiating Th1 or Th17 cells at indicated time points. (D) Cytokine secretion was quantified by Multiplex ELISA. The results are expressed as mean+SD. **p<0.01 as determined by Student’s t-test. (E) CD4+ T cells propagated under Th1 or Th17 polarizing conditions for 4 days were analyzed for cell surface expression of CD44. Numbers in quadrants indicate percent of T cells expressing CD44. The results are representative of at least three independent experiments.
Th17-DP cells have a selective growth advantage in a low IL-2 environment
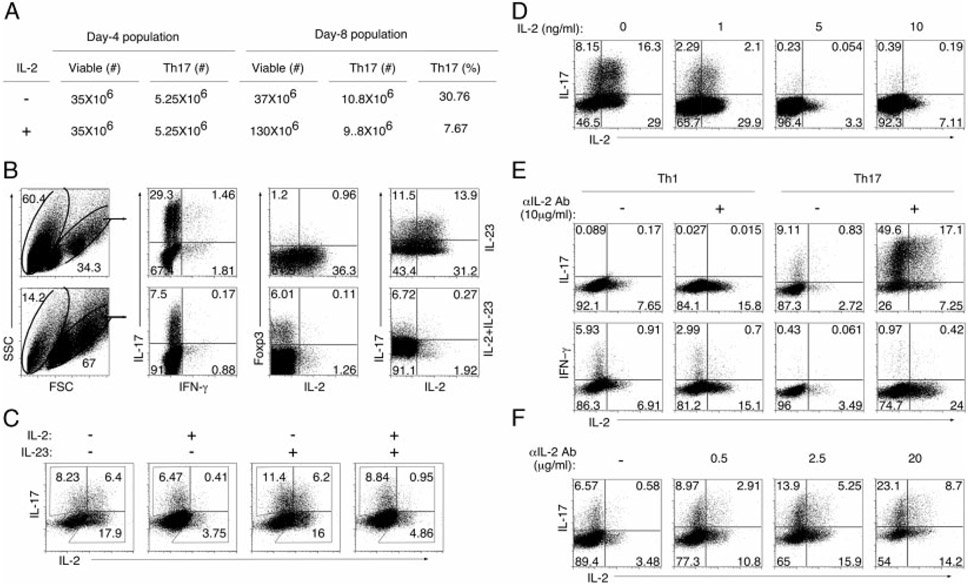
A possible explanation for the persistence of Th17-DP cells in blood and retina of mice with EAU compared with paucity of Th17-DP cells following in vitro stimulation may be related to the fact that stimulation with plate-bound anti-CD3/anti-CD28 induces strong TCR signals and the high amounts of IL-2 produced may antagonize Th17-DP expansion. Alternatively, T-cell priming during EAU is in context of APCs that produce inflammatory cytokines, such as IL-23, that may stabilize the Th17-DP phenotype in vivo. To test these possibilities, T cells from the 96 h Th17 cultures (see Fig. 3A) were propagated for four additional days in medium containing exogenous IL-23 alone or IL-23 plus IL-2. Of the 35 million T cells in the 96 h cultures, 5.25 million were Th17 and after 4 days in culture the Th17 cells increased to ~10 million, irrespective of whether the medium contained IL-2 or IL-23 (Fig. 4A). However, the total number of T cells in IL-2-containing cultures increased to 130 million. Because IL-2 induced tremendous expansion of non-Th17 T helper subsets, the Th17 percentage decreased substantially despite a net increase in absolute numbers of Th17 cells. However, substantial cell death (60.4%) occurred in cultures propagated in IL-23 alone compared with cultures supplemented with exogenous IL-2 (14.2%) (Fig. 4B). Substantial numbers of surviving T cells in IL-2-starved cultures were Th17 cells (29.3%) and their survival correlated with marked increase in Th17-DP cells (Fig. 4B). In fact, Th17-DP comprises 55% of Th17 cells in cultures without IL-2 compared with 3.9% in those with exogenous IL-2 (Fig. 4B), indicating that high amounts of IL-2 may antagonize expansion of Th17-DP cell numbers. Interestingly, the decrease in Th17-DP cell number in cultures containing exogenous IL-2 coincided with a corresponding increase in Foxp3+ T cells while an increase in Th17-DP cells in a IL-2 low environment (culture without exogenous IL-2) was associated with corresponding diminution in the number of Foxp3+ T cells (Fig. 4B), suggesting that IL-2 might promote plasticity of T-cell subsets, particularly Th17 cells [22-26].
Figure 4.
Th17-DP cells have a selective growth advantage in a low IL-2 environment. (A, B) CD4+ T cells were propagated under Th17 polarizing conditions for 4 days, washed and then expanded in medium containing IL-2 and/or IL-23 for an additional 4 days. (A) Viable cells were counted using a Cellometer® Automatic Cell Counter (Nexcelom Bioscience) and Neubauer hemocytometer. (B) Intracellular cytokine staining for detection of IFN-α, IL-17, IL-2 or Foxp3-expressing CD4+ T cells. (C) Cells propagated as in (B) were re-stimulated under Th17-polarizing conditions for 4 days, washed and expanded in IL-2 and/or IL-23 for an additional 4 days. Cells were then subjected to intracellular cytokine staining. (D) CD4+ T cells were propagated under Th17-polarization conditions for 4 days, washed and expanded for 4 days in medium containing indicated concentrations of IL-2. (E, F) T cells were activated with anti-CD3/CD28 for 4 days under Th1 or Th17 condition in medium containing varying amounts of anti-IL-2 neutralizing Abs. Numbers in quadrants indicate percent of T cells expressing IL-2, IL-17 or IFN-γ. The results are representative of more than three independent experiments.
To examine whether IL-23 is required for terminal differentiation of Th17-DP into Th17-SP cells that have lost capacity to express IL-2, we established 12 day mature Th17 cultures following two cycles of Th17 polarization. The mature Th17 cells were then propagated for 4 days in IL-23, IL-2 or IL-23+IL-2. We found that 44% of IL-17-expressing T cells retained capacity to produce IL-2 in the absence of exogenous cytokine (Fig. 4C; left most panel). Furthermore, Th17-DP cells comprised 35% of Th17 cells in cultures propagated in IL-23 (Fig. 4C), indicating that IL-23 plays a marginal role in inducing the loss of IL-2 expression by Th17-DP cells. In contrast, the percentage of T cells expressing IL-17 and IL-2 declined to 6 and 10%, respectively in medium containing IL-2 or IL-23 plus IL-2 (Fig. 4C). Interestingly, Th17-DP cells increased under IL-2-starvation conditions (Fig. 4C; left most panel), suggesting an inverse relationship between IL-2 levels and abundance of Th17-DP cells. We further show that the level of Th17-DP cells decreased in culture with increase in the concentration of exogenous IL-2 added to the culture medium (Fig. 4D). We performed reciprocal experiments to examine the effect of IL-2 depletion on Th1 or Th17 differentiation and expansion. Naïve T cells were propagated under Th1- or Th17-polarizing conditions for 4 days in the presence or absence of anti-IL-2 Abs. We observed a marked decrease in the percentage of IFN-γ-expressing T cells in cultures containing IL-2 neutralizing antibodies (Fig. 4E). In contrast, there was a dramatic increase in IL-17-expressing T cells, particularly the Th17-DP subtype (Fig. 4E), suggesting that IL-2 depletion may have differential effects on the clonal expansion of Th1 and Th17 cells. We further show that the percentage of the Th17-DP population increased markedly with increase in the concentration of anti-IL-2 Abs in the culture (Fig. 4F). Collectively, these results suggest that progressive diminution in Th17-DP levels observed in vivo during inflammatory responses [21] may be attributed to cumulative increase in IL-2 during the course of inflammation and that Th17-DP cells may have selective growth advantage in low IL-2 environment due to their capacity for sustained production of IL-2.
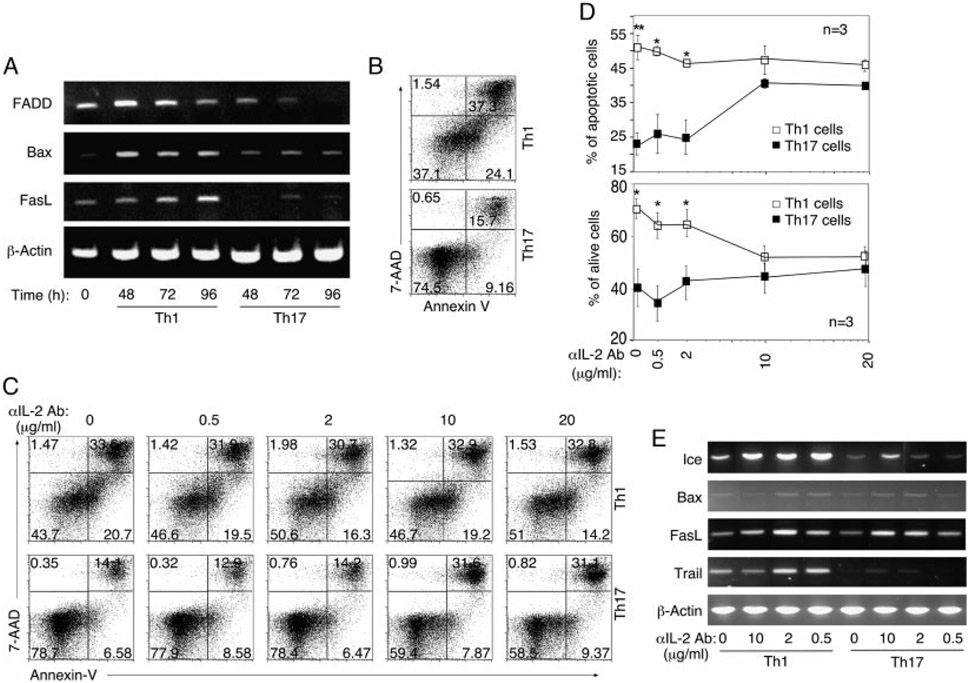
Th17 cells are more resistant to AICD
Enhanced survival of Th17-DP cells under IL-2-deprivation conditions (Fig. 4) prompted us to investigate the role of IL-2 in the survival of Th1 and Th17 cells. We stimulated naïve CD4+ T cells under Th1- or Th17-polarization conditions for 4 days and RT-PCR analysis of T cells harvested at various time points revealed enhanced expression of pro-apoptotic genes, FADD, Bax and FASL in Th1 compared with Th17 cultures (Fig. 5A). The enhanced transcription of cell death-associated genes was underscored by ~3-fold increase in apoptotic cells in Th1 cultures compared with Th17 (Fig. 5B). To further characterize the role of IL-2 in Th1 and Th17 longevity, T cells were propagated under Th1- or Th17-polarization conditions in the presence or absence of varying doses of anti-IL-2 Abs as indicated (Fig. 5C-E). Annexin-V staining assay performed after 4 days in culture showed increased cell death in Th1 compared to Th17 cells (Fig. 5B and C). Because Th1 cells produce relatively large amounts of IL-2 (see Fig. 3), the addition of anti-IL-2 Abs had marginal effects, as the level of apoptosis in Th1 cultures remained relatively constant at all anti-IL-2 concentrations (Fig. 5C; top panel). On the other hand, Th17 were relatively resistant to apoptosis at the low anti-IL-2 concentrations. However, the Th17 cells became more susceptible to apoptosis at the higher doses of IL-2 Abs, with levels of necrotic and apoptotic cell death approaching those of Th1 cells (Fig. 5C; lower panel). The effect of IL-2 on the survival of Th1 and Th17 cells is presented graphically in Fig. 5D. Similar to the results presented in Fig. 5A, expression of pro-apoptotic genes is still low in Th17 cells propagated at low anti-IL-2 Ab concentrations (Fig. 5E) and correlates with reduced cell death (Fig. 5C). Together, these results suggest that Th1 and Th17 cells differ in sensitivity to apoptosis and that ability of Th17 cells to constitutively produce IL-2 at levels below threshold for inducing AICD may confer survival advantages to Th17, vis-à-vis Th1.
Figure 5.
Th17 cells are more resistant to AICD. (A) Naïve CD4+ T cells were activated with anti-CD3/CD28 for 4 days under Th1 or Th17 conditions and the expression of pro-apoptotic genes was analyzed after 24 h, 72 h or 96 h by RT-PCR. (B) AICD was quantified after 4 days polarization by Annexin-V staining. (C) Annexin-V/7-AAD staining was performed on T cells that were activated with anti-CD3/CD28 for 4 days under Th1 or Th17 conditioning in medium containing varying concentrations of anti-IL-2 neutralizing Ab. (D) Effect of IL-2 on the survival of Th1 and Th17 is also presented graphically. (E) RT-PCR analysis of pro-apoptotic genes expressed by T cells activated with anti-CD3/CD28 for 4 days under Th1 or Th17 conditions in the absence or presence of various amounts of IL-2 Abs. The results are expressed as mean ± SD. *p<0.05 and p<0.01 as determined by Student’s t-test. The results are representative of more than three independent experiments.
Discussion
Data presented in this report provides mechanistic explanation for the high pathogenic potential of Th17 cells in autoimmune diseases. We show that Th17 cells secrete much less IL-2 compared with Th1 (Fig. 3D) and that a subset of the Th17 phenotype constitutively produces very low levels of IL-2 (Fig. 2). The level of IL-2 produced by Th17 cells was below threshold level for inducing AICD and yet was sufficient to promote homeostatic expansion of Th17 cells. We have also shown that expression of much lower levels of IL-2 during antigen priming correlated with their decreased susceptibility to AICD. Sensitivity of Th17 cells to IL-2 regulation was underscored by profound inhibition of Th17 cells by high IL-2 concentrations (Fig. 4D) and while IL-2 neutralization inhibited Th1 cells, it induced dramatic expansion of Th17 cells (Fig. 4E and F). It is, however, remarkable that Th17 expansion was triggered by IL-2-deprivation conditions (Fig. 4C and F), suggesting that low IL-2 availability in peripheral tissues and organs following Ag clearance may favor homeostatic expansion of Th17-DP cells. Thus, sustained production of low levels of IL-2 by Th17 cells may contribute to the maintenance of potentially pathogenic Th17 cells in the retina and contribute to intraocular inflammation. This is consistent with our finding of substantial numbers of Th17-DP cells persisting in blood and retina of mice with EAU (Fig. 2D) and in line with a recent study showing that during the resolution phase of acute uveitis, neither the clinical appearance nor the number of infiltrating leukocytes returned to pre-disease levels [27]. Physiological relevance of IL-2-expressing Th17 (Th17-DP) is suggested by our finding that substantial percentage of autoreactive T cells that mediate EAU was Th17-DP cells. We further show that the majority of human memory Th17 cells are Th17-DP and were inhibited by daclizumab (anti-IL-2Rα Abs), establishing for the first time potential clinical relevance of Th17-DP and suggesting that therapeutic IL-2 blockage may ameliorate Th17-mediated diseases.
Th17-DP cells were initially described in mice immunized with OVA [21]. However, it was then suggested that Th17-DP cells were an early Th17 developmental stage and that IL-23 subsequently induced its terminal differentiation into Th17-SP phenotype that no longer produced IL-2. We addressed this point in this study and found that loss of IL-2 expression by Th17-DP cells may be mediated by IL-2-dependent mechanisms (Fig. 4C). Whereas, IL-2 profoundly inhibited expansion of Th17-DP cells (Fig. 4C and D), highest amounts of Th17-DP cells were detected in Th17 cultures propagated under IL-2-starvation condition (Fig. 4C, E and F), suggesting that the capacity of Th17 cells to constitutively express IL-2 may be negatively regulated by high IL-2 levels. Thus, decrease in Th17-DP cells that had been observed at the peak of in vivo immune responses to OVA [21], may have derived, in part, from the elevated IL-2 levels and antagonism of Th17-DP expansion in the high IL-2 environment.
Antagonistic effect of IL-2 on the expansion of Th17-DP cells begs the question as to the functional relevance of constitutive IL-2 expression by the Th17-DP subset. The paradox of IL-2 inhibiting expansion of Th17-DP cells while promoting its expansion in IL-2 deprived environment, may suggest that there may be selective pressure for the expansion of Th17-DP cells during contraction phase of the immune response, a period associated with IL-2 withdrawal. In that respect, Th17-DP cells may be akin to memory T cells that typically arise coincident with Ag clearance at late stages of immune responses. Thus like memory T cells, Th17-DP cells possess the attribute of sustained production of low amounts of IL-2 that promotes survival and homeostatic expansion of memory T cells [28]. In fact, Th17-DP cells persisted in the blood and retina of mice at late stages of EAU when the level of IL-2 is at its nadir (Fig. 2D), providing suggestive evidence that sustained production of IL-2 by the Th17-DP cells, albeit at low levels, may contribute to the maintenance of potentially pathogenic Th17 cells in the immune privileged neuroretina. In this context, it is of note that the recent studies suggest that autoreactive memory CD4+ T lymphocytes induced during acute uveitis can mediate a chronic form of the disease [29]. It is also important to note that autoimmunity in Dry Eye Disease has recently been shown to due to the resistance of Th17 to Treg suppression [30]. Although the exact mechanism underlying Treg-mediated suppression of CD4+ effector T cells is unknown, it is thought to derive in part from the prodigious consumption of IL-2 by Treg cells, resulting in the depletion of IL-2 required for expansion of CD4+CD25− effector T cells. Interestingly, CD4+CD25+ Treg cells obtained from mice with EAU inhibited proliferation and the production of IFN-γ by CD4+ CD25− Th1 cells but did not affect IL-17 production by Th17 cells [31], raising the intriguing possibility that resistance of Th17 cells to inhibitory effects of Tregs may derive from the capacity of the Th17-DP to produce IL-2 in the IL-2-depleted environment of the retina. Similar to the mouse, Th17-DP cells are also present in humans. The human Th17-DP cells expressed surface markers characteristic of effector and central memory T cells (Fig. 1B) and comprised >80% of Th17 cells expanded by IL-2 in human blood (Fig. 1A). However, our finding that IL-2 induces homeostatic expansion of human Th17-DP memory T cells seems at odds with our in vitro mouse studies showing the inhibition of Th17-DP cells by IL-2. Similar apparently contradictory results have previously been reported [20] and this led to the suggestion that IL-2 may exert divergent effects on human and mouse Th17 cells [32].
In summary, data presented here suggest that low constitutive IL-2 expression confers survival advantage to Th17 cells by allowing their homeostatic expansion without inducing FasL and AICD. Persistence of Th17-DP cells in the blood and retina of mice with EAU and increase in Th17 cells in the blood of patients with uveitis suggest involvement of Th17 cells in pathophysiology of ocular inflammatory diseases. The relevance of IL-2 production by Th17 cells to Th17-mediated disease is further underscored by a recent report that MBP-specific IL-2-expressing Th17 cells provoked severe EAE compared with conventional Th17 that do not constitutively produce IL-2 [33]. In that report, it was also found that the Th17-DP cells preferentially trafficked to the CNS, while the conventional Th17 trafficked to the spleen, suggesting that Th17-DP cells express distinct patterns of chemokine receptor expression that allow them to home into CNS tissues. In humans, Th17-DP cells comprise majority of Th17 cells in PBMCs and may constitute the pool of pathogenic Th17-cells that perpetuate cycles of recurrent uveitis. Although mechanistic basis for efficacy of daclizumab therapy in uveitis is not fully understood [2], identifying Th17-DP memory T cells as important targets of daclizumab therapy, suggest that daclizumab may be useful for treating uveitis and other chronic inflammatory diseases.
Materials and methods
Normal human subjects
Blood samples were obtained after Institutional Review Board approval and consent from more than 20 normal human subjects at the NIH Department of Transfusion Medicine. PBMCs were isolated from heparin-treated whole blood by density-gradient centrifugation and CD4+ T cells were isolated using the Rosette Sep kit (Stem Cell Tech, VA, Canada). PBMCs or CD4+ cells were stimulated for 4 days with human anti-CD3 Abs (1 μg/mL), anti-CD28 Abs (3 μg/mL), human recombinant IL-2 (rIL-2), IL-6 or IL-23 at 10 ng/ml (R&D Systems Minneapolis, MN, USA).
Mice
C57BL/6 or B10A mice (6–8 wks old) were from Jackson Laboratory (Bar Harbor, ME, USA). STAT3KO mice have previously been described [19]. Animal care and use was in compliance with the NIH guidelines.
Induction of EAU
EAU was induced in C57BL/6 mice by active immunization with 150 μg bovine IRBP and human IRBP peptide (amino acid residues 1–20; 300 μg) in 0.2 mL emulsion 1:1 v/v with CFA containing Mycobacterium tuberculosis strain H37RA (2.5 mg/mL). For active immunization of B10.A mice, 50 μg bovine IRBP was used without human IRBP peptide. Mice also received Bordetella pertussis toxin (0.3 μg/mouse) concurrent with immunization. Clinical disease was established and scored by fundoscopy and histology as described previously [34]. Eyes were harvested for histological EAU evaluation 0, 14 and 21 days post-immunization, fixed in 10% buffered formalin and stained with Hematoxylin and eosin (H&E).
Analysis of CD4+ T-helper cells
We stimulated CD4+ T cells (>98%) from blood, spleen, retina or LNs as described [19]. In some experiments, cells were activated with plate-bound anti-CD3 Abs (10 μg/mL) and soluble anti-CD28 Abs (3 μg/mL) without exogenous cytokines or Abs (Th0 condition); under Th1 condition (anti-CD3/CD28 Abs, anti-IL-4 Abs (10 μg/mL) and IL-12 (10 ng/mL)) or Th17 condition (anti-CD3/CD28 Abs, IL-6 (10 ng/mL), TGF-β1 (2 ng/mL), anti-IFN-γ Abs (10 μg/mL), anti-IL-4 Abs (10 μg/mL)). For analysis of human Th17 cells, cells were cultured for 4 days in IL-2 or medium containing anti-CD3/CD28 Abs (Th0). For some cultures, exogenous cytokines (IL-23 (10 ng/mL) and IL-6 (10 ng/mL), 10 μg/mL Daclizumab) (Xenopax; Hoffmann-La Roche Basel, Switzerland) or anti-IL-2 neutralizing Abs were added. For intracellular cytokine detection, cells were re-stimulated for 5 h with PMA (20 ng/mL)/ionomycin (1 μM). Golgi-stop was added in the last hour and intracellular cytokine staining was performed using BD Biosciences Cytofix/Cytoperm kit as recommended (BD Pharmingen, San Diego, CA, USA). FACS analysis was performed on a Becton-Dickinson FACSCalibur (BD Biosciences) using monoclonal antibodies specific for CD3, CD4, CD8, CD25, CD62L, IL-17, CCR7, CD45RA, CD45RO, IFN-γ and the corresponding isotype control Abs (PharMingen, San Diego, CA, USA) as previously described [15]. Four-color FACS analysis was performed on samples stained with mAbs conjugated with fluorescent dyes with distinct fluorescence emission peaks and each experiment was color compensated. Samples were subjected to side-scatter & forward-scatter analysis and quadrant gates were set using isotype controls with <0.2% background.
Cytokine analysis
Mouse or human T cells were cultured for 4 days in IL-2 or medium containing anti-CD3/CD28 Abs under Th0-, Th1- or Th17-polarization condition. For some cultures, exogenous cytokines (IL-23, IL-6) were added. Multiplex ELISA of supernatants for IL-17, IFN-γ or IL-2 secretion was performed by Pierce Service Laboratory (Pierce Woburn, MA, USA).
Lymphocyte proliferation assay
Human T cells were cultured for 4 days with anti-CD3/CD28 and/or cytokine as indicated. CFSE dilution assay was performed using a commercially available CFSE Cell Proliferation Kit (Molecular Probes, Eugene, OR, USA). Establishment of cell cycling parameters and calculation of the number of cell divisions were as previously described [35].
Reverse transcription (RT) PCR and quantitative RT-PCR analysis
All RNA samples were DNA free. cDNA synthesis, RT-PCR and qPCR analyses were performed as described [15]. Each gene-specific primer pair used for RT-PCR analysis spans at least an intron and primers and probes used for RT-PCR or qPCR were purchased from Applied Biosystems. mRNA expression was normalized to the levels of β-Actin and GAPDH genes.
Apoptosis analysis
The activated Th1 and Th17 cells were harvested and the apoptotic cells were detected by FACS analysis by using PE-Annexin V apoptosis detection kit I (BD Bioscience).
Statistical analysis
Statistical analysis was performed by Student’s t-test. The results were expressed in mean ± SD. The number of samples analyzed is indicated as n in the figure. Asterisks denote p-value (*p ≤ 0.05 or **p ≤ 0.01) as indicated in the figures.
Acknowledgements:
The National Eye Institute and NIH Intramural Research Programs funded this research. Authors thank Dr. Igal Gery for critical reading of manuscript and Rafael Villasmil for technical assistance with FACS analysis (National Eye Institute, NIH).
Abbreviations:
- AICD
activation-induced cell death
- EAU
experimental autoimmune uveitis
- IRBP
interphotoreceptor retinoid-binding protein
- STAT3KO
mice conditionally deficient in STAT3 in the CD4+ T-cell compartment
- Th17-DPcells
Th17 cells constitutively expressing IL-2
- VKH
Vogt–Koyanagi–Harada
Footnotes
Conflict of interest: The authors have declared no financial or commercial conflict of interest.
References
- 1.Nussenblatt RB, Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int. Rev. Immunol 2002. 21: 273–289. [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB, Fortin E, Schiffman R, Rizzo L, Smith J, Van Veldhuisen P, Sran, et al. , Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc. Natl. Acad. Sci. USA 1999. 96: 7462–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho JH, The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol 2008. 8: 458–466. [DOI] [PubMed] [Google Scholar]
- 4.Nakae S, Saijo S, Horai R, Sudo K, Mori S and Iwakura Y, IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 2003. 100: 5986–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T et al. , IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med 2005. 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, Huang X and Kijlstra A, Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest. Ophthalmol. Vis. Sci 2008. 49: 3058–3064. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Park JA, Lee EY, Lee YJ, Song YW and Lee EB, Imbalance of Th17 to Th1 cells in Behcet’s disease. Clin. Exp. Rheumatol 2010. 28: S16–S19. [PubMed] [Google Scholar]
- 8.Jiang S, Liu X, Luo L, Qu B, Huang X, Lin Y, Ye S and Liu Y, Serum levels of Th17-related cytokines in Behcet disease patients after cataract surgery. Mol. Vis 2011. 17: 1425–1430. [PMC free article] [PubMed] [Google Scholar]
- 9.Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, Chen L et al. , IL-23 promotes CD4+ T cells to produce IL-17 in Vogt–Koyanagi–Harada disease. J. Allergy Clin. Immunol 2007. 119: 1218–1224. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S, Liu X, Luo L, Qu B, Huang X, Xu L, Lin Y et al. , Elevated serum IL-23 correlates with intraocular inflammation after cataract surgery in patients with Vogt–Koyanagi–Harada disease. Br. J. Ophthalmol 2010. 94: 1078–1082. [DOI] [PubMed] [Google Scholar]
- 11.Chi W, Yang P, Zhu X, Wang Y, Chen L, Huang X and Liu X, Production of interleukin-17 in Behcet’s disease is inhibited by cyclosporin A. Mol. Vis 2010. 16: 880–886. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang K, Wen J, Liu X, Kijlstra A, Chen L, Chi W, Zhou H et al. , Inhibitory effect of rapamycin and dexamethasone on production of IL-17 and IFN-gamma in Vogt–Koyanagi–Harada patients. Br. J. Ophthalmol 2009. 93: 249–253. [DOI] [PubMed] [Google Scholar]
- 13.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z and Nussenblatt RB, A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol 1988. 140: 1490–1495. [PubMed] [Google Scholar]
- 14.Nussenblatt RB, Experimental autoimmune uveitis: mechanisms of disease and clinical therapeutic indications. Invest. Ophthalmol. Vis. Sci 1991. 32: 3131–3141. [PubMed] [Google Scholar]
- 15.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I et al. , T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med 2007. 13: 711–718. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Qian J, Guo J, Yuan YF and Xue K, Suppression of experimental autoimmune uveoretinitis by Anti-IL-17 antibody. Curr. Eye Res 2009. 34: 297–303. [DOI] [PubMed] [Google Scholar]
- 17.Dong C, TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol 2008. 8: 337–348. [DOI] [PubMed] [Google Scholar]
- 18.Korn T, Bettelli E, Oukka M and Kuchroo VK, IL-17 and Th17 Cells. Annu. Rev. Immunol 2009. 27: 485–517. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Lee YS, Yu CR and Egwuagu CE, Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol 2008. 180: 6070–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB et al. , Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007. 26: 371–381. [DOI] [PubMed] [Google Scholar]
- 21.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK et al. , The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol 2009. 10: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B et al. , Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008. 29: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF and Gery I, Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J. Immunol 2008. 181: 7205–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowell E and Wilson CB, Programming perpetual T helper cell plasticity. Immunity 2009. 30: 7–9. [DOI] [PubMed] [Google Scholar]
- 25.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO and Weaver CT, Late developmental plasticity in the T helper 17 lineage. Immunity 2009. 30: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Chong MM and Littman DR, Plasticity of CD4+ T cell lineage differentiation. Immunity 2009. 30: 646–655. [DOI] [PubMed] [Google Scholar]
- 27.Copland DA, Wertheim MS, Armitage WJ, Nicholson LB, Raveney BJ and Dick AD, The clinical time-course of experimental autoimmune uveoretinitis using topical endoscopic fundal imaging with histologic and cellular infiltrate correlation. Invest. Ophthalmol. Vis. Sci 2008. 49: 5458–5465. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Geginat J and Lanzavecchia A, Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol 2004. 22: 745–763. [DOI] [PubMed] [Google Scholar]
- 29.Oh HM, Yu CR, Lee Y, Chan CC, Maminishkis A and Egwuagu CE, Autoreactive memory CD4+ T lymphocytes that mediate chronic uveitis reside in the bone marrow through STAT3-dependent mechanisms. J. Immunol 2011. 187: 3338–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR and Dana R, Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol 2009. 182: 1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M, Yang P, Du L, Zhou H, Ren X and Kijlstra A, Contribution of CD4+CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Invest. Ophthalmol. Vis. Sci 2010. 51: 383–389. [DOI] [PubMed] [Google Scholar]
- 32.Laurence A and O’Shea JJ, T(H)-17 differentiation: of mice and men. Nat. Immunol 2007. 8: 903–905. [DOI] [PubMed] [Google Scholar]
- 33.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL et al. , Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 2010. 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takase H, Yu CR, Liu X, Fujimoto C, Gery I and Egwuagu CE, Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J. Neuroimmunol 2005. 168: 118–127. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF and Hodgkin PD, Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat. Protoc 2007. 2: 2057–2067. [DOI] [PubMed] [Google Scholar]