Abstract
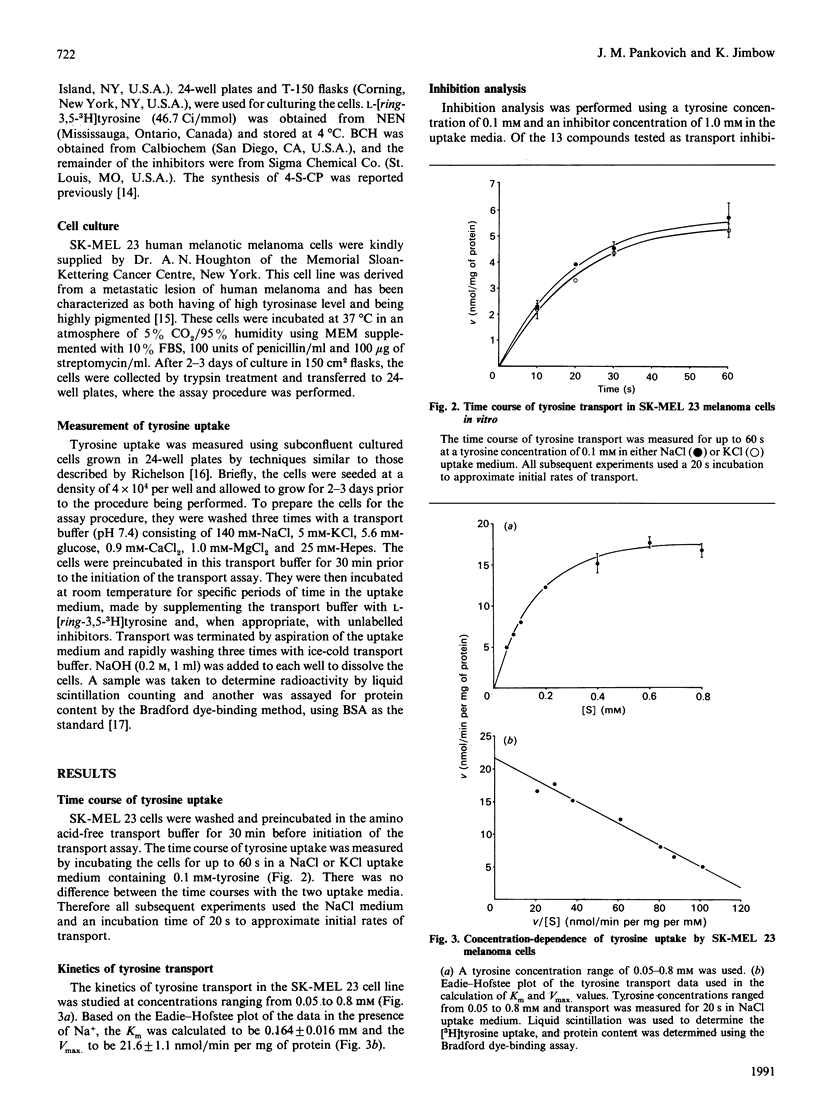
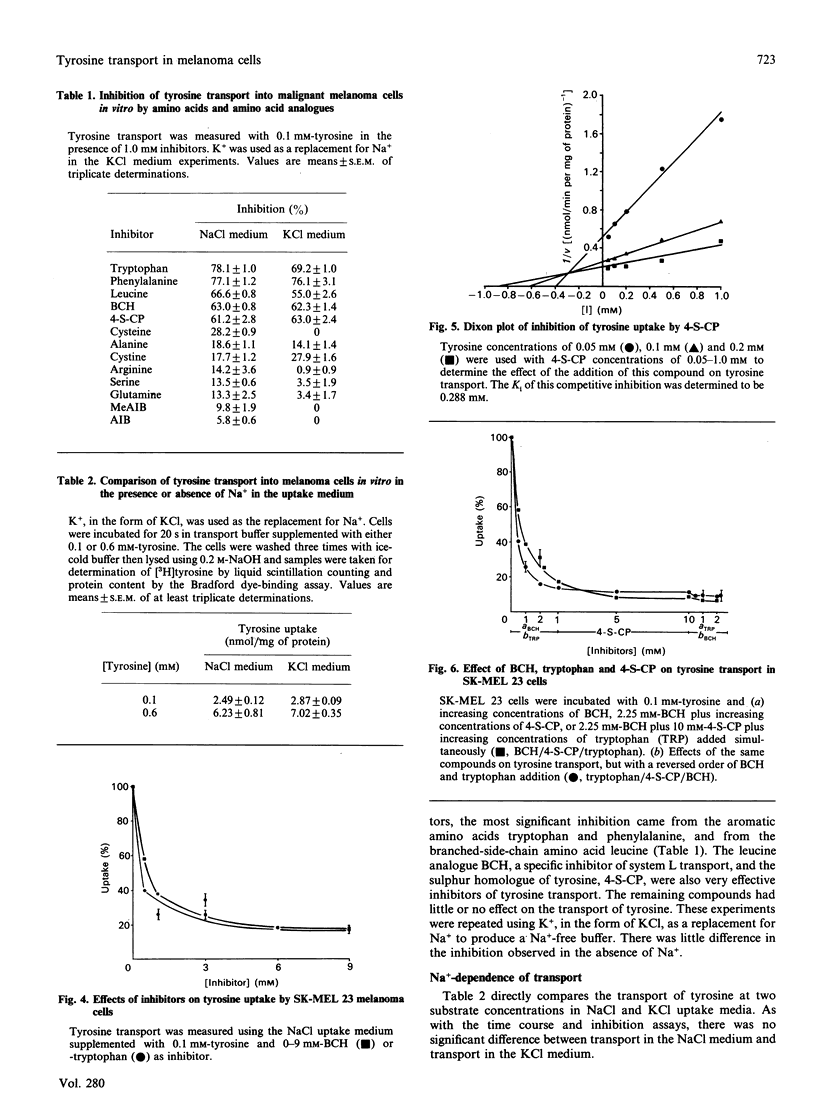
Tyrosine is an essential amino acid for the initial step of melanin synthesis, yet little is known concerning its transport in melanocytes. As an important first step in the development of new anti-melanoma agents based upon chemical and pharmacological modifications of melanin synthesis, the present study characterized the transport mechanism of tyrosine in vitro using the human melanoma cell line SK-MEL 23. Several tyrosine transport systems may be involved in melanocytes: systems L and T, which transport neutral amino acids with branched or aromatic side chains, and systems A and ASC, which transport neutral amino acids with smaller side chains. In order to determine which system or combination of systems is involved in tyrosine transport in melanoma cells, studies of kinetics, Na(+)-dependence and competitive inhibition were undertaken. The Km and Vmax. for the Na(+)-independent transport system were found to be 0.164 +/- 0.016 mM and 21.6 +/- 1.1 nmol/min per mg of protein respectively. This transport was preferentially inhibited by the system L specific analogue, 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid, the system T substrate tryptophan, and the sulphur homologue of tyrosine, 4-S-cysteinylphenol. Sequential addition of these inhibitors at increasing concentrations indicated that they inhibit the same transporter. Our results suggest that tyrosine transport in SK-MEL 23 melanoma cells is similar to system L transport previously characterized in other cell types. This one transport system appears to supply all the tyrosine required for both cell growth and melanin synthesis. The transport system may be subject to manipulation by melanogenic stimulating factors, making the transport of cytotoxic tyrosine analogues an important area for further study.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alena F., Jimbow K., Ito S. Melanocytotoxicity and antimelanoma effects of phenolic amine compounds in mice in vivo. Cancer Res. 1990 Jun 15;50(12):3743–3747. [PubMed] [Google Scholar]
- Begleiter A., Lam H. Y., Grover J., Froese E., Goldenberg G. J. Evidence for active transport of melphalan by two amino acid carriers in L5178Y lymphoblasts in vitro. Cancer Res. 1979 Feb;39(2 Pt 1):353–359. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. On the strategy of kinetic discrimination of amino acid transport systems. J Membr Biol. 1985;84(2):97–103. doi: 10.1007/BF01872207. [DOI] [PubMed] [Google Scholar]
- Dufour M., Panasci L. C., St Germain J., Boulet L. Effects of amino acids on the transport and cytotoxicity of melphalan by human bone marrow cells and human tumor cells. Cancer Chemother Pharmacol. 1985;15(2):125–131. doi: 10.1007/BF00257522. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Tiffany S. M., Vogel F. S. The toxicity of melanin precursors. J Invest Dermatol. 1978 Feb;70(2):113–116. doi: 10.1111/1523-1747.ep12541249. [DOI] [PubMed] [Google Scholar]
- Hoare D. G. The transport of L-leucine in human erythrocytes: a new kinetic analysis. J Physiol. 1972 Mar;221(2):311–329. doi: 10.1113/jphysiol.1972.sp009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N., Real F. X., Davis L. J., Cordon-Cardo C., Old L. J. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med. 1987 Mar 1;165(3):812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Kato T., Ishikawa K., Kasuga T., Jimbow K. Mechanism of selective toxicity of 4-S-cysteinylphenol and 4-S-cysteaminylphenol to melanocytes. Biochem Pharmacol. 1987 Jun 15;36(12):2007–2011. doi: 10.1016/0006-2952(87)90501-6. [DOI] [PubMed] [Google Scholar]
- Ito Y., Jimbow K. Selective cytotoxicity of 4-S-cysteaminylphenol on follicular melanocytes of the black mouse: rational basis for its application to melanoma chemotherapy. Cancer Res. 1987 Jun 15;47(12):3278–3284. [PubMed] [Google Scholar]
- Jara J. R., Martinez-Liarte J. H., Solano F., Peñafiel R. Transport of L-tyrosine by B16/F10 melanoma cells: the effect of the intracellular content of other amino acids. J Cell Sci. 1990 Nov;97(Pt 3):479–485. doi: 10.1242/jcs.97.3.479. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Miura T., Ito S., Ishikawa K. Phenolic melanin precursors provide a rational approach to the design of antitumor agents for melanoma. Pigment Cell Res. 1989 Jan-Feb;2(1):34–39. doi: 10.1111/j.1600-0749.1989.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Miura S., Ueda T., Jimbow K., Ito S., Fujita K. Synthesis of cysteinylphenol, cysteaminylphenol, and related compounds, and in vivo evaluation of antimelanoma effect. Arch Dermatol Res. 1987;279(4):219–225. doi: 10.1007/BF00417318. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Lee M., Moore P. A., Cecchini G. Neutral amino acid transport systems of tissue culture cells. J Biol Chem. 1977 Apr 25;252(8):2675–2679. [PubMed] [Google Scholar]
- Pawelek J. M. Factors regulating growth and pigmentation of melanoma cells. J Invest Dermatol. 1976 Apr;66(4):201–209. doi: 10.1111/1523-1747.ep12482134. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M., Lerner A. B. 5,6-Dihydroxyindole is a melanin precursor showing potent cytotoxicity. Nature. 1978 Dec 7;276(5688):626–628. doi: 10.1038/276627a0. [DOI] [PubMed] [Google Scholar]
- Richelson E. A microwell assay method for the biochemical study of cultured cells. Anal Biochem. 1973 Apr;52(2):563–573. doi: 10.1016/0003-2697(73)90062-6. [DOI] [PubMed] [Google Scholar]
- Richelson E. Studies on the transport of L-tyrosine into an adrenergic clone of mouse neuroblastoma. J Biol Chem. 1974 Oct 10;249(19):6218–6224. [PubMed] [Google Scholar]
- Rosenberg R., Young J. D., Ellory J. C. L-Tryptophan transport in human red blood cells. Biochim Biophys Acta. 1980 May 23;598(2):375–384. doi: 10.1016/0005-2736(80)90015-2. [DOI] [PubMed] [Google Scholar]
- Saga K., Shimojo T. Studies on the transport of tyrosine, leucine, and methionine in cultured B-16 mouse melanoma cells. J Biochem. 1982 Aug;92(2):343–355. doi: 10.1093/oxfordjournals.jbchem.a133940. [DOI] [PubMed] [Google Scholar]
- Slominski A. L-tyrosine induces synthesis of melanogenesis related proteins. Life Sci. 1989;45(19):1799–1803. doi: 10.1016/0024-3205(89)90520-1. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R. Are L-tyrosine and L-dopa hormone-like bioregulators? J Theor Biol. 1990 Mar 8;143(1):123–138. doi: 10.1016/s0022-5193(05)80292-9. [DOI] [PubMed] [Google Scholar]
- Souhami R., Peters W. High dose chemotherapy in solid tumours in adults. Clin Haematol. 1986 Feb;15(1):219–234. doi: 10.1016/s0308-2261(86)80013-3. [DOI] [PubMed] [Google Scholar]
- Słominski A., Moellmann G., Kuklinska E., Bomirski A., Pawelek J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J Cell Sci. 1988 Mar;89(Pt 3):287–296. doi: 10.1242/jcs.89.3.287. [DOI] [PubMed] [Google Scholar]
- Thody A. J., Oliver I., Sherbet G. V. Cytotoxicity of 4-hydroxyanisole and tyrosinase activity in variant cell lines of B16 melanoma. Eur J Cancer Clin Oncol. 1988 Dec;24(12):1879–1884. doi: 10.1016/0277-5379(88)90101-0. [DOI] [PubMed] [Google Scholar]
- Vadgama J. V., Christensen H. N. Discrimination of Na+-independent transport systems L, T, and asc in erythrocytes. Na+ independence of the latter a consequence of cell maturation? J Biol Chem. 1985 Mar 10;260(5):2912–2921. [PubMed] [Google Scholar]
- Van Winkle L. J., Christensen H. N., Campione A. L. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem. 1985 Oct 5;260(22):12118–12123. [PubMed] [Google Scholar]
- Vistica D. T. Cytotoxicity as an indicator for transport mechanism: evidence that melphalan is transported by two leucine-preferring carrier systems in the L1210 murine leukemia cell. Biochim Biophys Acta. 1979 Jan 19;550(2):309–317. doi: 10.1016/0005-2736(79)90217-7. [DOI] [PubMed] [Google Scholar]
- Wick M. M. An experimental approach to the chemotherapy of melanoma. J Invest Dermatol. 1980 Feb;74(2):63–65. doi: 10.1111/1523-1747.ep12519812. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jones S. E., Ellory J. C. Amino acid transport in human and in sheep erythrocytes. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):355–375. doi: 10.1098/rspb.1980.0100. [DOI] [PubMed] [Google Scholar]
- Young J. D., Wolowyk M. W., Jones S. M., Ellory J. C. Red-cell amino acid transport. Evidence for the presence of system ASC in mature human red blood cells. Biochem J. 1983 Nov 15;216(2):349–357. doi: 10.1042/bj2160349. [DOI] [PMC free article] [PubMed] [Google Scholar]


