Abstract
Abnormal intraglandular stromal-epithelial interactions have been known as a main key contributing factor for development of Benign Prostatic Hyperplasia (BPH). However, the underlying mechanism for the dysregulated intercellular communication remains unclear. In this study we compared the proteomic profiles of hyperplastic tissue with adjacent normal tissue of BPH and identified Rab27B small GTPase, a key regulator of exocytosis, as a protein that was overexpressed in the epithelium of BPH tissue. Overexpression of Rab27B in prostatic epithelial cells strongly increased the signaling activities of the PI3K/AKT and ERK1/2 pathways, whereas, downregulation of Rab27B expression in the epithelial cells of BPH reduced the signaling activities and decreased cell proliferation. The elevated Rab27B expression caused an overall increase in cell surface presentation of growth factor receptors without affecting their expression. However, the small GTPase also possesses an inhibitory activity against mTORC1 independent of its role in cell surface presentation of growth factor receptors. Our findings demonstrate a pivotal role of the small GTPase in autocrine and paracrine signaling and suggest that its abnormal expression underlies the dysregulated stromal-epithelial interactions in BPH.
Keywords: Rab27B, Benign Prostatic Hyperplasia, membrane receptors, AKT, ERK1/2, mTORC1
1. Introduction
Benign prostatic hyperplasia (BPH) is one of the most common disease conditions in elderly men, affecting ~42% of men in their 50s and >80% in their 80s. The disease condition is characterized by development of hyperplastic nodules composed of proliferative prostatic stromal and epithelial cells in the transitional zone of the prostate, leading to bladder outlet obstruction and consequently, deterioration in urinary function and quality of life (Chughtai et al., 2016).
The homeostasis of the prostate depends on mutual interactions between prostatic epithelial and stromal cells, which are mediated by paracrine signaling between both types of cells (Timms and Hofkamp, 2011). In aged men changes in intraprostatic hormone levels alter expression of various growth factors, cytokines and their receptors, resulting in perturbation of paracrine signaling (Banerjee et al., 2018; Ho and Habib, 2011). During prostate inflammation, the intraglandular T-lymphocytes and macrophages further alter the paracrine signaling through releasing a large amount of cytokines (De Nunzio et al., 2016). These altered intraprostatic epithelial-stromal interactions, as a consequence of hormone changes and inflammation, are believed to be a major driving factor for development of BPH (La Vignera et al., 2016).
Paracrine signaling requires secretion of signaling molecules, such as growth factors and cytokines, into the extracellular space. It also depends on surface presentation of receptors for various signaling molecules secreted by other cells. Both processes are mediated by exocytosis, in which membrane bound vesicles are generated and delivered to the cell surface (Jahn and Sudhof, 1999). The fusion of the vesicles with the plasma membrane results in the release of the soluble contents of the vesicles into the extracellular space and insertion of vesicular membrane proteins into the plasma membrane. The levels of the secretion and membrane presentation are thus a function of exocytosis activity. Exocytosis is a complex process that involves vesicle budding, trafficking, docking and fusion with their target membrane compartments (Rothman, 1994). The process is regulated at virtually every step by small GTPases of the Rab family that function as molecule switches to direct membrane-bounded vesicles to their destinations (Fukuda, 2008; Stenmark, 2009). The late stages of exocytosis that involve transport and docking of vesicles to the plasma membrane are regulated by two members of the Rab GTPase family, Rab27A and Rab27B (Fukuda, 2013). The two small GTPases are highly similar in their amino acid sequence and play redundant roles in controlling vesicular transport and docking in constitutive and regulated secretion. However, they also have distinct cellular distribution in different types of cells and function at different steps of the vesicular trafficking in the same type of cells (Chen et al., 2003; Dong et al., 2012; Kimura and Niki, 2011). The small GTPase also regulates the fusion of multivesicular bodies (MVBs) of the endocytic pathway with the plasma membrane (Ostrowski et al., 2010), hence playing a role in exosome release.
In an attempt to understand the molecular basis underlying the pathogenesis of BPH, we isolated epithelial tissues from BPH patients using a laser-captured approach and compared the proteomic profiles of BPH tissues with adjacent normal prostatic tissues by mass spectrometry (O’Malley et al., 2014). The analysis identified Rab27B as a protein that was overexpressed in the epithelia of BPH tissues. To determine how this abnormal expression of a key factor in exocytosis contributes to BPH development, we examined its effects on autocrine and paracrine signaling in prostatic epithelial cells.
2. Materials and Methods
2.1. Cell lines and antibodies
HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS). BPH-1 cells were cultured in RPMI-1640 supplemented with 10% and 100U ml−1 penicillin/streptomycin. BHPrE-1 cells were cultured in DMEM:F12 in 1:1 ratio supplemented with 0.4% bovine pituitary extract, insulin-transferrin-selenium mix, epidermal growth factor (10 ng/mL), antimycotic mix and 5% FBS. Prostatic epithelial RWPE-1 cells (cat# CRL-11609) were obtained from ATCC (Manassas, VA) and cultured in Keratinocyte Serum Free Medium supplemented with 0.05 mg/ml BPE and 5 ng/ml EGF. Prostatic stromal WPMY-1 cells were obtained from ATCC (CRL-2854) and cultured in DMEM medium supplemented with 5% FBS. The following antibodies were purchased from Cell Signaling Technology (Beverly, MA): 4E-BP1 (#9452), AKT (Cat# 9272), β-actin (#4970), EGFR (#4267), ERK1/2 (#9102), GAPDH (#5174), IGF-1Rβ (#3027), NaK-ATPase (#3010), Rab27A (#69295), S6 (#2217), TGF-βR2 (#79424), phospho-4EBP1 at T37/46 (#2855), phospho-AKT at T308 (# 4056) and S473 (# 4051), phospho-ERK1/2 at T202/Y204 (#9101) and phospho-S6 at S235/236 (# 4958). Antibody for Rab27B (Cat# SAB2107195) was from Sigma-Aldrich (St. Louis, MO). Alexa-488 Fluor labeled phalloidin (#A12379) was from Fisher Scientific (Pittsburgh, PA).
2.2. Immunohistochemistry of BPH specimens
Formalin-fixed paraffin embedded sections from a cohort of ten archival BPH specimens from patients naïve to androgen manipulation were obtained from the Pitt Biospecimen Core at the University of Pittsburgh Medical Center. The BPH specimens were from patients over 60 years of age with clinical symptoms of BPH and who also underwent prostatectomy because of BPH and were collected under an approved University of Pittsburgh Institutional Review Board protocol. No incidental foci of carcinoma were present in this cohort. Samples were sectioned at 5 μm, and immunostaining was performed with primary Rab27B antibody using the ImmunoCruz rabbit ABC staining System (Santa Cruz Biotechnology, Santa Cruz, CA) followed by Vector NovaRED substrate (Vector Laboratories, Burlingame, CA). Slides were then counterstained in hematoxylin and cover-slipped. Immunostained sections were imaged with a Leica DM LB microscope (Leica Microsystems Inc, Bannockburn, IL) equipped with an Imaging Source NII 770 camera (The Imaging Source Europe GmbH, Bremen, Germany) and NIS-Elements Documentation v 4.6 software (Nikon Instruments, Inc., Mellville, NY).
Immunostained slides were evaluated quantitatively using the H-Score method by assessing protein expression as a function of staining intensity, where no staining=0, weak=1, moderate=2, strong=3, times the percentage of cells exhibiting each level of intensity as previously (Jain et al., 2013). A minimum of 5 fields from each section was analyzed and an average score for each tissue type was calculated for each patient. All scores were reviewed and confirmed by a board-certified genitourinary pathologist (R.D.).
2.3. GEO data analysis of Rab27B expression
Four datasets of gene expression profiling (GSE5377, GSE104749, GSE65343, GSE38241) from Gene-Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/), which includes 17 BPH and 21 normal tissue samples, were analyzed for Rab27 expression. Data quantile normalization was carried out by median polish algorithm and missing value imputation by k-nearest neighbor (KNN) algorithm. Batch effects in the expression data were adjusted using empirical Bayes methods according to previous studies (Johnson et al., 2007). Violin plots were generated using the R (version 4.0.0) program.
2.4. Plasmid construction
GFP-tagged Rab27A (#89237) and Rab27B (#89447) plasmids were obtained from Addgene (Watertown, MA). The Rab27A and Rab27B ORFs in the plasmids were amplified by PCR and cloned into pLenti6 vector to create plasmids expressing an untagged version of the genes. The cloned genes were verified by DNA sequencing. For expression Rab27A and Rab27B in HEK293 cells, pLenti6-Rab27A and pLenti6-Rab27B plasmids were transfected using lipofectamine 2000 (#11668019) from Fisher Scientific (Pittsburgh, PA). For expression of Rab27B in RWPE-1 cells, the Lenti6-Rab27B plasmid was packaged with the Lenti-X™ Packaging Single Shots kit (#631276) from Takara Bio and the resulting viral particles were used for infection of RWPE-1 cells. Plasmids expressing Rab27B specific shRNAs TRCN0000286658 (shRNA658), TRCN0000047691(shRNA691), TRCN0000293978 (shRNA978) and TRCN0000380112(shRNA112) were purchased from Sigma-Aldrich (St. Louis, MO).
2.5. Isolation of plasma membrane proteins
For analyzing the effect of Rab27B overexpression on cell surface presentation of membrane proteins, cultured cells at ~70% confluence were transfected with empty vector or plasmids expressing Rab27B or Rab27A (for HEK293 cells) or infected with lentiviral particles expressing control or Rab27B (for RWPE-1 cells). The cells were harvested two days after the transfection/infection. For analyzing the effect of Rab27B downregulation, BPH-1 cells stably expressing Rab27B specific shRNAs were grown to 90% confluence and harvested. Plasma membrane proteins were isolated from the harvested cells using the Qproteome Plasma Membrane Protein kit (#37601) from Qiagen (Germantown, MD) or the Plasma Membrane Protein Extraction Kit #ab65400) from Abcam (Branford, CT) following the instructions provided by the manufacturers. The two different kits used different concepts for plasma membrane protein isolation but yielded comparable results. The results from the Qiagen kit were shown.
2.6. Confocal microscopy
Cultured cells were fixed with 4% paraformaldehyde, permeabilized and stained with primary and fluorophore-conjugated secondary antibodies as described previously (Zhong et al., 2016). Anti-IGF-1Rβ antibody (1:400 dilution), Alexa fluor-488 phalloidin (1:400 dilution) and DAPI (0.1mg/ml) were used to stain IGF-1Rβ, actin fibers and nuclei, respectively. Fluorescent images of the stained cells were acquired using Olympus BX61WI Fluoview FV1000 confocal microscope (Olympus, Center Valley, PA) with an Olympus PlanAbo 60/1.45 oil objective. Images were processed using Olympus FV10-ASW software (ver 4.2) and Adobe Photoshop (CS5.1).
2.7. Measurement of IGF-1 level and cell proliferation in conditioned media
Conditioned media were made by culturing cells at ~80% confluence in serum-free media (DMEM for BPH-1 cells and base Keratinocyte Serum Free Medium for RWPE-1 cells) for 24 hr. The conditioned media were removed and cleared of cells by centrifugation. IGF-1 levels in the conditioned media are measured using human IGF-1 ELISA kit (#ELH-IGF1-1) from Raybiotech (Peachtree Corners, GA) following manufacturer’s instruction. To measure the growth of WPMY-1 cells in the conditioned media, 1.25 ×105 cells were seeded in each well of a 24-well plate and grown for 24 hr in 0.5 ml of DMEM supplemented with 5% FBS. The cells were rinsed 2x with serum-free DMEM medium followed by incubation with 0.5ml of the conditioned media. At indicated time points, two duplicated wells of the cells form each condition media were trypsinized, stained with trypan blue, and counted for viable cells using a hemocytometer.
2.8. Statistical analyses
Statistical analysis was performed using GraphPad Prism 8.0 software. Differences between two independent sample groups were analyzed with t-test. A value of P<0.05 is considered as statistically significant.
3. Results
3.1. Rab27B is overexpressed in epithelia of BPH
In an attempt to understand the molecular mechanisms underlying the pathophysiology of BPH, we isolated hyperplastic and adjacent normal tissues of BPH using laser capture approach and compared the protein expression profile of the two samples using mass spectrometry (O’Malley et al., 2014). This analysis identified 15 proteins that were overexpressed more than 3-fold in the epithelia of BPH in comparison with the normal tissues. All the proteins but one fall into three functional groups, including amino acid metabolism, translation, and cytoskeleton. Their overexpression reflects altered cell metabolism and cytoskeletal organization in the epithelial cells of BPH, which was not further pursued. The only exception is Rab27B, a small GTPase of the Rab family that is involved in exocytosis. This small GTPase was overexpressed more than 3.5 fold in the epithelia of the hyperplastic tissues than in normal tissues (data not shown).
To confirm the result from the proteomic analysis, we examined Rab27B expression in a separate cohort of paraffin-embedded tissue specimens from patients who had undergone prostatectomy for BPH using immunohistochemistry. A significantly increased expression of Rab27B was observed in the epithelium (p=0.0001) of BPH tissues (n=10) in comparison with adjacent normal tissues (n=9) (Fig. 1A and 1B). In contrast, no significant difference (p=0.325) in the expression was seen in the stromal regions between BPH and adjacent normal tissues (Fig. 1A and 1B). Similarly, a search of Gene-Expression Omnibus (GEO) database revealed a significant (p<0.001) overexpression of Rab27B in prostate tissues from patients with BPH in comparison with those from healthy controls (Fig. 1C). In cells of two prostatic epithelial cell lines, BPH-1 and BHPrE-1, that were respectively derived from prostatic tissues of BPH patients and a BPH patient and a benign human prostate surgical sample (Hayward et al., 1995; Jiang et al., 2010), we also observed a significantly higher expression of Rab27B than that of RWPE-1 prostatic epithelial cells (Fig. 1D), which were derived from a healthy human prostate (Bello et al., 1997). These results confirm the abnormal expression of Rab27B in the epithelia of BPH.
Figure 1. Rab27B overexpression in epithelia of BPH.
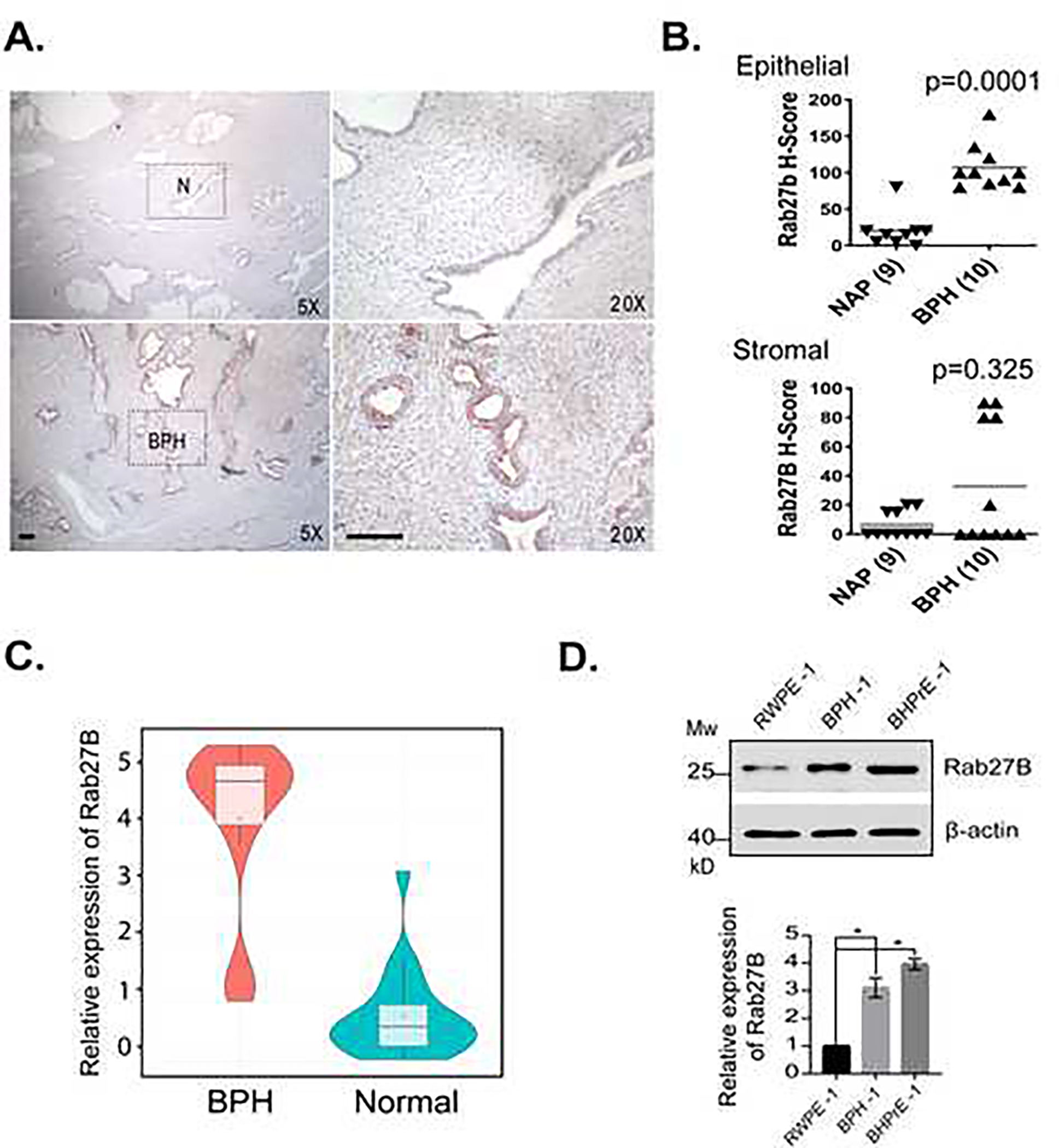
A. Immunohistochemistry (IHC) images showing the expression of Rab27B in BPH and adjacent normal (N) prostatic tissues. Scale bar = 100 μM. B. Quantitative presentations of the Rab27B expression levels in BPH and normal prostatic tissues (NAP) analyzed by IHC. C. Violin plot showing the centrality analysis of the expression of Rab27B in BPH and healthy (normal) prostates. D. Western blots and quantitative presentations showing the expression levels of Rab27B in prostatic epithelial cells derived from normal prostate (RWPE-1), BPH tissues (BPH-1) and benign prostate tissues (BHPrE-1). Quantitative presentation data are from three independent experiments and expressed as mean ± SD. * P < 0.01.
3.2. Overexpression of Rab27B stimulates growth factor signaling
Given the key role of Rab27B in controlling exocytosis, its overexpression is expected to have a profound impact on cell surface presentation of various growth factor receptors and secretion of signaling molecules, and consequently, on cell autocrine and paracrine signaling. To test the notion, we first examined the effects of Rab27B overexpression on cell signaling in HEK293 cells. We found that the overexpression increased activation-dependent phosphorylation of AKT at T308 and S473, and that of S6 at 235/236 (Fig. 2A and 2B), indicating an enhanced activation of the PI3K/AKT/mTORC1 pathway. The overexpression also activated the MAPK pathway, as manifested by an elevated level of phosphorylation of ERK1/2 (Fig. 2A and 2B). These observations suggest that Rab27B overexpression is able to augment growth factor signaling activities. Overexpression of Rab27A, the homologue of Rab27B, produced similar effects on AKT and MAPK phosphorylation (Fig. 2A and 2B). Interestingly, despite an increase in AKT phosphorylation, the mTORC1-dependent phosphorylation of S6 was not affected by Rab27A overexpression (Fig. 2A and 2B), suggesting that Rab27A may have a role in mTORC1 regulation independent of AKT. When Rab27B was overexpressed in prostatic epithelial RWPE-1 cells, similar activation effects were observed on the PI3K/AKT/mTORC1 and MAPK pathways (Fig. 2C and 2D). Collectively, these findings demonstrate that overproduced Rab27B is able to increase growth factor signaling.
Figure 2. Rab27B overexpression stimulates growth factor signaling.
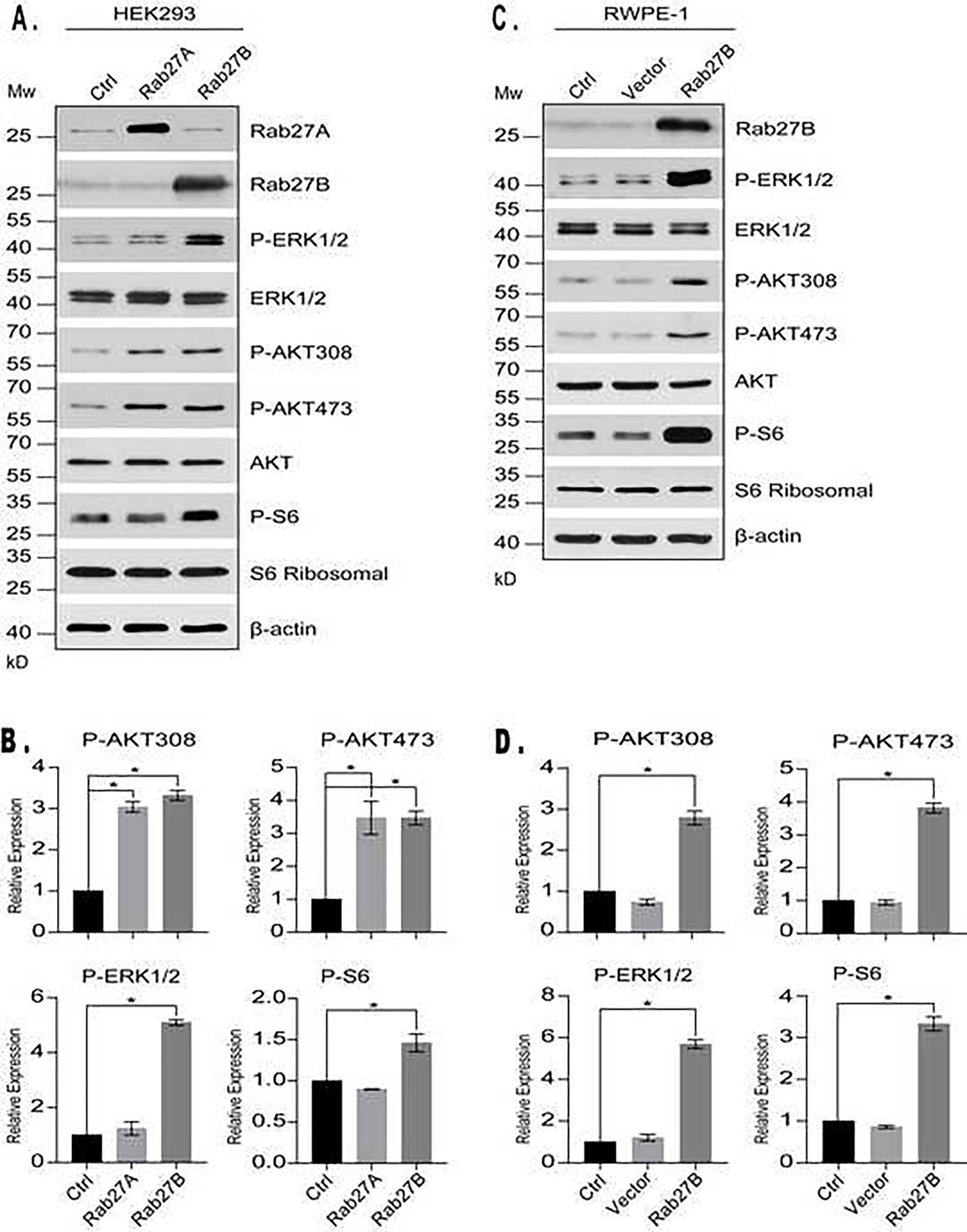
A. Western blotting analysis of activation-dependent phosphorylation of ERK1/2, AKT and S6 in HEK293 cells overexpressing Rab27A, Rab27B or control vector. B. Quantitative presentations of activation-dependent phosphorylation of ERK1/2, AKT and S6 in the cells shown in A. The data were obtained by comparing the ratio of phosphorylated / total levels of the indicated proteins in cells overexpressing Rab27A or Rab27B with that of the cells expressing a control vector. Data are from three independent experiments and expressed as mean ± SD. * P < 0.01. C. Western blotting analysis of activation-dependent phosphorylation of ERK1/2, AKT and S6 in uninfected RWPE-1 cells (ctrl) or those infected with lentiviral particles expressing empty vector or Rab27B. D. Quantitative presentations of activation-dependent phosphorylation of ERK1/2, AKT and S6 in the cells shown in C. Data are from three independent experiments and expressed as mean ± SD. * P < 0.01.
3.3. Overexpression of Rab27B increases cell surface presentation of growth factor receptors
We next determined if Rab27B overexpression was able to increase cell surface presentation of growth factor receptors. Accordingly, we overexpressed Rab27B, Rab27A or control vector in HEK293 cells and isolated the plasma membrane proteins from the cells. Analysis of a few major growth factor receptors, including EGFR, IGF-1Rβ, and TGF-βR2, revealed a drastic increase of their levels in the plasma membrane of the cells overexpressing Rab27A and Rab27B (Fig. 3A and 3B). The effect of the overexpression was not limited to growth factor receptors, as we found that it also increased the level of NaK-ATPase, a plasma membrane ion pump. However, the overexpression did not affect the total amount of the receptors, indicating that the increased cell surface presentation of the receptors was caused by an enhanced exocytosis but not protein synthesis. Similar increases of the receptors in the plasma membrane were also observed in normal prostatic epithelial RWPE-1 cells overexpressing Rab27B (Fig. 3C and 3D). These results demonstrate that Rab27B overexpression is able to increase the amount of growth factor receptors in the plasma membrane without affecting their expression levels. In addition, overexpression of Rab27B in RWPE-1 cells increased the amount of IGF-1 secreted into the culture media (Fig. 3E). Collectively, the observations suggest that Rab27B overexpression increases exocytosis of RWPE-1 cells.
Figure 3. Rab27B overexpression increases cell surface presentation of growth factor receptors.
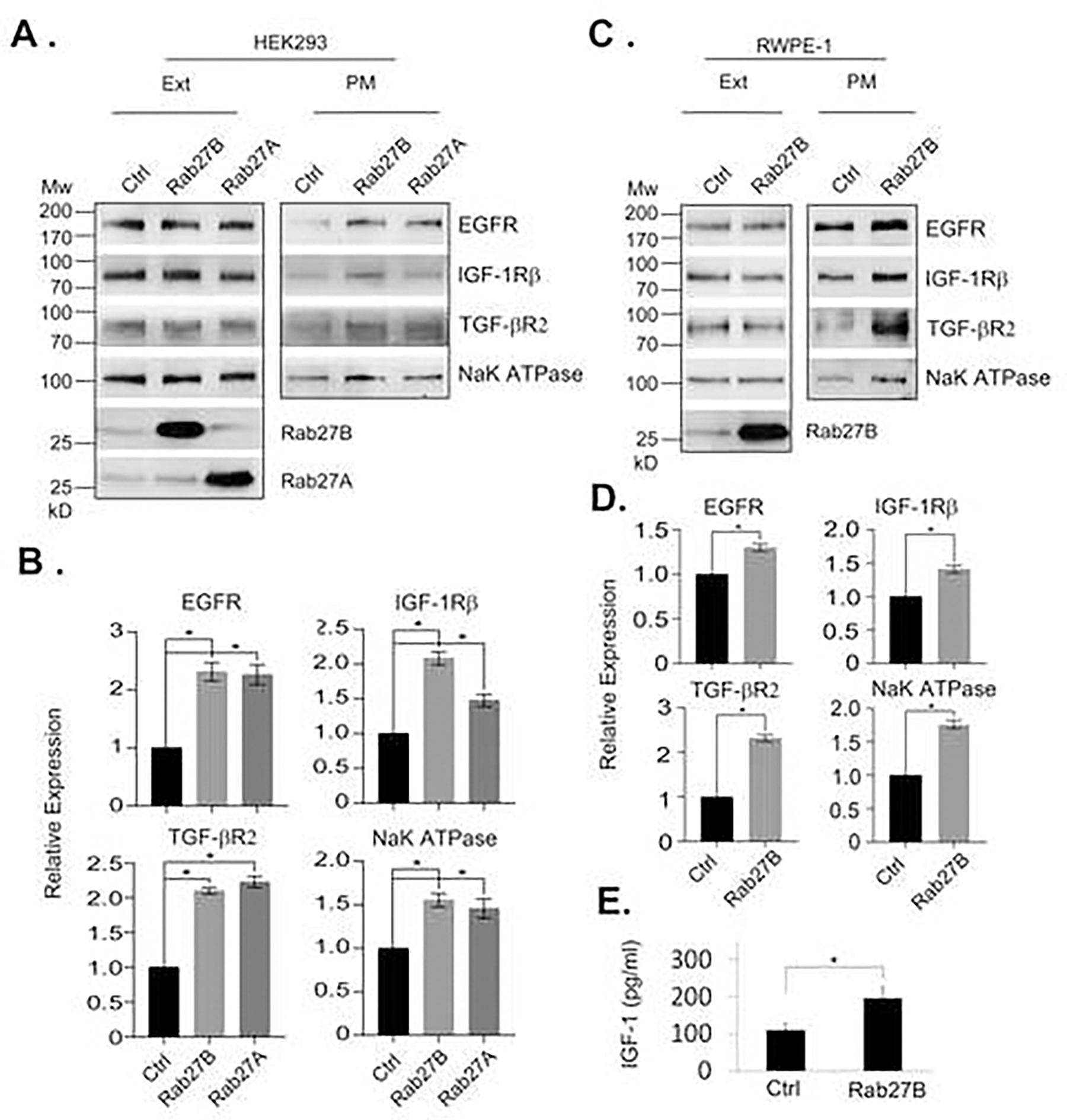
A. Plasma membrane proteins were isolated from HEK293 cells overexpressing Rab27A, Rab27B or control vector. The levels of the indicated membrane proteins in total cell extracts (Ext) and plasma membrane (PM) fractions were analyzed by western blotting. B. Quantitative presentations of the relative levels of the plasma membrane proteins from the cells shown in A. Data are from three independent experiments and expressed as mean ± SD. * P < 0.01. C. Plasma membrane proteins were isolated from RWPE-1 cells overexpressing Rab27B or control vector. The levels of the indicated membrane proteins in total cell extracts and plasma membrane fractions were analyzed by western blotting. D. Quantitative presentations of the relative levels of the plasma membrane proteins from the cells shown in C. Data are from three independent experiments and expressed as mean ± SD. * P < 0.01. E. The levels of IGF-1 in media conditioned by RWPE-1 expressing Rab27B or control vector. Data are from three independent experiments and expressed as mean ± SD. * P < 0.05.
3.4. Downregulation of Rab27B in BPH-1 cells reduces growth factor signaling
In the BPH-1 cells, as in BPH tissues, Rab27B is abnormally overexpressed. To determine how the abnormal expression affects the prostatic epithelial cells of BPH, we used shRNA-mediated knockdown to reduce the expression of Rab27B in BPH-1 cells and examined the effect of knockdown on growth factor signaling and proliferation of the cells. We established several BPH-1 cell lines stably expressing Rab27B specific shRNAs and selected two lines with the Rab27B expression reduced to a level similar to that in normal prostatic epithelial cell line, RWPE-1 (Fig. 4A). In comparison with the control BPH-1 cells, those with the Rab27 knockdown cells had a reduced growth rate (Fig. 4B), suggesting that the overproduced Rab27B promoted BPH-1 cell growth. Analysis of the growth factor signaling in the knockdown cells revealed that the signaling activities of the PK3K/AKT and MAPK pathways, as reflected by the activation-dependent phosphorylation of AKT and ERK1/2, were greatly reduced (Fig. 4C and 4D). These findings demonstrate that reduction of Rab27B expression in BPH-1 cells is able to reduce the growth factor signaling and decrease cell growth. Surprisingly, while knockdown of Rab27B caused AKT inactivation, it led to a strong increase in mTORC1 signaling activity, which was manifested by high levels of phosphorylated S6 and eIF4E binding protein 1 (4E-BP1). This observation suggests that like Rab27A, Rab27B has a negative role in mTORC1 activity independent of AKT.
Figure 4. Rab27B downregulation in BPH-1 cells reduces growth factor signaling.
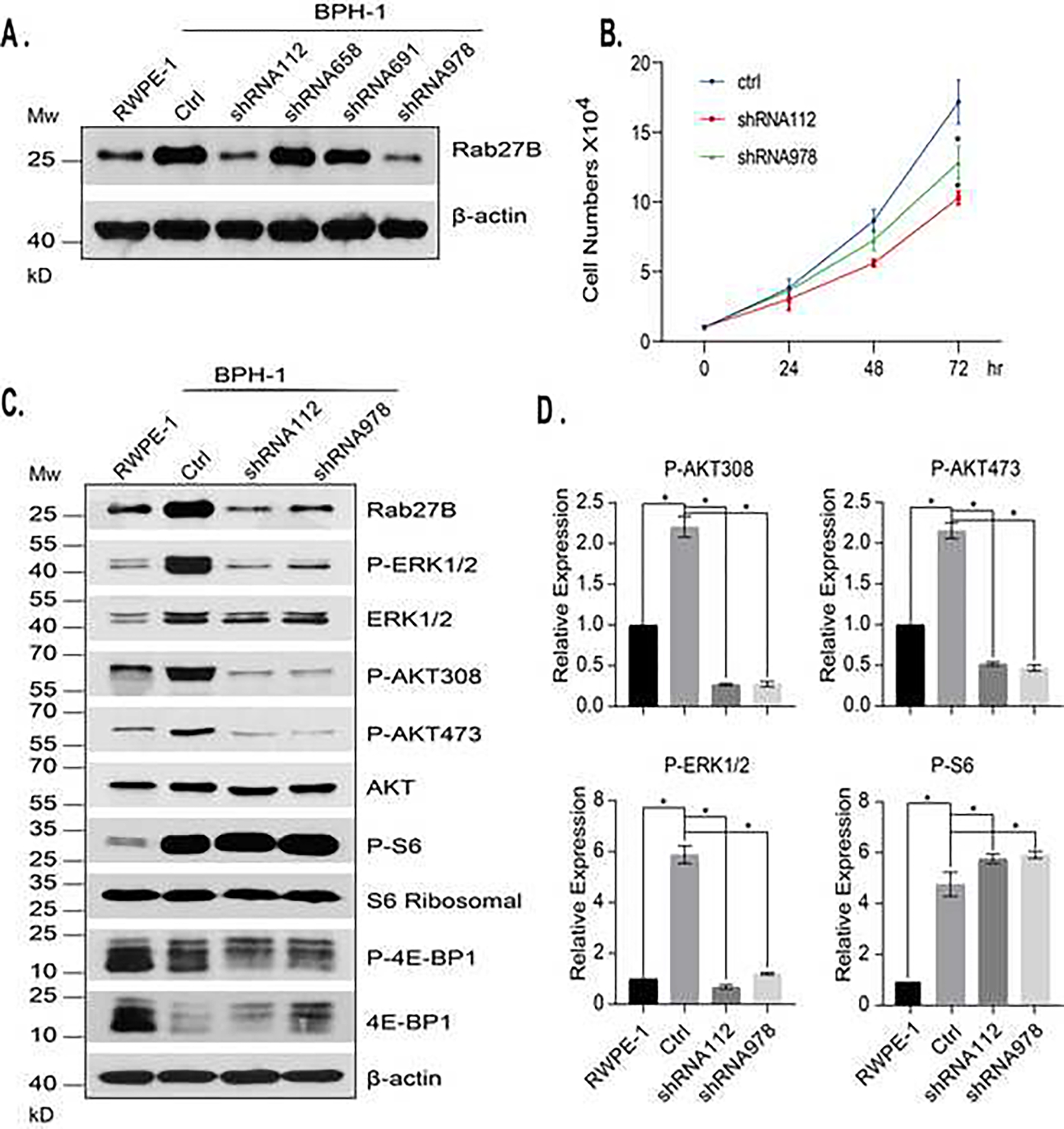
A. BPH-1 cells stably expressing scrambled control shRNA (ctrl) or Rab27B specific shRNAs were analyzed by western blotting for the levels of Rab27B. The expression level of Rab27B in RWPE-1 was included for comparison. B. The growth rates of BPH-1 cells stably expressing control shRNA (ctrl) or Rab27B specific shRNAs were analyzed. The data are from four independent experiments and the growth differences at 72 hr between the control and the two Rab27B shRNAs expressed as mean ± SD. * P < 0.01. C. The levels of the activation-dependent phosphorylation of ERK1/2, AKT, S6, 4E-BP1 and their expression in cells stably expressing control or Rab27B specific shRNAs were analyzed by western blotting. D. Quantitative presentations of the phosphorylation levels of ERK1/2, AKT and S6 in the cells shown in C. The data were obtained by comparing the ratio of phosphorylated / total protein level of the Rab27 shRNA expressing cells with that of the control cells. Data are from three independent experiments and expressed as mean ± SD. * P < 0.01.
3.5. Downregulation of Rab27B reduced cell surface presentation of growth factor receptors
To determine the mechanism underlying the reduced growth factor signaling activities upon Rab27B downregulation, we examined the surface presentation of a few growth factor receptors in cells with Rab27B downregulated by shRNA. As shown in Fig. 5, we found that knockdown of Rab27B drastically reduced the levels of EGFR, IGF-1Rβ, TGF-βR2 and NaK-ATPase in the plasma membrane (Fig. 5A and 5B). However, the downregulation did not affect the total levels of the membrane proteins. These observations suggest that the decrease of Rab27B affects the surface presentation of the receptors by reducing exocytosis. A confocal microscopic examination of IGF-1Rβ revealed a tight distribution of the receptor along the cell boundary in BPH-1 cells but a diffused distribution in cells expressing Rab27B specific shRNAs (Fig. 5C). This observation indicates a defect in the plasma membrane deposition of the receptor, consistent with the reduced plasma membrane level of the receptor in Rab27B knockdown cells (Fig. 5A and 5B). To assess whether downregulation of Rab27B also reduce secretion of growth factors, we compared the amounts of IGF-1 secreted into the culture media by BPH-1 control cells and those with downregulated Rab27B. We found that Rab27B downregulation significantly reduced the amount of IGF-1 secreted into the culture media (Fig. 5D). Next, we examined that ability of culture media conditioned by the knockdown cells to stimulate growth of prostatic stromal cells. We found that media conditioned by Rab27B downregulated BPH-1 cells possessed a weaker activity to stimulate the growth of prostatic stromal WPMY-1 cells than those from BPH-1 control cells (Fig. 5E). This observation suggests that Rab27B downregulation reduced the amount of growth factors secreted into the media, hence lowering the growth promoting activity in the conditioned media.
Figure 5. Rab27B downregulation reduces cell surface presentation of growth factor receptors.
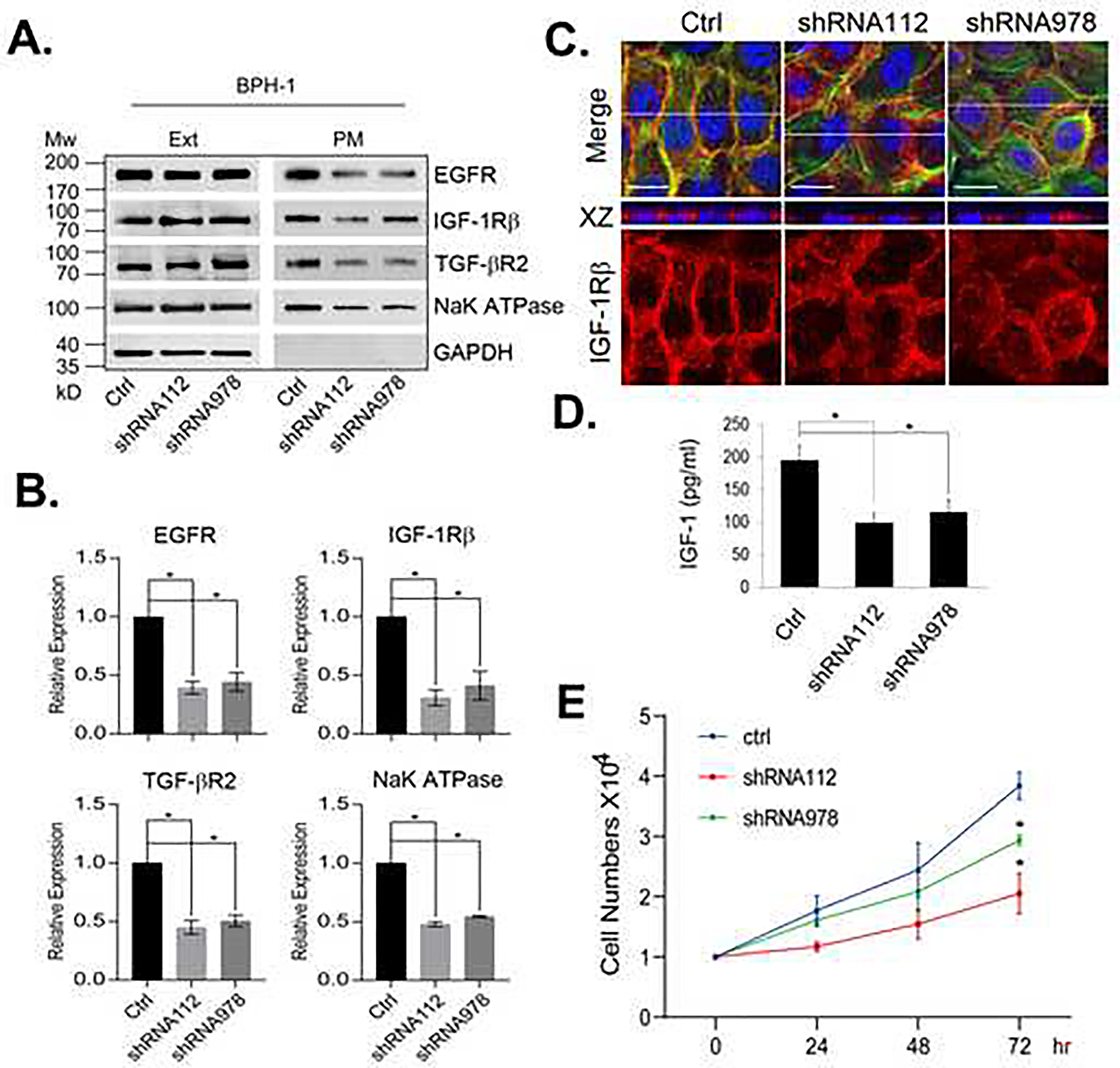
A. The levels of the indicated proteins in the total cell extracts (Ext) and plasma membrane (PM) of BPH-1 cells expressing control or Rab27B specific shRNAs were analyzed by western blotting. B. Quantitative presentations of the relative levels of the plasma membrane proteins from the cells shown in A. The data are from three independent experiments and expressed as mean ± SD. * P < 0.01. C. Confocal images of IGF-1Rβ distribution in BPH-1 cells expressing control or Rab27B specific shRNAs. IGF-1Rβ was detected with IGF-1Rβ antibody (red), actin fibers with Alexa fluor-488 phalloidin (green) and nuclei (blue) with DAPI. White lines in the merged images mark where the cross-sections of XZ plane were taken. Scale bar = 20 μm. D. The levels of IGF-1 in media conditioned by BPH-1 cells expressing control or Rab27B specific shRNAs. Data are from three independent experiments and expressed as mean ± SD. * P < 0.01. E. The growth curves of WPMY-1 cells in media conditioned by BPH-1 cells expressing control or Rab27B specific shRNAs. Data are from four independent experiments. The growth differences at 72 hr between the cells cultured by conditioned media from the control and the two Rab27B shRNAs knockdown cells are expressed as mean ± SD. * P < 0.01.
4. Discussion
Prostatic epithelial and stromal cells have been known to produce a large amount of secretory proteins and signaling molecules, which are essential for epithelial-stromal interactions and homeostasis of the prostate (Lee and Peehl, 2004). For many years, studies of prostatic epithelial-stromal interactions in BPH have focused mainly on androgen-induced expression of growth factors and other signaling molecules (Ho and Habib, 2011). In the present study we show that altered exocytosis can also plays a key role in the BPH development. This abnormal exocytosis, caused by overexpression of a small GTPase, Rab27B, in the epithelium of BPH, induces an increase in the cell surface presentation of growth factor receptors and secretion of growth factors. This increased presentation of growth factors and their receptors are expected to stimulate both autocrine and paracrine signaling of the prostatic epithelial cells, hence promoting BPH development. Consistent with the notion, our data show that Rab27B overexpression leads to enhanced growth factor signaling activities in normal prostatic epithelial cells (Fig. 2), whereas its downregulation reduces the signaling activities and cell growth of BPH-1 cells (Fig. 4).
Abnormal expression of a few other Rab GTPases involved in exocytosis, including Rab1A, 2A and Rab35, has been found to affect cell signaling (Tzeng and Wang, 2016). However, in those cases, the effects of the small GTPases are mediated through their direct interactions with components of signaling pathways, and hence their effect is limited to one particular pathway (Luo et al., 2015; Thomas et al., 2014; Wheeler et al., 2015). On the other hand, the impact of Rab27B overexpression on cell signaling is much broader than that of other Rab GTPases. It is not limited to secretion of certain signaling molecules or to a particular pathway. Instead, the overexpression affects general growth factor signaling by altering the cell surface presentations of many different receptors. Given the fact that the small GTPase is only one of the factors that act at late stages of exocytosis, it is surprising that its overexpression alone is able to sustain an increased exocytosis, which requires increased protein synthesis and vesicular transport. One possibility is that the augmented growth factor signaling in Rab27B overexpressing cells stimulates protein synthesis, and hence exocytosis. However, we did not observe any significant changes in the total levels of the receptors in the cells. Alternatively, the overexpression may alter the sorting of the receptors before or after they reach the plasma membrane, and consequently increase the retention of the receptors on the plasma membrane. The latter possibility is consistent with the fact that Rab27B also controls MVBs, a unique endosomal compartment involved in turnover of membrane proteins (Fukuda, 2013; Ostrowski et al., 2010).
In addition to controlling growth factor signaling through receptor presentation, Rab27B and its homologue Rab27A appear to have a negative role in regulating mTORC1 activity independent of the growth factor signaling. This novel role is manifested by our observation that downregulated Rab27B expression in BPH-1 cells resulted in a drastic increase in mTORC1 signaling activity, despite a decreased AKT activation (Fig. 4B). In our previous study, we have found that an association between Rab27A and mTORC1 (Zou et al., 2019), which suggests that Rab27A and Rab27B may have a shared function in controlling mTORC1 activity unrelated to their role in exocytosis. Since mTORC1 requires lysosome localization for its activation, it is possible that both Rab27B and Rab27A are involved in regulating the mTORC1 localization through their role in sorting of MVBs.
Abnormal expression of Rab27B has been found in many types of cancers, including breast, colorectal, pancreatic, ovarian, and prostate cancers (Bao et al., 2014; Hendrix et al., 2010b; Li et al., 2017; Ren et al., 2016; Worst et al., 2017; Zhao et al., 2016). In most cases, a high expression of the small GTPase is associated with cancer metastasis and poor prognosis and has been suggested as a marker for cancer progression (Bao et al., 2014; Hendrix et al., 2010a). However, in some cancers downregulated Rab27B expression is found to correlate with advanced stages of cancer progression (Dong et al., 2015; Worst et al., 2017). These observations indicate a critical yet complex function of Rab27B in cancer development. The overexpressed Rab27B in breast cancer cells has been found to increase invasive growth through secretion of pro-invasive factors that modify tumor microenvironment (Hendrix et al., 2010b). This mechanism may underlie its role in cancer metastasis. Our findings that Rab27B controls cell autocrine and paracrine signaling further suggest that an altered Rab27B expression plays a critical role in cancer cell proliferation and survival.
5. Conclusion
In summary, our study identifies the small GTPase Rab27B as a key factor involved in development of BPH. The abnormal expression of this small GTPase alters the cell surface presentation of many growth factors receptors and hence impacts prostatic epithelial-stromal interactions.
Acknowledgements:
This study is supported in part by U54 DK112079 pilot grant and GM132127 to YJ and U54 DK112079 to ZW. We would like to thank Dr. Simon Hayward (NorthShore University HealthSystem) for kindly providing BPH-1 and BHPrE1 cell lines. This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by award P30 CA047904.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Banerjee PP, Banerjee S, Brown TR, and Zirkin BR (2018). Androgen action in prostate function and disease. Am J Clin Exp Urol 6, 62–77. [PMC free article] [PubMed] [Google Scholar]
- Bao J, Ni Y, Qin H, Xu L, Ge Z, Zhan F, Zhu H, Zhao J, Zhou X, and Tang X (2014). Rab27b is a potential predictor for metastasis and prognosis in colorectal cancer. Gastroenterology research and practice 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello D, Webber MM, Kleinman HK, Wartinger DD, and Rhim JS (1997). Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 18, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guo X, Deng FM, Liang FX, Sun W, Ren M, Izumi T, Sabatini DD, Sun TT, and Kreibich G (2003). Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci U S A 100, 14012–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, Woo HH, Te AE, and Kaplan SA (2016). Benign prostatic hyperplasia. Nat Rev Dis Primers 2, 16031. [DOI] [PubMed] [Google Scholar]
- De Nunzio C, Presicce F, and Tubaro A (2016). Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol 13, 613–626. [DOI] [PubMed] [Google Scholar]
- Dong W-W, Mou Q, Chen J, Cui J-T, Li W-M, and Xiao W-H (2012). Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World journal of gastroenterology: WJG 18, 1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Cui J, Yang J, Li W, Wang S, Wang X, Li X, Lu Y, and Xiao W (2015). Decreased expression of Rab27A and Rab27B correlates with metastasis and poor prognosis in colorectal cancer. Discov Med 20, 357–367. [PubMed] [Google Scholar]
- Fukuda M (2008). Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci 65, 2801–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M (2013). Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 14, 949–963. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, and Narayan P (1995). Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim 31, 14–24. [DOI] [PubMed] [Google Scholar]
- Hendrix A, Braems G, Bracke M, Seabra MC, Gahl WA, De Wever O, and Westbroek W (2010a). The secretory small GTPase Rab27B as a marker for breast cancer progression. Oncotarget 1, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, et al. (2010b). Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst 102, 866–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CK, and Habib FK (2011). Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol 8, 29–41. [DOI] [PubMed] [Google Scholar]
- Jahn R, and Sudhof TC (1999). Membrane fusion and exocytosis. Annu Rev Biochem 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Jain S, Roy S, Amin M, Acquafondata M, Yin M, Laframboise W, Bastacky S, Pantanowitz L, Dhir R, and Parwani A (2013). Amylase alpha-1A (AMY1A): a novel immunohistochemical marker to differentiate chromophobe renal cell carcinoma from benign oncocytoma. Am J Surg Pathol 37, 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, and Hayward SW (2010). Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells 28, 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, and Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Kimura T, and Niki I (2011). Rab27a, actin and beta-cell endocytosis. Endocr J 58, 1–6. [DOI] [PubMed] [Google Scholar]
- La Vignera S, Condorelli RA, Russo GI, Morgia G, and Calogero AE (2016). Endocrine control of benign prostatic hyperplasia. Andrology 4, 404–411. [DOI] [PubMed] [Google Scholar]
- Lee KL, and Peehl DM (2004). Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol 172, 1784–1791. [DOI] [PubMed] [Google Scholar]
- Li J, Jin Q, Huang F, Tang Z, and Huang J (2017). Effects of Rab27A and Rab27B on invasion, proliferation, apoptosis, and chemoresistance in human pancreatic cancer cells. Pancreas 46, 1173–1179. [DOI] [PubMed] [Google Scholar]
- Luo ML, Gong C, Chen CH, Hu H, Huang P, Zheng M, Yao Y, Wei S, Wulf G, Lieberman J, et al. (2015). The Rab2A GTPase promotes breast cancer stem cells and tumorigenesis via Erk signaling activation. Cell Rep 11, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley KJ, Eisermann K, Pascal LE, Parwani AV, Majima T, Graham L, Hrebinko K, Acquafondata M, Stewart NA, Nelson JB, et al. (2014). Proteomic analysis of patient tissue reveals PSA protein in the stroma of benign prostatic hyperplasia. Prostate 74, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12, 19–30; sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- Ren P, Yang XQ, Zhai XL, Zhang YQ, and Huang JF (2016). Overexpression of Rab27B is correlated with distant metastasis and poor prognosis in ovarian cancer. Oncology letters 12, 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE (1994). Mechanisms of intracellular protein transport. Nature 372, 55–63. [DOI] [PubMed] [Google Scholar]
- Stenmark H (2009). Rab GTPases as coordinators of vesicle traffic. Nature reviews Molecular cell biology 10, 513–525. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Zhang YJ, Wei YH, Cho JH, Morris LE, Wang HY, and Zheng XF (2014). Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell 26, 754–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms BG, and Hofkamp LE (2011). Prostate development and growth in benign prostatic hyperplasia. Differentiation 82, 173–183. [DOI] [PubMed] [Google Scholar]
- Tzeng HT, and Wang YC (2016). Rab-mediated vesicle trafficking in cancer. J Biomed Sci 23, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Zoncu R, Root DE, Sabatini DM, and Sawyers CL (2015). Identification of an oncogenic RAB protein. Science 350, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worst TS, Meyer Y, Gottschalt M, Weis C-A, Von Hardenberg J, Frank C, Steidler A, Michel MS, and Erben P (2017). RAB27A, RAB27B and VPS36 are downregulated in advanced prostate cancer and show functional relevance in prostate cancer cells. International journal of oncology 50, 920–932. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wang Q, Wang X, Zhu H, Zhang S, Wang W, Wang Z, and Huang J (2016). Correlation between RAB27B and p53 expression and overall survival in pancreatic cancer. Pancreas 45, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Zhao X, Li J, Yuan W, Yan G, Tong M, Guo S, Zhu Y, Jiang Y, Liu Y, et al. (2016). Tumor Suppressor Folliculin Regulates mTORC1 through Primary Cilia. J Biol Chem 291, 11689–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Lai M, Zhang Y, Zheng L, Xing Z, Li T, Zou Z, Song Q, Zhao X, Xia L, et al. (2019). Exosome Release Is Regulated by mTORC1. Adv Sci (Weinh) 6, 1801313. [DOI] [PMC free article] [PubMed] [Google Scholar]


