Abstract
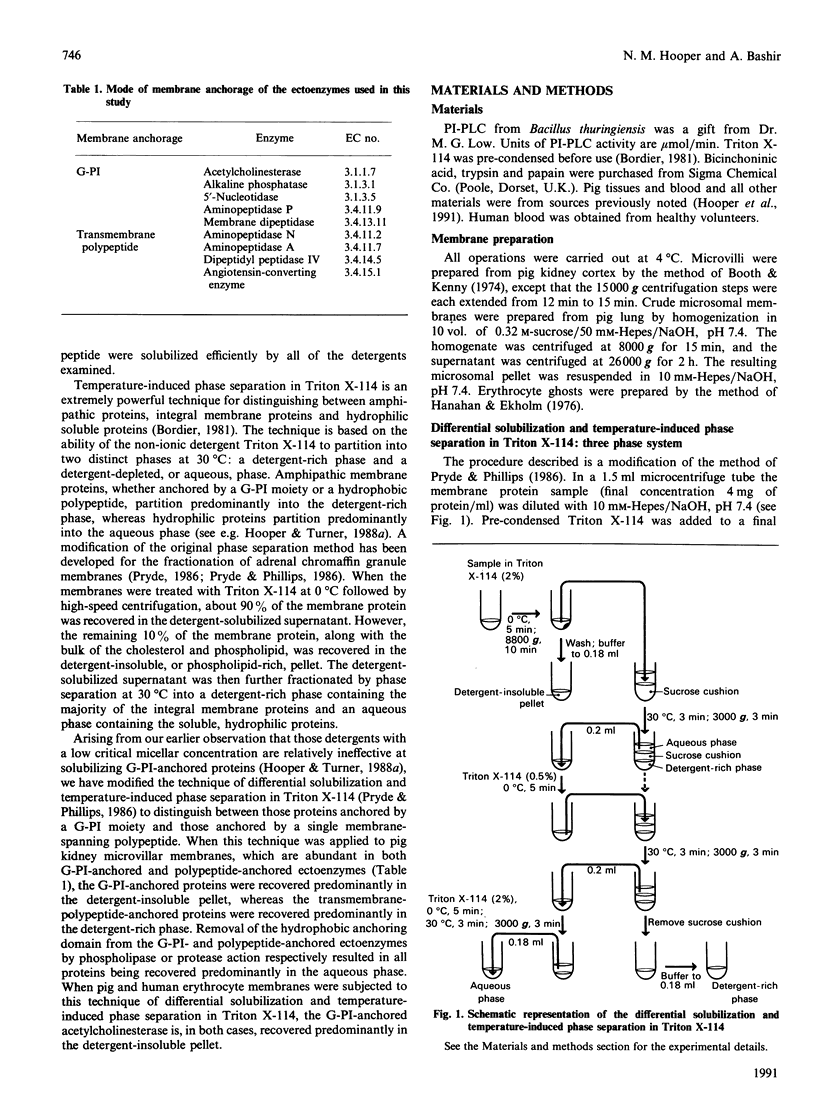
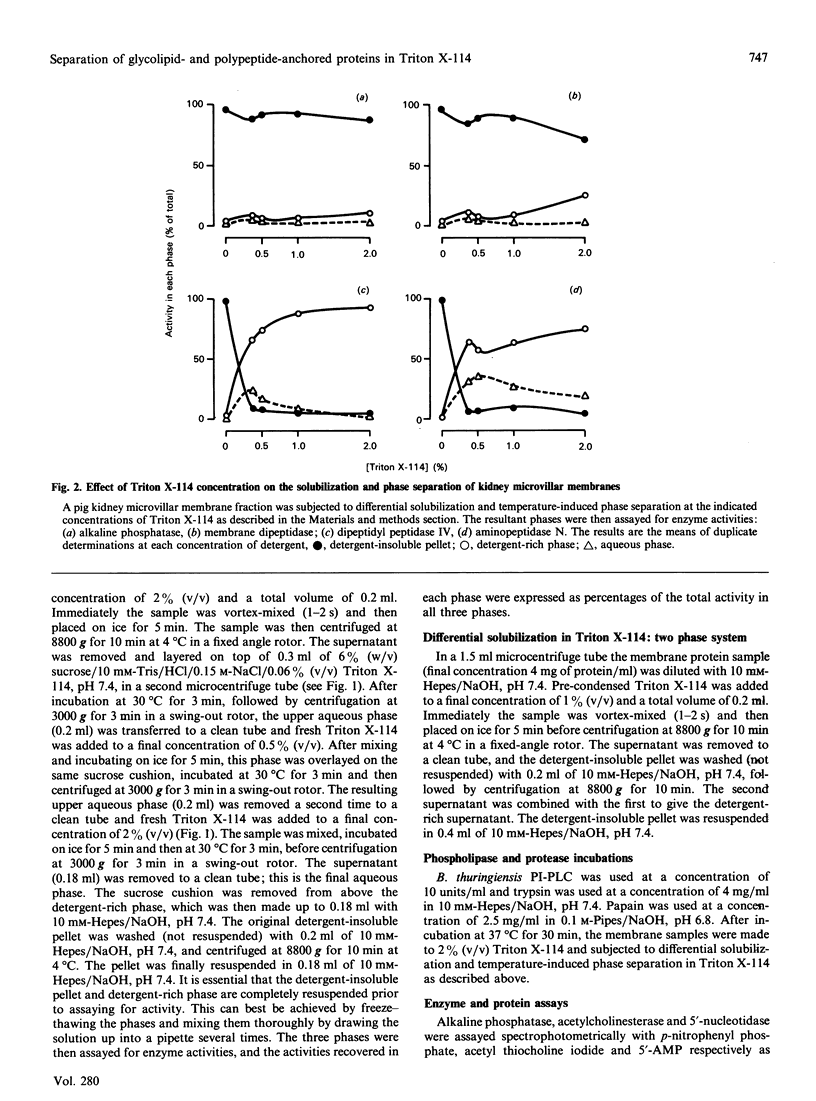
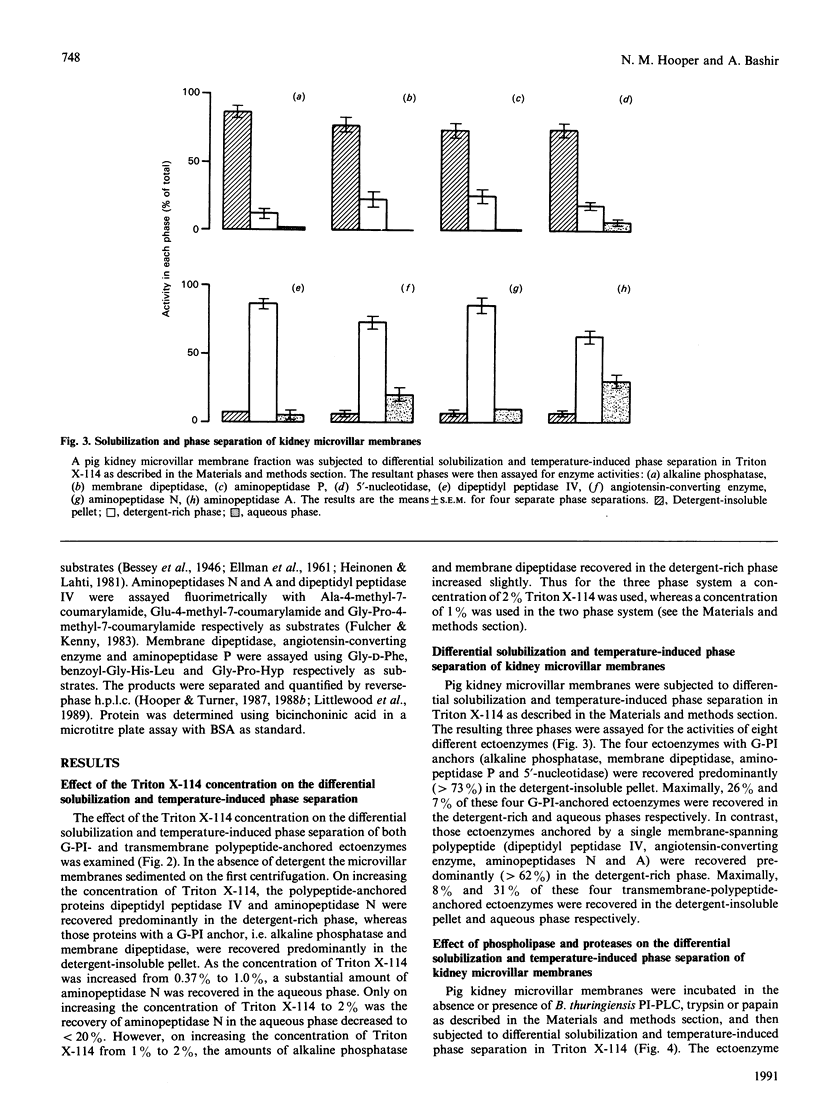
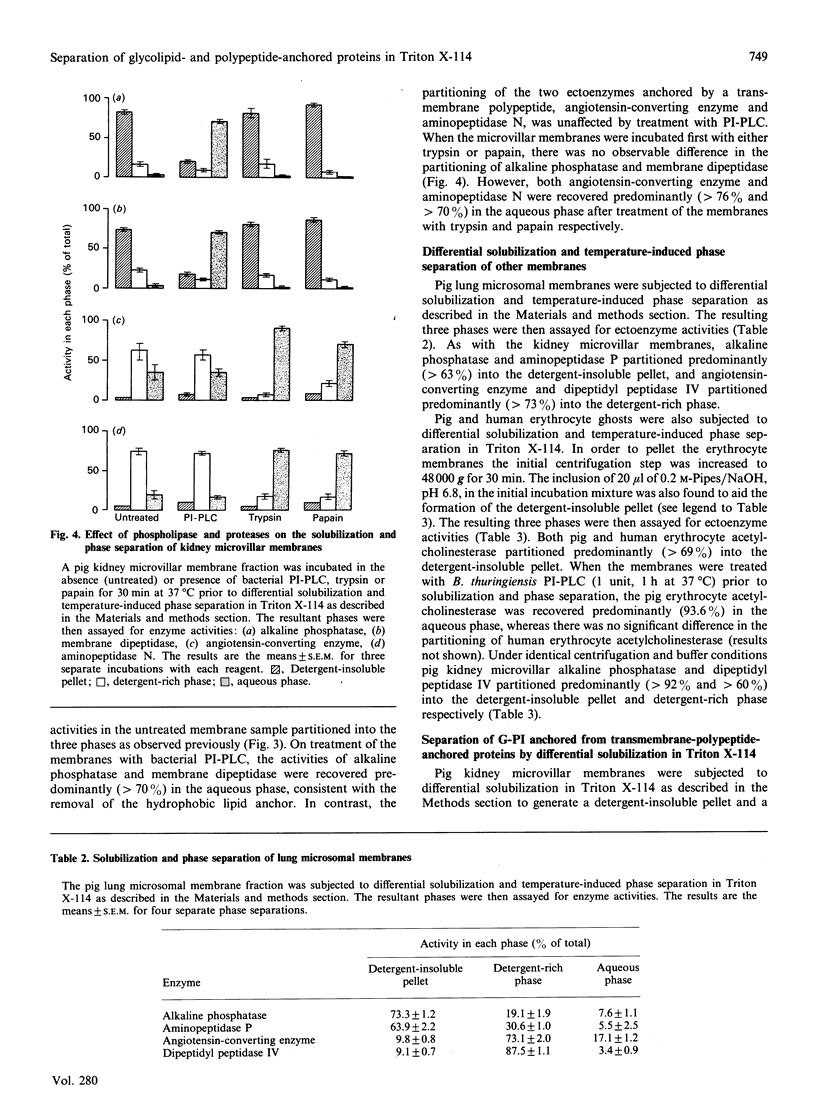
Treatment of kidney microvillar membranes with the non-ionic detergent Triton X-114 at 0 degrees C, followed by low-speed centrifugation, generated a detergent-insoluble pellet and a detergent-soluble supernatant. The supernatant was further fractionated by phase separation at 30 degrees C into a detergent-rich phase and a detergent-depleted or aqueous phase. Those ectoenzymes with a covalently attached glycosyl-phosphatidylinositol (G-PI) membrane anchor were recovered predominantly (greater than 73%) in the detergent-insoluble pellet. In contrast, those ectoenzymes anchored by a single membrane-spanning polypeptide were recovered predominantly (greater than 62%) in the detergent-rich phase. Removal of the hydrophobic membrane-anchoring domain from either class of ectoenzyme resulted in the proteins being recovered predominantly (greater than 70%) in the aqueous phase. This technique was also applied to other membrane types, including pig and human erythrocyte ghosts, where, in both cases, the G-PI-anchored acetylcholinesterase partitioned predominantly (greater than 69%) into the detergent-insoluble pellet. When the microvillar membranes were subjected only to differential solubilization with Triton X-114 at 0 degrees C, the G-PI-anchored ectoenzymes were recovered predominantly (greater than 63%) in the detergent-insoluble pellet, whereas the transmembrane-polypeptide-anchored ectoenzymes were recovered predominantly (greater than 95%) in the detergent-solubilized supernatant. Thus differential solubilization and temperature-induced phase separation in Triton X-114 distinguished between G-PI-anchored membrane proteins, transmembrane-polypeptide-anchored proteins and soluble, hydrophilic proteins. This technique may be more useful and reliable than susceptibility to release by phospholipases as a means of identifying a G-PI anchor on an unpurified membrane protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chajek-Shaul T., Halimi O., Ben-Naim M., Stein O., Stein Y. Phosphatidylinositol-specific phospholipase C releases lipoprotein lipase from the heparin releasable pool in rat heart cell cultures. Biochim Biophys Acta. 1989 Nov 20;1014(2):178–183. doi: 10.1016/0167-4889(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hom J., Schenkman S. Purification of a glycosyl-phosphatidylinositol-specific phospholipase D from human plasma. J Biol Chem. 1989 Aug 15;264(23):13760–13764. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Michaelson D. M., Silman I. Solubilization of membrane-bound acetylcholinesterase by a phosphatidylinositol-specific phospholipase C. J Neurochem. 1985 Nov;45(5):1487–1494. doi: 10.1111/j.1471-4159.1985.tb07217.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J., Ekholm J. E. The preparation of red cell ghosts (membranes). Methods Enzymol. 1974;31:168–172. doi: 10.1016/0076-6879(74)31018-x. [DOI] [PubMed] [Google Scholar]
- Heinonen J. K., Lahti R. J. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981 May 15;113(2):313–317. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- Hooper N. M. Angiotensin converting enzyme: implications from molecular biology for its physiological functions. Int J Biochem. 1991;23(7-8):641–647. doi: 10.1016/0020-711x(91)90032-i. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Broomfield S. J., Turner A. J. Characterization of antibodies to the glycosyl-phosphatidylinositol membrane anchors of mammalian proteins. Biochem J. 1991 Jan 15;273(Pt 2):301–306. doi: 10.1042/bj2730301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Aminopeptidase P is anchored by a glycosyl-phosphatidylinositol moiety. FEBS Lett. 1988 Mar 14;229(2):340–344. doi: 10.1016/0014-5793(88)81152-9. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1988 Mar 15;250(3):865–869. doi: 10.1042/bj2500865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Isolation of two differentially glycosylated forms of peptidyl-dipeptidase A (angiotensin converting enzyme) from pig brain: a re-evaluation of their role in neuropeptide metabolism. Biochem J. 1987 Feb 1;241(3):625–633. doi: 10.1042/bj2410625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Li S., Fung W. J., Hulmes J. D., Reik L., Pan Y. C., Low M. G. Purification and characterization of glycosyl-phosphatidylinositol-specific phospholipase D. J Biol Chem. 1990 Oct 15;265(29):17738–17745. [PubMed] [Google Scholar]
- Jemmerson R., Low M. G. Phosphatidylinositol anchor of HeLa cell alkaline phosphatase. Biochemistry. 1987 Sep 8;26(18):5703–5709. doi: 10.1021/bi00392a019. [DOI] [PubMed] [Google Scholar]
- Jäger K., Meyer P., Stieger S., Brodbeck U. Production and characterization of antibodies against the cross-reacting determinant of glycosyl-phosphatidylinositol-anchored acetylcholinesterase. Biochim Biophys Acta. 1990 Jul 6;1039(3):367–373. doi: 10.1016/0167-4838(90)90272-h. [DOI] [PubMed] [Google Scholar]
- Littlewood G. M., Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Affinity purification, characterization and localization of the phospholipase C-solubilized form of renal dipeptidase. Biochem J. 1989 Jan 15;257(2):361–367. doi: 10.1042/bj2570361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Non-lytic release of acetylcholinesterase from erythrocytes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1977 Oct 1;82(1):143–146. doi: 10.1016/0014-5793(77)80905-8. [DOI] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Pryde J. G., Phillips J. H. Fractionation of membrane proteins by temperature-induced phase separation in Triton X-114. Application to subcellular fractions of the adrenal medulla. Biochem J. 1986 Jan 15;233(2):525–533. doi: 10.1042/bj2330525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Zamze S. E., Ferguson M. A., Collins R., Dwek R. A., Rademacher T. W. Characterization of the cross-reacting determinant (CRD) of the glycosyl-phosphatidylinositol membrane anchor of Trypanosoma brucei variant surface glycoprotein. Eur J Biochem. 1988 Oct 1;176(3):527–534. doi: 10.1111/j.1432-1033.1988.tb14310.x. [DOI] [PubMed] [Google Scholar]


