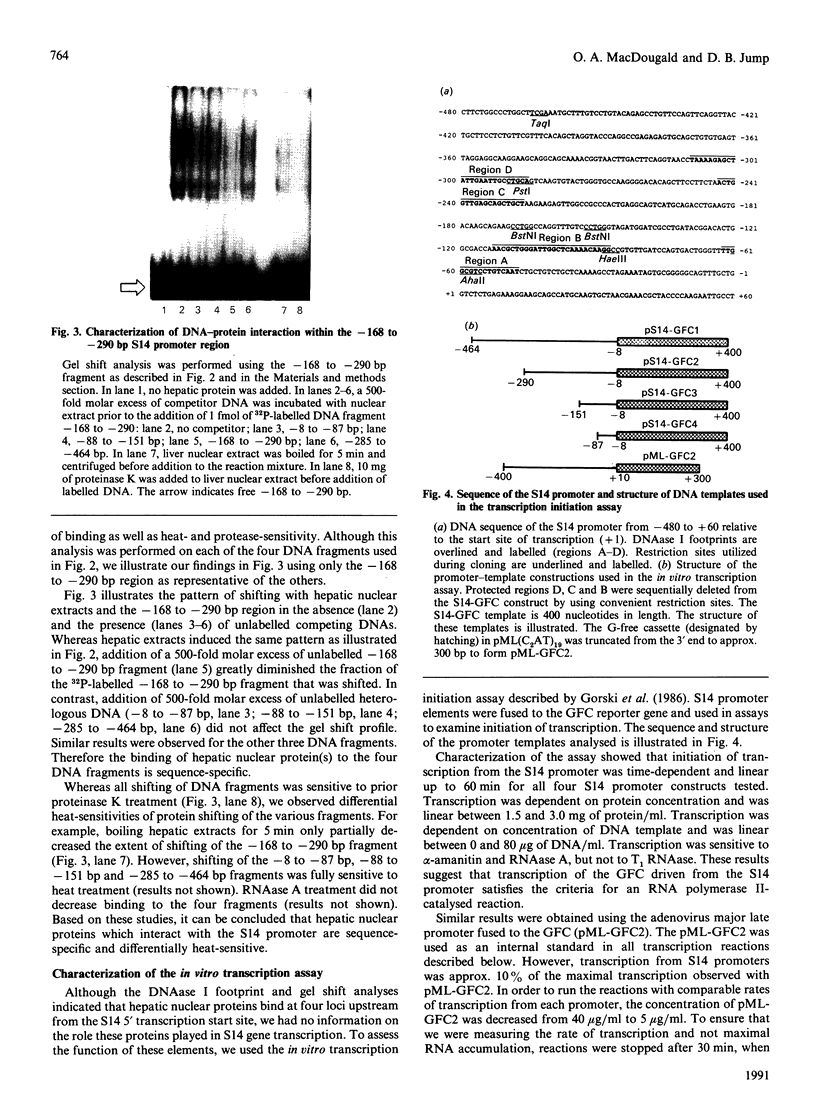
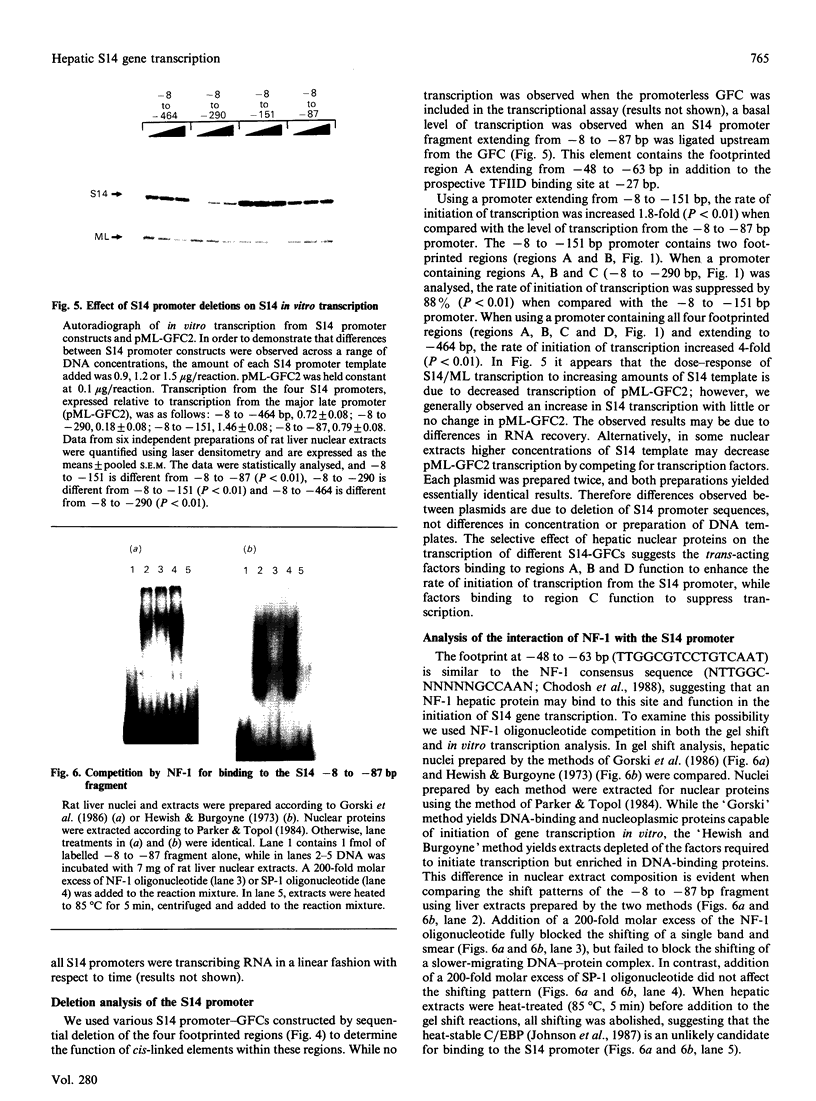
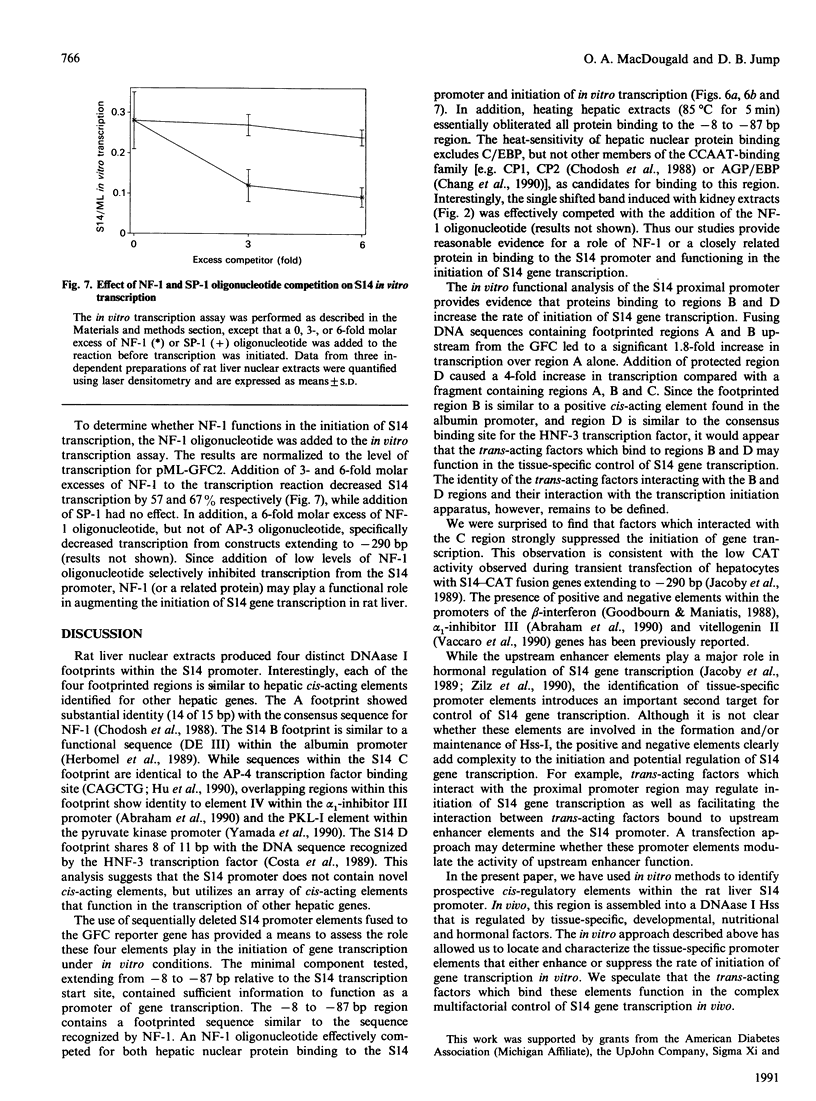
Abstract
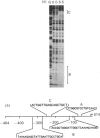
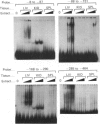
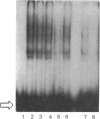
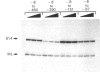
The structure of DNAase I hypersensitive site 1 (Hss-1), located adjacent to the 5' end of the rat liver S14 gene, is regulated by tissue-specific factors, and its formation correlates with the transcriptional activation of the S14 gene. We propose that tissue-specific trans-acting factors interacting with key cis-linked elements within this site function in the initiation of S14 gene transcription. To examine this hypothesis we used DNAase I footprint, gel shift and in vitro transcriptional analyses to identify cis-linked elements that function in the control of S14 gene transcription. Binding of rat liver nuclear proteins to the S14 promoter (from -8 to -464 bp) produced four DNAase I footprints (designated A-D). Gel shift studies showed that DNA-protein binding was tissue- and sequence-specific, differentially heat-sensitive, and abolished by proteinase K. The function of the four cis-acting elements was assessed by using an in vitro transcription initiation assay in which the S14 promoter was fused to a reporter gene (G-free cassette). Deletion studies showed that nuclear factors binding to regions A (-48 to -63 bp), B (-88 to -113 bp) and D (-286 to -310 bp) enhanced the rate of initiation of transcription, while proteins binding to region C (-227 to -244 bp) suppressed the rate of initiation of transcription. Based on oligonucleotide competition studies, we suggest that hepatic NF-1 (or a related protein) binding to the A region enhances the rate of initiation of S14 gene transcription. Since trans-acting factors interacting with regions B and D are found in liver but not in spleen or kidney, we suggest that the proteins interacting with these regions may be involved in the tissue-specific augmentation of S14 gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. J., Bradshaw A. D., Shiels B. R., Northemann W., Hudson G., Fey G. H. Hepatic transcription of the acute-phase alpha 1-inhibitor III gene is controlled by a novel combination of cis-acting regulatory elements. Mol Cell Biol. 1990 Jul;10(7):3483–3491. doi: 10.1128/mcb.10.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hamblin P. S., Ozawa Y., Jefferds A., Mariash C. N. Interaction between fructose and glucose on the regulation of the nuclear precursor for mRNA-S14. J Biol Chem. 1989 Dec 25;264(36):21646–21651. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Rollier A., Tronche F., Ott M. O., Yaniv M., Weiss M. C. The rat albumin promoter is composed of six distinct positive elements within 130 nucleotides. Mol Cell Biol. 1989 Nov;9(11):4750–4758. doi: 10.1128/mcb.9.11.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hu Y. F., Lüscher B., Admon A., Mermod N., Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990 Oct;4(10):1741–1752. doi: 10.1101/gad.4.10.1741. [DOI] [PubMed] [Google Scholar]
- Jacoby D. B., Zilz N. D., Towle H. C. Sequences within the 5'-flanking region of the S14 gene confer responsiveness to glucose in primary hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17623–17626. [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Bell A., Lepar G., Hu D. Insulin rapidly induces rat liver S14 gene transcription. Mol Endocrinol. 1990 Nov;4(11):1655–1660. doi: 10.1210/mend-4-11-1655. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Bell A., Santiago V. Thyroid hormone and dietary carbohydrate interact to regulate rat liver S14 gene transcription and chromatin structure. J Biol Chem. 1990 Feb 25;265(6):3474–3478. [PubMed] [Google Scholar]
- Jump D. B., Narayan P., Towle H., Oppenheimer J. H. Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem. 1984 Mar 10;259(5):2789–2797. [PubMed] [Google Scholar]
- Jump D. B., Oppenheimer J. H. High basal expression and 3,5,3'-triiodothyronine regulation of messenger ribonucleic acid S14 in lipogenic tissues. Endocrinology. 1985 Dec;117(6):2259–2266. doi: 10.1210/endo-117-6-2259. [DOI] [PubMed] [Google Scholar]
- Jump D. B. Rapid induction of rat liver S14 gene transcription by thyroid hormone. J Biol Chem. 1989 Mar 15;264(8):4698–4703. [PubMed] [Google Scholar]
- Jump D. B., Veit A., Santiago V., Lepar G., Herberholz L. Transcriptional activation of the rat liver S14 gene during postnatal development. J Biol Chem. 1988 May 25;263(15):7254–7260. [PubMed] [Google Scholar]
- Jump D. B., Wong N. C., Oppenheimer J. H. Chromatin structure and methylation state of a thyroid hormone-responsive gene in rat liver. J Biol Chem. 1987 Jan 15;262(2):778–784. [PubMed] [Google Scholar]
- Lepar G. J., Jump D. B. Hormonal regulation of the S14 gene in 3T3-F442A cells. Mol Endocrinol. 1989 Aug;3(8):1207–1214. doi: 10.1210/mend-3-8-1207. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro M., Pawlak A., Jost J. P. Positive and negative regulatory elements of chicken vitellogenin II gene characterized by in vitro transcription competition assays in a homologous system. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3047–3051. doi: 10.1073/pnas.87.8.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N. C., Deschamps B. J., Yeomans Z. A., Cannon P. D. Binding of nuclear proteins to the rat liver S14 gene is influenced by thyroid state. J Biol Chem. 1990 May 25;265(15):8775–8781. [PubMed] [Google Scholar]
- Wong N. C., Perez-Castillo A. M., Sanders M. M., Schwartz H. L., Oppenheimer J. H. Thyroid hormone and circadian regulation of the binding activity of a liver-specific protein associated with the 5'-flanking region of the S14 gene. J Biol Chem. 1989 Mar 15;264(8):4466–4470. [PubMed] [Google Scholar]
- Yamada K., Noguchi T., Matsuda T., Takenaka M., Monaci P., Nicosia A., Tanaka T. Identification and characterization of hepatocyte-specific regulatory regions of the rat pyruvate kinase L gene. The synergistic effect of multiple elements. J Biol Chem. 1990 Nov 15;265(32):19885–19891. [PubMed] [Google Scholar]
- Zilz N. D., Murray M. B., Towle H. C. Identification of multiple thyroid hormone response elements located far upstream from the rat S14 promoter. J Biol Chem. 1990 May 15;265(14):8136–8143. [PubMed] [Google Scholar]