Abstract
The 34-kDa product of adenovirus E4 region open reading frame 6 (E4orf6) dramatically enhances transduction by recombinant adeno-associated virus vectors (rAAV). This is achieved by promoting the conversion of incoming single-stranded viral genomes into transcriptionally competent duplex molecules. The molecular mechanism for enhancing second-strand synthesis is not fully understood. In this study, we analyzed the cellular consequences of E4orf6 expression and the requirements for efficient rAAV transduction mediated by E4orf6. Expression of E4orf6 in 293 cells led to an inhibition of cell cycle progression and an accumulation of cells in S phase. This was preceded by specific degradation of cyclin A and p53, while the levels of other proteins involved in cell cycle control remained unchanged. In addition, the kinase activity of cdc2 was inhibited. We further showed that p53 expression is not necessary or inhibitory for augmentation of rAAV transduction by E4orf6. However, overexpression of cyclin A inhibited E4orf6-mediated enhancement of rAAV transduction. A cyclin A mutant incapable of recruiting protein substrates for cdk2 was unable to inhibit E4orf6-mediated augmentation. In addition, we created an E4orf6 mutant that is selectively defective in rAAV augmentation of transduction. Based on these findings, we suggest that cyclin A degradation represents a viral mechanism to disrupt cell cycle progression, resulting in enhanced viral transduction. Understanding the cellular pathways used during transduction will increase the utility of rAAV vectors in a wide range of gene therapy applications.
There is increasing interest in adeno-associated virus (AAV) as a potential gene delivery vector for human gene therapy (10, 27, 35, 68). AAV is a small human parvovirus with a single-stranded linear DNA genome, and recombinant vectors consist of the viral inverted terminal repeats (ITRs) flanking the foreign gene of interest. rAAV is packaged into AAV particles by cotransfection, together with a plasmid containing the AAV rep and cap genes, into cells in which a lytic infection is induced by infection with adenovirus (Ad) or transfection of helper plasmids (53, 69). The virtues of AAV as a vector include its lack of pathogenicity, high titer, ease of manipulation, absence of all viral open reading frames, and ability to transduce nondividing cells. Transduction with rAAV has been demonstrated with many recombinant genes and in numerous cell types, including differentiated and nondividing cells (27, 68). The mechanisms of rAAV-mediated transduction are poorly understood and variable results for transduction efficiencies have been reported. Transduction into nondividing cells in vivo has recently been demonstrated to be surprisingly effective, although in all settings there is a delay before gene expression is detected (61a, 68). In contrast, transduction into cells in culture is relatively inefficient but can be enhanced by treatment with inhibitors of DNA synthesis, genotoxic agents, and DNA-damaging agents such as UV irradiation and hydroxyurea (2, 22, 51). In addition, it has been suggested that rAAV preferentially transduces cells in S phase (52). It has been shown that transduction with purified rAAV is limited by conversion of the incoming single-stranded genome into a transcriptionally active double-stranded form (22, 23). This rate-limiting step can be considerably enhanced by the expression of Ad E4 region open reading frame 6 (E4orf6), which promotes second-strand synthesis (22, 23).
These observations suggest that there may be a link between E4orf6 and the cell cycle. Many viral oncoproteins deregulate cell cycle control by interfering with functions of nuclear cell cycle regulatory proteins (reviewed in reference 26). Most small DNA viruses replicate their genomes only when the infected cell progresses into the S phase. Examples include the autonomous parvoviruses, which have an absolute requirement for S-phase transition for their replication. This may be partially determined by the necessity for duplex formation, which is probably dependent on a cellular function expressed early in S phase (13). The dependent parvoviruses, such as AAV, harness the changes in cellular milieu caused by helper viruses, such as Ad, for their own replication (3, 9). Exactly how the helper virus affects the cell to create an environment permissive for AAV remains unclear. Although the links between the Ad E1 gene products and cell cycle control have been well established, the connections for other early Ad proteins which are also necessary for AAV helper activity have been less closely examined.
Progression through the mammalian cell cycle is controlled by the interplay of distinct positive and negative regulators. These function in part by coordinating the phosphorylation of key proteins by cyclin-dependent kinases (CDKs). CDKs are in turn regulated in a complex fashion by phosphorylation, dephosphorylation, and their association with cyclins or specific CDK inhibitors (reviewed in references 30 and 33). Cyclin levels oscillate throughout the cell cycle and are restricted spatially within a cell, thus restricting CDK activity both temporally and spatially. Cyclins and CDKs are divided into functional subgroups based on the phase of the cell cycle they regulate. The cyclin E-cdk2 and cyclin A-cdk2 complexes are necessary for entry and progression through S phase, while the cyclin B-cdc2 complex is required for the G2/M transition. Cyclin A associates with cdk2 during S phase and with cdc2 during G2 phase (41, 42, 45, 61), and a number of observations suggest that cyclin A is involved in controlling DNA replication (8, 18, 28). Cyclins also play a role in substrate selection for kinase action. For example, the RXL motif has been found in both substrates and inhibitors of cyclin A-cdk2 and mediates the interaction with a hydrophobic patch on the surface of cyclin A (1, 12, 57).
Little is known about functions of E4orf6 that would explain its role in second-strand synthesis. We hypothesized that there might be a link between augmentation of rAAV transduction by E4orf6 and cell cycle regulation, replication, and/or DNA repair, and we set out to define relevant functions for E4orf6. The E4orf6 protein physically associates with the E1b 55-kDa protein in productively infected cells (56), and this complex is involved in the transport of adenovirus mRNAs to the cytoplasm (5, 44). It is estimated that 50% of the total E4orf6 in infected cells is complexed with E1b (14), while noncomplexed E4orf6 may have additional functions not dependent on E1b (39). Products of the E4 region have also been implicated in regulating Ad DNA synthesis (6, 64, 65). In addition, the E4orf6 protein inhibits transcriptional activation by p53 (16), results in degradation of p53 (59), and has oncogenic potential (32). One mechanism suggested for enhancing rAAV transduction by E4orf6 is dephosphorylation of a single-stranded-DNA-binding protein that recognizes the D sequence of the viral ITR and inhibits second-strand synthesis (47, 48), although the identity of the protein and its kinase are not yet known. To begin to decipher the function of E4orf6 in creation of an environment suitable for AAV transduction, we examined the effect of this protein on key regulators of the cell cycle. In this report we show that induction of E4orf6 expression in 293 cells results in an accumulation of cells in S phase. Concomitant with disruption of cell cycle progression is a decrease in the level of the cyclin A protein, induced posttranscriptionally by E4orf6 expression. Overexpression of cyclin A by cotransfection inhibits the augmentation of rAAV transduction mediated by E4orf6. In addition, we have identified a motif in the E4orf6 protein that is essential for rAAV augmentation. A greater understanding of the pathways used by E4orf6 to augment rAAV transduction will suggest strategies to improve the efficiency of rAAV vectors for human gene therapy applications.
MATERIALS AND METHODS
Plasmids.
The E4orf6 coding region was amplified from Ad type 5 (Ad5) DNA by PCR and subcloned under the control of the cytomegalovirus immediate-early promoter in expression vector pcDNA3.1 (Invitrogen) or pRK5. The E4orf6.AXA mutant was created by using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) to change residues R243 and L245 to alanine and then subcloned into pRK5. pRK5.cyclinA was constructed by subcloning an EcoRI fragment of pCycA into pRK5. The pRK5.cyclinB, pRK5.cyclinE, and pCMV.cdk2 constructs were generously provided by T. Hunter. pcDNA.cyclinAhpm was kindly provided by B. Schulman. Wild-type human p53 was expressed from the simian virus 40 promoter in pSV2.p53 (63), and the reporter for p53-specific transactivation was pPG13.Luc (20). These plasmids were gifts from T. Halozonetis and W. El-Deiry, respectively.
Cell culture, transfection, and reporter assays.
All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The inducible E4orf6 cell line 10-3 has been previously described and characterized (23, 24). It was generated by stable transfection of 293 cells with the plasmid pMT-E4orf6, which expresses the E4orf6 gene under control of the zinc-inducible sheep metallothionein promoter. Mouse embryonic fibroblasts (MEFs) (passage 9 or less) from knockout mice, p53+/− or p53−/−, were kindly provided by G. Wahl and C. Barlow. All other cell lines were obtained from the American Type Culture Collection. Where indicated, cells were irradiated with a Stratalinker UV cross-linker (Stratagene).
Transfections were performed in duplicate by calcium phosphate precipitation by standard methods and repeated multiple times. The transfections were performed on cells plated in 35-mm-diameter plates. Total amounts of DNA were maintained at 4.5 μg in all transfection assays. For luciferase assays, cells were transfected with 1.0 μg of the pPG13.Luc reporter, 1.0 μg of pSV2.hp53 or pSV2.hp53.C273, and 1.5 μg of pcDNA, pRK5.E4orf6 or pRK5.E4orf6.AXA. The cells were harvested for luciferase activity 30 h posttransfection as specified by the manufacturer (Promega). Luciferase activity was measured in a Luminometer (Berthold) and normalized for transfection efficiency by determining β-galactosidase activity from cotransfected pCMVβ.
Viruses and transduction assays.
Recombinant AAV expressing the Escherichia coli lacZ gene or the Aequorea victoria green fluorescent protein (GFP) under the control of the cytomegalovirus promoter were generated and purified by standard methods as previously described (23, 69). Titers of virus were determined by dot blot hybridization. Cells were infected with rAAV (1,000 genomes/cell) for 2 h in DMEM–2% FBS and then replaced with fresh medium containing 10% serum. For rAAV.LacZ, transduction was determined by assessing β-galactosidase expression with histochemical staining in situ (23, 66) or by measuring β-galactosidase activity in cell extracts following a 24-h infection with rAAV-LacZ, using the GalactoLight kit (TROPIX). For rAAV.GFP, cells were viewed under a confocal microscope (kindly provided by F. Gage). When indicated, the cells were either induced to express E4orf6 for 24 h by addition of zinc (ZnSO4) or transfected 24 h prior to infection. Wild-type Ad5 and E1-deleted recombinant viruses expressing GFP or p53 were propagated in 293 cells and purified by sequential rounds of ultracentrifugation in CsCl gradients. Titers were determined by plaque assays on 293 cells. The E4 mutant dl1004 was propagated and subjected to titer determination on W162 complementing cells.
Western blotting.
Cells were lysed in lysis buffer (50 mM Tris-HCl [pH 7.6], 1% Nonidet P-40, 150 mM NaCl, 0.1 mM ZnOAc) with proteinase inhibitors and normalized for protein concentration by the Lowry assay (Bio-Rad). Equal amounts of protein (50 to 100 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting was performed to detect specific proteins. For time course experiments, treated and untreated samples were normalized for protein content at each time point and an equal volume of lysate was loaded for all time points. E4orf6 protein was detected with monoclonal antibody 45 (MAb45) (38). Primary antibodies specific to the following proteins were purchased from Santa Cruz (Santa Cruz, Calif.): p53 (DO-1), cyclin A (BF683), cyclin B (GSN-1), cyclin D (HD11), cyclin E (HE111), cdk2 (M2), and proliferating-cell nuclear antigen (PCNA) (PC10). Antibodies to p21 (Ab-1) and cdc2 (Ab-1) were purchased from Oncogene. The antiphosphotyrosine antibody was purchased from Upstate Biotechnology. Proteins were detected by enhanced chemiluminescence (NEN, Boston, Mass.) as specified by the manufacturer.
Northern blot analysis.
Total RNA was extracted with ULTRASPEC (BIOTECX), supplemented with linear acrylamide (Ambion, Austin, Tex.), as specified by the manufacturer. A modification of the Northern blot method described by Burnett (7) was used. Briefly, RNA was denaturated and electrophoresed as published (7). It was transferred to a nylon filter in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and was fixed with a UV cross-linker (Stratagene). Filters were stained with methylene blue to ensure equal loading and transfer. Hybridization was performed with the rapid-hybridization buffer (Amersham, Little Chalfont, United Kingdom) as specified by the manufacturer. Radiolabeled probes were synthesized with the Rediprime kit (Amersham) and purified on G-50 columns (Amersham).
FACS analysis.
To establish exponential-phase cultures of 10-3 cells, the cells were seeded at a low density in six-well plates containing DMEM supplemented with 10% FBS. At 24 h later the cells were fed with fresh growth medium containing either zinc at various concentrations, aphidicolin, or zinc plus alphidicolin. At various time points posttreatment, the cells were trypsinized, collected, and washed twice with cold phosphate-buffered saline. The cell pellets were thoroughly resuspended in 2 ml of cold 80% ethanol and stored at 4°C. On the day of the fluorescence-activated cell sorter (FACS) analysis, cells were collected by centrifugation and the pellets were resuspended in 0.5 ml of propidium iodide staining buffer (5 μg of propidium iodide per ml, 0.5% Tween 20, and 1% bovine serum albumin in phosphate-buffered saline). The cell suspension was incubated in the dark at room temperature for 15 min and analyzed on an EPICS XL flow cytometer (Coulter Corp., Hialeah, Fla.) for DNA content. Excitation was carried out with the 488-nm lines of an argon ion laser operating at a continuous output of 200 mW. Cell cycle analysis by DNA distribution was performed by the MultiCycle AV software (Phenix Flow Systems, San Diego, Calif.).
Kinase assays.
Cdc2 kinase assays were performed with the cdc2 kinase assay kit (Upstate Biotechnology) as specified by the manufacturer. For cdk2 kinase assays, cells were lysed in RIPA buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 0.2% Nonidet P-40, 0.2% Triton X-100, 0.2% deoxycholate, 1 mM dithiothreitol, 10% glycerol) and supplemented with protease inhibitors. cdk2 proteins were immunoprecipitated, and the immune complexes were washed twice in kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2, 1 mM dithiothreitol, 10 mM NaF, 10 mM β-glycerophosphate). After a 60-min incubation at 30°C in 25 μl of kinase buffer containing 25 μM ATP, 5 μCi of [γ-32P]ATP, and 1 μg of histone H1, the kinase reaction was terminated by adding an equal volume of 2× sample buffer (100 mM Tris [pH 6.8], 20% glycerol, 4% SDS, 0.1% bromophenol blue). Kinase activity was assessed by gel electrophoresis and PhosphorImager analysis (Molecular Dynamics).
RESULTS
Induction of E4orf6 disrupts progression through the cell cycle.
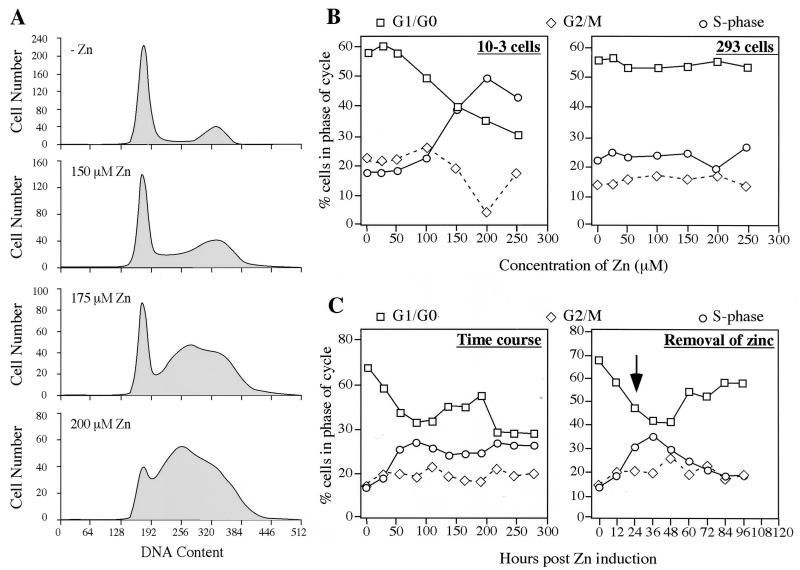
We have generated a cell line called 10-3, which is derived from the human embryonic kidney cell line 293 and expresses the E4orf6 protein under control of a zinc-inducible promoter (24). We have previously demonstrated dramatic augmentation of rAAV transduction in these 10-3 cells upon induction of E4orf6 (23). This cell line is therefore an excellent model system in which to study both the effect of E4orf6 expression on the host cell and the requirements for efficient rAAV transduction. In the present study we first investigated the impact of E4orf6 expression on cell cycle progression. Using FACS analysis, we analyzed the effect of zinc-induced E4orf6 expression on the distribution of cells at each stage of the cell cycle. Induction of E4orf6 in 10-3 cells resulted in significant alterations to the cell cycle profile (Fig. 1A). With increasing concentrations of zinc, we observed an increase in the number of 10-3 cells in S phase, combined with a decrease in the number of these in G1 and G2. The proportion of cells in each of the different phases of the cell cycle was quantitated as a function of different concentrations of zinc, as shown in Fig. 1B. Some experimental variation was observed, but the S-phase accumulation was observed in all experiments. The number of dead cells was consistently below 8% under all conditions. The parental 293 cell line was treated and analyzed in parallel. No significant change in cell cycle profile was observed in 293 cells with similar concentrations of zinc (Fig. 1B). This demonstrated that the accumulation in S phase observed for 10-3 cells can be attributed to E4orf6 expression and not to zinc treatment. Cell cycle profiles were analyzed over a time course of E4orf6 induction with 150 μM zinc (this concentration produced the S-phase accumulation but showed minimum toxicity). This showed that within 24 to 40 h of induction, the number of cells in S phase had peaked at almost 40% and that this was accompanied by a dramatic decrease in the number of cells in the G1 phase (Fig. 1C). This effect could be reversed by the removal of zinc at 24 h postinduction (Fig. 1C).
FIG. 1.
Effect of E4orf6 expression on cell cycle progression. The cell line 10-3 was derived from 293 cells after stable transfection with pMT-E4orf6 and expresses the E4orf6 protein under control of the zinc-inducible sheep metallothionein promoter (24). (A) Exponentially growing 10-3 cells in the presence or absence of zinc induction were studied for DNA content by propidium iodide staining and FACS. (B) Both 10-3 and 293 cells were incubated with increasing concentrations of zinc, and the proportion of cells in each stage of the cell cycle was determined after 24 h. (C) E4orf6 expression in 10-3 cells was induced with 150 μM zinc, and the time course of cell cycle alterations was observed by FACS. For one set of cells, zinc was removed after 24 h (indicated by an arrow), and the effect of E4orf6 expression was reversible.
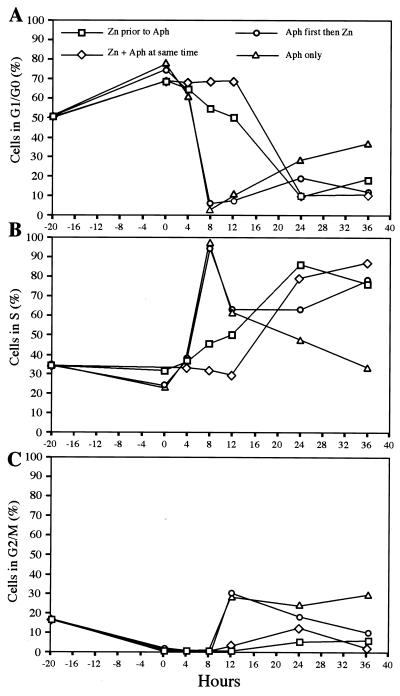
These results suggested that E4orf6 expression either promotes cell entry into S phase or prevents exit from S phase. To ascertain where in the cell cycle the block occurred, we performed further FACS analysis after synchronization of 10-3 cells with aphidicolin in the presence or absence of E4orf6 induction with zinc (Fig. 2). Treatment for 20 h with aphidicolin (0.5 μg/ml) led to a partial (60 to 80%) synchronization of cells in G1/G0 (Fig. 2A). In the absence of E4orf6 expression, release of the aphidicolin block allowed almost 100% of cells to enter S phase within 8 h (Fig. 2B). This was accompanied by a drop in the number of cells in G1, and after 8 h cells began to enter G2/M (Fig. 2C). Within 36 h, the cell cycle profile had returned to normal. However, for cells that were induced for E4orf6 expression with zinc either before, during, or after synchronization, this pattern was altered. For cells induced before or at the same time as aphidicolin treatment, the increase in the number in S phase is delayed, and this is accompanied by a gradual decrease in the number G1/G0. These cells express E4orf6 and fail to accumulate in G2/M (Fig. 2C). Cells which are first arrested with aphidicolin and then induced with zinc rapidly move into S phase but, unlike untreated cells, do not return to a normal cell cycle profile and continue to show an accumulation in S phase (Fig. 2B). Taken together, these results suggest that E4orf6 expression leads to a slow accumulation in S phase, with a block at exit from S phase and entry into G2/M. An S/G2 block induced by resveratrol in the absence of E4orf6 expression was not sufficient to enhance rAAV transduction significantly (data not shown). This suggests that in addition to arresting cells at the S/G2 block, E4orf6 expression results in further modifications to the cell in order to augment rAAV transduction.
FIG. 2.
Effect of combining aphidicolin synchronization and E4orf6 expression in 10-3 cells. Cells were synchronized by treatment with 0.5 μg of aphidicolin per ml in the absence of zinc, together with zinc induction, before or after zinc induction. Cells were harvested at intervals after release from the aphidicolin block, and their DNA content assessed by propidium iodide staining and FACS sorting. Data is plotted to show the proportion of cells in G1/G0 (A), S (B), or G2/M (C) phases of the cell cycle.
Effect of E4orf6 expression on the level of cellular proteins in 10-3 cells.
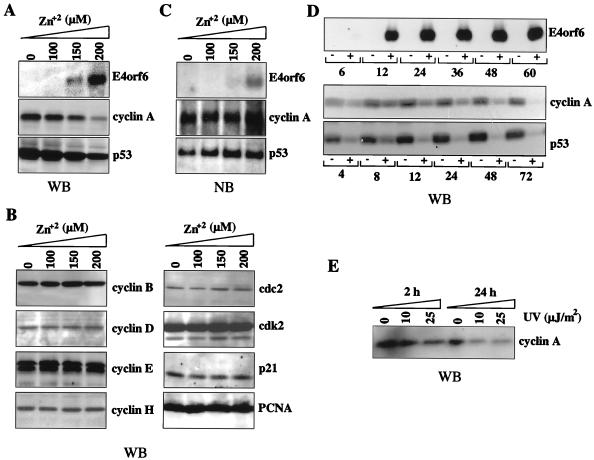
The above findings demonstrate that E4orf6 affects cell cycle regulation in the presence of the adenovirus E1 gene products expressed in 293 cells. To begin to characterize the molecular basis for arrest of cell cycle in 10-3 cells, we examined whether E4orf6 expression affects the level of cellular proteins that are key regulators of cell cycle progression. Western blotting with specific antibodies was used to investigate the steady-state levels of E4orf6 and cellular proteins in 10-3 cells treated with zinc. Upon incubation with increasing concentrations of zinc, E4orf6 induction was detected by Western blotting (Fig. 3A) and also by Northern blotting (Fig. 3B). The levels of p53 and cyclin A, two cellular proteins involved in the control of cell cycle progression, were down-regulated specifically upon E4orf6 expression (Fig. 3A). Moreover, cyclin A and p53 levels remained low in E4orf6-expressing cells 24 h after infection with rAAV (data not shown). Analysis of RNA by Northern blotting of total RNA extracted from zinc-treated cells suggested that the decrease in both p53 and cyclin A levels was a posttranscriptional event, since RNA levels did not diminish with E4orf6 expression (Fig. 3B). Time course studies showed that at a zinc concentration of 150 μM, E4orf6 expression could be demonstrated by 12 h postinduction. Alterations in the levels of cyclin A and p53 were very rapid, with detectable decreases in both by 12 h and some indication of a slight effect even earlier (Fig. 3D). The levels of both proteins increased in untreated cells over time, due to continued cell proliferation. To confirm that the effect on these protein levels was specific and not a general phenomenon, we assessed the levels of other cellular proteins involved in cell cycle progression and control. Western blotting could not detect any alterations in the steady-state levels of cyclin B, D, E, or H or in cdc2, cdk2, p21, pRb, or PCNA, 24 h following induction of E4orf6 (Fig. 3C and data not shown).
FIG. 3.
Expression of the Ad protein E4orf6 leads to the specific degradation of p53 and cyclin A. (A) Protein extracts were made from 10-3 cells grown in the absence or presence of increasing amounts of zinc, 24 h after induction. Proteins (50 μg per lane) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected by immunoblotting and enhanced chemiluminescence (Amersham). Proteins were detected with specific antibodies to E4orf6 (MAb45), p53 (DO-1), and cyclin A (BF683). (B) Total cellular RNA extracted from uninduced and zinc-induced 10-3 cells was separated on an agarose-formaldedyde gel, transferred to a nitrocellulose membrane, and hybridized with radiolabeled cDNA probes for E4orf6, cyclin A, and p53. (C) The protein levels of cyclin B, D, E, and H, as well as other cell cycle proteins such as cdc2, cdk2, p21, and PCNA, remained unchanged in 10-3 cells following a 24-h incubation with zinc. (D) Time course of E4orf6 induction and cyclin A and p53 degradation in 10-3 cells treated with 150 μM zinc. Equal volumes of cell lysate were loaded for each time point, and therefore there is an increase in protein levels for untreated cells. (E) Cyclin A was also down-regulated in 293 cells exposed to UV light at 10 or 25 μJ/m2. Treated or untreated cells were harvested 2 or 24 h following exposure, and cyclin A protein levels were detected by Western blotting using the BF683 antibody. WB, Western blotting; NB, Northern blotting.
Other treatments such as UV and genotoxic agents also enhance rAAV transduction (22, 51). It is interesting that UV treatment, at the level that enhances rAAV transduction, also leads to a decrease in the steady-state levels of cyclin A (Fig. 3E). In contrast to our observations with E4orf6, this decrease in cyclin A levels is due to down-regulation at the transcriptional level (46, 58).
p53 is neither essential nor inhibitory for augmentation of rAAV transduction by E4orf6.
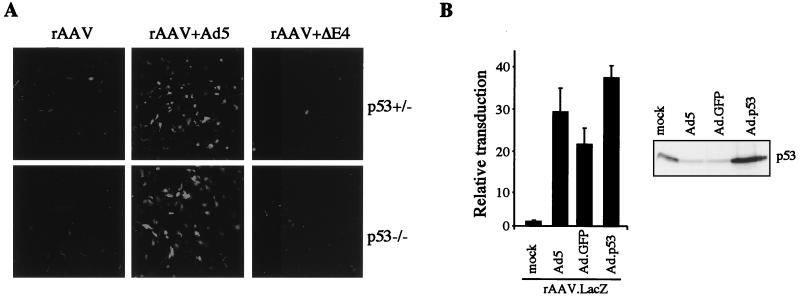
We were interested in determining whether these alterations in cellular proteins had any bearing on the augmentation of rAAV transduction by E4orf6 expression in the 10-3 cell line. The E4orf6 protein binds p53 and blocks its ability to activate transcription (16). Inhibition of p53 activity and degradation of the protein by E4orf6 might be functionally important for its augmentation effect. If this were the case, one might expect adenovirus-mediated augmentation of rAAV transduction not to require E4orf6 in cells that lack p53. Conversely, overexpression of p53 might be expected to prevent the E4orf6 augmentation effect. We therefore tested rAAV transduction in cell lines lacking p53. In the Saos2 cell line, which lacks functional p53, augmentation of rAAV transduction by adenovirus infection still required E4orf6 (66a). It is possible that this cell line has acquired further mutations that may affect the interpretation. Therefore, we performed the experiment in early-passage MEFs obtained from mutant mice. MEFs from knockout p53−/− mice showed transduction similar to MEFs from wild-type or heterozygous mice (Fig. 4A). The results with MEFs reflect those seen with all other cell lines so far examined (22, 23). Transduction by rAAV alone showed low levels of gene expression that could be dramatically enhanced by coinfection with wild-type Ad5 but not by a mutant adenovirus (dl1004) with the entire E4 region deleted (Fig. 4A). This result shows that even in the absence of p53, E4orf6 is necessary for the augmentation effect of adenovirus infection. To assess whether p53 might inhibit E4orf6-mediated augmentation of rAAV transduction, we transduced cells with rAAV.LacZ in the presence of p53 overexpression. This was done with 293 cells either by coinfection with a recombinant Ad-expressing p53 (Fig. 4B) or by cotransfection of E4orf6 with a p53 plasmid (Fig. 6B). In neither case was there inhibition of the E4orf6-mediated augmentation of rAAV transduction. Despite degradation of p53 during Ad infection (25, 49, 59), the levels in cells infected with the recombinant Ad-p53 remained high, due to the elevated level of overexpression from the constitutive CMV promoter, as confirmed by Western blotting (Fig. 4B). Together, these results show that p53 is neither necessary nor inhibitory for the augmentation of rAAV transduction by E4orf6.
FIG. 4.
p53 is neither necessary nor inhibitory for augmentation of rAAV transduction by Ad. (A) Augmentation of rAAV transduction by Ad in MEFs from p53−/− knockout mice requires E4orf6. MEFs (passage 9) were transduced with rAAV.GFP (1,000 genomes/cell) alone or in the presence of wild-type Ad5 (multiplicity of infection, 50 PFU/cell) or the E4 mutant dl1004 (AdΔE4). GFP expression was assessed after 48 h by using a confocal microscope. (B) Overexpression of p53 does not prevent augmentation of rAAV transduction by Ad. 293 cells were transduced with rAAV.LacZ (1,000 genomes/cell) alone or in the presence of Ad5, Ad.GFP, or Ad.p53 (multiplicity of infection, 100 PFU/cell). Transduction was assessed by measuring β-galactosidase activity in cell extracts after 24 h. Overexpression of p53 was confirmed by Western blotting of cell extracts with a p53-specific antibody (DO-1).
FIG. 6.
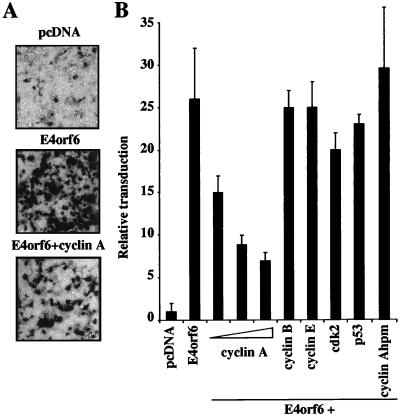
Cyclin A overexpression inhibits augmentation of rAAV transduction by E4orf6. (A) Human 293T cells were transfected with pcDNA-GFP (pcDNA) or E4orf6 or cotransfected with E4orf6 and cyclin A. Cells were infected with rAAV.LacZ (1,000 genomes/cell) at 24 h posttransfection, and transduction was assessed after a further 24 h by histochemical staining for β-galactosidase activity in situ. (B) Inhibition is specific to cyclin A, whereas other cell cycle proteins and the cyclin A hydrophobic patch mutant (hpm) had no effect on E4orf6-mediated augmentation. Plasmids expressing the indicated proteins were transfected into 293T cells, which were subsequently transduced with rAAV.LacZ, and β-galactosidase activity was assessed in cell extracts after a further 24 h. Presented are means and standard deviations for three to six independent experiments.
cdc2 kinase activity is inhibited upon E4orf6 expression.
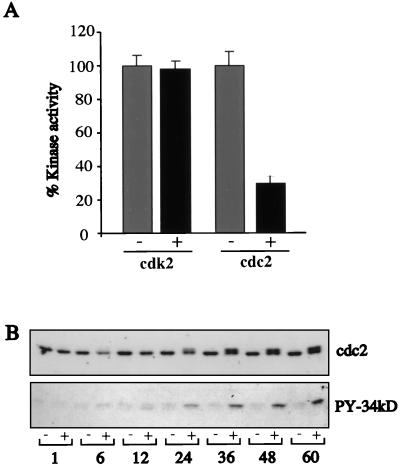
Cyclin A associates with cdk2 in S phase and with cdc2 in G2/M, to modulate the activity of these kinases. Their proper activation is crucial for cell cycle progression and DNA replication. Given the decrease in cyclin A levels, we asked whether the activity of these kinases was altered upon E4orf6 expression in 10-3 cells incubated with 150 μM zinc. Kinase activity was assessed by the ability of cdc2 or cdk2 immunoprecipitates to phosphorylate H1 histone after 36 h of E4orf6 induction in 10-3 cells. While no difference was observed for cdk2, cdc2 kinase activity was reduced by 70% (Fig. 5A). Western blot analysis of cell extracts showed that the overall steady-state levels of these two kinases were not altered upon E4orf6 induction in these cells (Fig. 3C). In addition to cyclin binding, cdc2 regulation is achieved mainly by reversible phosphorylation (29). To test whether the reduction in kinase activity was due to phosphorylation of cdc2, we analyzed the electrophoretic mobility of cdc2 after E4orf6 induction. In untreated 10-3 cells, cdc2 appeared as a single band, but a second form appeared after 24 h of E4orf6 expression (Fig. 5B). To test whether this second form of cdc2 was due to tyrosine phosphorylation, proteins phosphorylated on tyrosine residues were immunoprecipitated and immunocomplexes were subjected to Western blotting with antibodies specific for cdc2. A strong band corresponding to cdc2 was observed 24 h following E4orf6 induction (Fig. 5B). These results suggest that in the presence of E4orf6, the kinase activity of cdc2 may be inhibited due to phosphorylation on a tyrosine residue.
FIG. 5.
The kinase activity of cdc2 is inhibited following expression of E4orf6. (A) Immunoprecipitates from 10-3 cells cultured in the absence (−) or presence (+) of 150 μM zinc for 36 h were tested for their ability to phosphorylate H1 histone in vitro. Kinase activity was inhibited for cdc2 but not for cdk2. (B) Protein extracts were made from 10-3 cells in the absence or presence of zinc induction (150 μM) at different intervals between 1 and 60 h. Proteins (50 μg per lane) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected by immunoblotting with a cdc2 antibody (top). Note the appearance of a higher-molecular-weight species by 24 h after E4orf6 induction. Tyrosine-phosphorylated proteins (PY) were immunoprecipitated from these extracts and subjected to Western blot analysis with a cdc2-specific antibody (bottom).
Overexpression of cyclin A inhibits the augmentation of rAAV transduction by E4orf6.
We then asked whether the decrease in cyclin A levels observed in 10-3 cells contributed to the E4orf6 effect on rAAV transduction. Expression vectors for cyclin A, B, or E or cdk2 were cotransfected in 293 cells together with the E4orf6 plasmid. Cells were subsequently infected with rAAV.LacZ at 24 h posttransfection and assessed for transduction after a further 24 h (Fig. 6). Cyclin A overexpression had a dramatic inhibitory effect on the ability of E4orf6 to augment rAAV transduction. This was dose dependent and clearly visible by both histochemical staining of transduced cells with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Fig. 6A) and β-galactosidase assays on cellular extracts (Fig. 6B). This effect was specific to cyclin A, since none of the other proteins had any significant effect.
A conserved hydrophobic patch has been identified on the surface of cyclin A, and this region has been demonstrated to mediate binding to RXL-containing proteins (57). We tested the ability of a cyclin A hydrophobic patch mutant, CyAhpm (with mutations M210A, L214A, and W217A), which does not interact with the RXL domain (57), to inhibit the E4orf6-mediated augmentation of rAAV transduction. This mutant had no inhibitory effect on E4orf6-mediated augmentation (Fig. 6B). The specific degradation of cyclin A upon E4orf6 induction, together with the inhibition of E4orf6 augmentation by cyclin A overexpression, suggests a link between cyclin A and E4orf6-mediated enhancement of rAAV transduction.
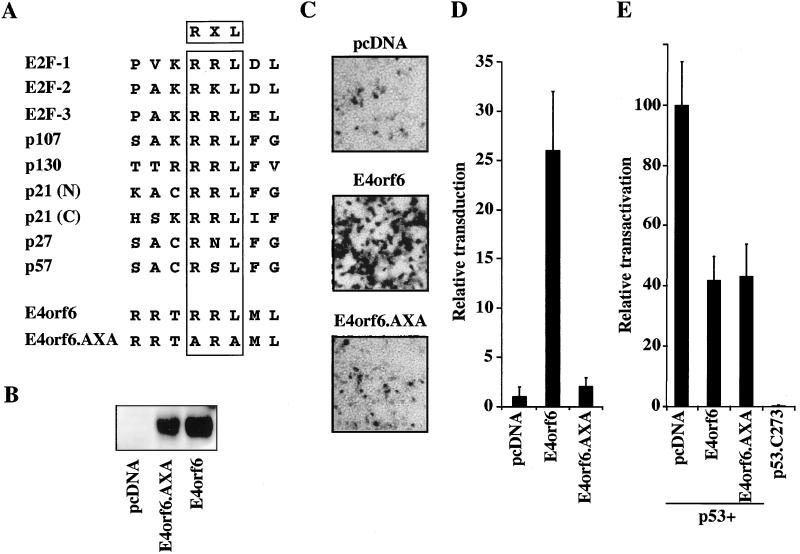
The E4orf6 protein contains a putative RXL motif that is essential for augmentation of rAAV transduction but not other functions.
We noted that the E4orf6 protein contained a domain similar to the RXL motif identified in proteins that either interact with cyclin A or are substrates for cdk-cyclin A-mediated phosphorylation (Fig. 7A). To assess whether this motif played any role in augmentation of rAAV transduction by the E4orf6 protein, the RXL sequence was changed to AXA by site-directed mutagenesis (mutations R243A and L245A). Western blotting with MAb45 showed expression of the mutant protein at a similar level to that of the wild-type protein. (Fig. 7B). In an assay for augmentation, wild-type or mutant E4orf6 was transiently expressed in 293 cells, which were infected with rAAV.LacZ after 24 h, and the degree of transduction was assessed by X-Gal staining of cell monolayers (Fig. 7C) or β-galactosidase assays on cell extracts (Fig. 7D). The E4orf6.AXA mutant had completely lost its ability to augment rAAV transduction, suggesting that this region may play a role in the enhancement effect.
FIG. 7.
The E4orf6 protein contains a putative RXL domain that is necessary for augmentation of rAAV transduction but not for other functions of the protein. (A) E4orf6 contains a putative RXL domain. Sequence alignments of the putative RXL domains of cyclin A binding proteins and E4orf6. This region was altered in the E4orf6.AXA mutant, as indicated. (B) Western blot of cell extracts from 293T cells transfected with expression vectors pcDNA-GFP, E4orf6, or E4orf6.AXA confirmed expression of the E4orf6 proteins. (C) The E4orf6.AXA mutant is unable to augment rAAV transduction. Human 293T cells were transfected with either pcDNA-GFP (pcDNA), E4orf6, or E4orf6.AXA and infected with rAAV-LacZ (1,000 genomes/cell) at 24 h posttransfection. The cells were fixed 24 h postinfection and stained for β-galactosidase activity in situ. (D) Cells were treated as in panel C and quantitated for β-galactosidase activity. (E) The E4orf6.AXA mutant retains its ability to inhibit p53-mediated transactivation. Saos2 cells were transfected with expression vectors for wild-type or mutant p53, E4orf6, E4orf6.AXA, empty vector, and the PG13.Luc reporter plasmid. After 30 h, cells were harvested for luciferase activity.
A number of activities have been ascribed to the E4orf6 protein in addition to its ability to act as a helper for AAV replication and transduction. One of the functions reported for this protein is its ability to inhibit p53-mediated transcriptional activation (16). We examined the effect of the AXA mutation on E4orf6 inhibition of transcriptional activation by p53 in transient-transfection assays. Saos2 osteosarcoma cells, which lack endogenous p53, were transfected with a plasmid expressing the E4orf6 protein, together with a plasmid encoding p53, and a luciferase reporter plasmid containing p53-binding sites cloned upstream of a minimal promoter. As previously reported, transactivation by p53 was inhibited by the wild-type E4orf6 protein (16) to about 40% of its normal activity (Fig. 7E). The E4orf6.AXA mutant inhibited p53-mediated transcriptional activation in a similar manner to the wild-type protein (Fig. 7E). These results demonstrate that the E4orf6.AXA mutant retains at least some of the functions of the wild-type protein.
DISCUSSION
To understand how the Ad E4orf6 protein modifies the cellular millieu to enhance rAAV transduction, we have examined the effect of this protein on cell cycle regulators. We found that induction of E4orf6 expression in a cell line based on 293 cells, leads to accumulation of cells in S phase. A number of observations suggest that this alteration in cell cycle progression may play a role in the AAV life cycle. Transduction by rAAV vectors is known to occur preferentially in cells which are in S phase (52), and so accumulation in this phase could be partly responsible for enhanced rAAV transduction obtained in the presence of E4orf6. Duplex formation for the related autonomous parvoviruses also requires factors present in S-phase cells (13). Thus, AAV may benefit from the alteration in cell cycle induced by Ad helper proteins.
Concomitant with disruption of cell cycle progression, we observed a decrease in the levels of cyclin A and p53 proteins, induced posttranscriptionally by E4orf6 expression. Others have reported a similar degradation of p53 in BRK and 293 cell lines upon E4orf6 expression and also in Ad-infected cells (32, 36, 49, 59). However, the augmentation of rAAV transduction in p53−/− MEFs and in the presence of p53 overexpression suggests that degradation of p53 is not a prerequisite for rAAV transduction or E4orf6-mediated augmentation. In contrast, overexpression of cyclin A by cotransfection inhibits the augmentation of rAAV transduction mediated by E4orf6. This suggests that cyclin A levels are relevant to augmentation of rAAV transduction by E4orf6.
Checkpoints maintain the order and fidelity of cell cycle events. Although most of the regulators of cell cycle checkpoints were initially characterized in yeast, several mammalian homologues have been described (21, 54). The DNA replication checkpoint ensures that only cells which have successfully completed one round of DNA replication will undergo cell division. While crucial to cell viability, these checkpoints are not necessary during viral replication. The cyclin A-cdk2 complex phosphorylates many proteins substrates associated with cell cycle progression and DNA replication. Cells which enter S phase due to the action of the cyclin E-cdk2 complex would not go through a successful replication process in the absence of cyclin A and would therefore not proceed with the cell cycle. This situation would be ideal for both AAV transduction and replication, since the cellular DNA replication machinery assembled by the cell can then be harnessed by the virus. Down-regulation of cyclin A-cdk2 and cyclin A-cdc2 kinase activities by specific degradation of cyclin A by E4orf6 may represent a viral mechanism that interrupts cellular DNA replication to enhance viral production. This may play a role in Ad replication, and the helper-dependent AAV takes advantage of the effect. Expression of E4orf6 inhibits the activity of cdc2 in two ways, by degrading cyclin A and also by affecting the phosphorylation status of cdc2 directly. It has been suggested that failure to pass a DNA replication checkpoint results in the inhibition of cdc25 activity by the action of protein kinases such as Cds1 (34, 43, 70) and its recently identified mammalian homologue Chk2 (31). The inhibition of the cdc25 phosphatases ensures that cdc2 is kept inactive until the completion of DNA replication to prevent the transition to mitosis in the presence of unreplicated DNA. If E4orf6-expressing cells are not able to complete cellular DNA replication successfully due to the lack of cyclin A, the cdc25 phosphatases will probably be phosphorylated on inhibitory residues, which will render them unable to dephosphorylate cdc2 and initiate mitosis. We have observed hyperphosphorylated forms of cdc25 proteins following E4orf6 expression, which is consistent with this model (12a). The outcome of E4orf6 expression is thus inactivation of cdc2 kinase activity, which may be responsible for the block to exit from S phase. It is interesting that after UV irradiation, which causes a cell cycle arrest due to a failure to pass a DNA damage checkpoint, cyclin A levels also fall and cdks are inactivated by a combination of p21 and phosphorylation (46). A similar situation has been reported for wild-type AAV infection of primary human cells, in which cells arrest due to transcriptional down-regulation of cyclin A and induction of p21 (25b).
We noted that the E4orf6 protein contains a sequence that resembles an RXL motif. Mutation of this region abolishes the ability to enhance rAAV transduction, suggesting that the RXL motif may play a role in the augmentation effect. Transient-transfections and reporter gene assays showed that the E4orf6.AXA mutant retained the ability of the wild-type protein to inhibit transcriptional activation by the cellular p53 protein. This demonstrates that the protein is at least partly functional. This mutant protein presents a tool to be used in determining which functions of E4orf6 are relevant to enhancement of rAAV transduction. One of the proteins that associates with E4orf6 is the Ad E1b 55-kDa protein (14, 56). Viruses with the E1b gene deleted also show a decrease in their ability to augment rAAV transduction, suggesting that E1b may play a role (23). In this report, we have analyzed E4orf6 augmentation only in the context of 293 cells which also express Ad E1 genes. One explanation for the lack of augmentation by the E4orf6.AXA mutant might be its inability to form a functional complex with the E1b 55-kDa protein. The region responsible for the interaction between these two proteins had been previously mapped to the N-terminal 55 amino acids of the E4orf6 protein (50), and the mutations incorporated into the AXA protein are at residues 243 and 245. We have found that E4orf6.AXA is unable to bind to E1b, relocate E1b to the nucleus, and lead to p53 degradation (11a). In agreement with these observations, it has been recently reported that a region of the E4orf6 protein between amino acids 241 and 250 forms an arginine-faced α-helix which is required for E1b nuclear localization by E4orf6 (40). It is possible that the RXL domain in the amphipathic α-helix of E4orf6 is necessary for interaction with a cellular protein required for relocalization of E1b and replication of Ad.
Alternatively, phosphorylation of either protein may be important for their association. Both the E1b 55-kDa protein and E4orf6 are phosphoproteins (4, 60), and the AXA mutation may result in altered phosphorylation. The putative RXL motif in E4orf6 raises the intriguing possibility that it can act as a substrate and bind to cyclin A. We have been unable to detect a direct interaction between these two protein (25a), but this could be due to the transient nature of the association and to the fact that E4orf6 expression leads to rapid cyclin A degradation. Phosphorylation may be a clue to how E4orf6 achieves its enhancement of rAAV transduction. One cellular protein suggested to be a target of E4orf6 is a single-stranded DNA binding protein that recognizes the D region of the viral ITR and prevents second-strand synthesis (48). The tyrosine phosphorylation status of this protein correlates well with the efficiency of rAAV transduction in human cells in vitro and murine cells in vivo (47). Treatments that augment rAAV transduction, such as E4orf6 expression or hydroxyurea, result in dephosphorylation of the single-stranded DNA binding protein as assessed by a shift in the complex in an electrophoretic gel mobility shift assay (48). Our data suggests that there may be links between E4orf6 and the regulation of phosphorylation for additional cellular proteins. A potential cellular target for E4orf6 might be the human replication protein A (RPA), although there is presently no published data to support this. RPA is composed of three subunits of 70, 34, and 11 kDa and plays an essential role in initiation and elongation of DNA replication (reviewed in reference 67). The protein is phosphorylated in a cell cycle-dependent manner in human cells, primarily on the 34-kDa subunit, and is mediated by the cdc and cdk kinases (15, 19). By using in vitro replication assays, it has been shown that RPA is involved in AAV replication (37, 62). It is possible that part of the E4orf6 enhancement effect is mediated through RPA, perhaps by changes to its phosphorylation status. It is interesting that some of the effects on cellular proteins that we have uncovered for E4orf6 are shared with other treatments known to enhance rAAV transduction, such as UV irradiation and DNA-damaging agents. These treatments also lead to down-regulation of cyclin A levels, phosphorylation and negative regulation of cdc25 proteins, a block to entry into mitosis, and changes in phosphorylation of RPA (11). Transduction by rAAV vectors in vivo can occur efficiently in nondividing cells and may occur by a different mechanism. However, since there is a delay to gene expression in most in vivo settings, even in vivo transduction will benefit from understanding the pathways involved and the requirements for efficient gene expression from rAAV vectors. Although there may be multiple routes to enhancement of rAAV transduction (17, 55), there may be common pathways that are activated by enhancing agents. Understanding the requirements for second-strand synthesis and the ways in which helper viral proteins can promote this step, will greatly enhance the usefulness of rAAV as a gene therapy vector.
ACKNOWLEDGMENTS
We thank T. Hunter, B. Schulman, W. El-Deiry, and T. Halozonetis for plasmids; P. Hearing for E4orf6-specific antibody MAb45; G. Wahl and C. Barlow for MEFs; G. Ketner and W. El-Deiry for viruses; W. Cordier for technical assistance in virus production; and F. Gage for use of the confocal microscope. We also thank T. Hunter and T. Stracker for helpful discussions and comments on the manuscript. M.D.W. is grateful to I. Verma and F. Gage for continued support and encouragement.
This work was supported by the Oracle Corporate Giving Program (M.D.W.), an Innovation Grant from the President’s Club of the Salk Institute (M.D.W.), Odette Wurzburger (M.D.W.), the National Institute of Diabetes, Digestive and Kidney Disorders of NIH (J.M.W.), the Cystic Fibrosis Foundation (M.D.W. and J.M.W.), and by Genovo, Inc., a company that J.M.W. founded and holds equity in.
REFERENCES
- 1.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W G., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander I E, Russell D W, Miller A D. DNA-damaging agents greatly increase the transduction of non-dividing cells by adeno-associated virus vectors. J Virol. 1994;68:8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berns K I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2173–2197. [Google Scholar]
- 4.Boivin D, Morrison M R, Marcellus R C, Querido E, Branton P E. Analysis of synthesis, stability, phosphorylation, and interacting polypeptides of the 34-kilodalton product of open reading frame 6 of the early region 4 protein of human adenovirus type 5. J Virol. 1999;73:1245–1253. doi: 10.1128/jvi.73.2.1245-1253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridge E, Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 6.Bridge E, Medghalchi S, Ubol S, Leesong M, Ketner G. Adenovirus early region 4 and viral DNA synthesis. Virology. 1993;193:794–801. doi: 10.1006/viro.1993.1188. [DOI] [PubMed] [Google Scholar]
- 7.Burnett W. Northern blotting of RNA denatured in glycerol without buffer recirculation. BioTechniques. 1997;22:668–671. doi: 10.2144/97224st01. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso M C, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- 9.Carter B J. Adeno-associated virus helper functions. In: Tijssen P, editor. Handbook of parvoviruses. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 255–282. [Google Scholar]
- 10.Carter B J. Adeno-associated virus vectors. Curr Opin Biotechnol. 1992;3:533–539. doi: 10.1016/0958-1669(92)90082-t. [DOI] [PubMed] [Google Scholar]
- 11.Carty M P, Zernik-Kobak M, McGrath S, Dixon K. UV light-induced DNA synthesis arrest in HeLa cells is associated with changes in phosphorylation of human single-stranded DNA-binding protein. EMBO J. 1994;13:2114–2123. doi: 10.1002/j.1460-2075.1994.tb06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Cathomen, T., and M. D. Weitzman. Unpublished observations.
- 12.Chen J, Saha P, Kornbluth S, Dynlacht B D, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Chen, N. N. Unpublished observations.
- 13.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 14.Cutt J R, Shenk T, Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987;61:543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Din S, Brill S J, Fairman M P, Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990;4:968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- 16.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 17.Duan D, Sharma P, Dudus L, Zhang Y, Sanlioglu S, Yan Z, Yue Y, Ye Y, Lester R, Yang J, Fisher K J, Engelhardt J F. Formation of adeno-associated virus circular genomes is differentially regulated by adenovirus E4 ORF6 and E2a gene expression. J Virol. 1999;73:161–169. doi: 10.1128/jvi.73.1.161-169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Urso G, Marraccino R L, Marshak D R, Roberts J M. Cell cycle control of DNA replication by a homologue from human cells of the p34cdc2 protein kinase. Science. 1990;250:786–791. doi: 10.1126/science.2173140. [DOI] [PubMed] [Google Scholar]
- 19.Dutta A, Stillman B. cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 1992;11:2189–2199. doi: 10.1002/j.1460-2075.1992.tb05278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 21.Elledge S. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao G-P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grand R J, Grant M L, Gallimore P H. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology. 1994;203:229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- 25a.Grifman, M. Unpublished data.
- 25b.Hermanns J, Schulze A, Jansen-Durr P, Kleinschmidt J A, Schmidt R, zur Hausen H. Infection of primary cells by adeno-associated virus type 2 results in a modulation of cell cycle-regulating proteins. J Virol. 1997;71:6020–6027. doi: 10.1128/jvi.71.8.6020-6027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen-Dürr P. How viral oncogenes make the cell cycle. Trends Genet. 1996;12:270–275. doi: 10.1016/0168-9525(96)81455-7. [DOI] [PubMed] [Google Scholar]
- 27.Kotin R M. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 28.Krude T, Jackman M, Pines J, Laskey R A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 29.Lew D J, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 30.MacLachlan T K, Sang N, Giordano A. Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Crit Rev Eukaryotic Gene Expression. 1995;5:127–156. doi: 10.1615/critreveukargeneexpr.v5.i2.20. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 32.Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 34.Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- 35.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 36.Nevels M, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein contributes to malignant transformation by antagonizing E1A-induced accumulation of the tumor suppressor protein p53. Oncogene. 1999;18:9–17. doi: 10.1038/sj.onc.1202284. [DOI] [PubMed] [Google Scholar]
- 37.Ni T H, McDonald W F, Zolotukhin I, Melendy T, Waga S, Stillman B, Muzyczka N. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J Virol. 1998;72:2777–2787. doi: 10.1128/jvi.72.4.2777-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obert S, O’Connor R J, Schmid S, Hearing P. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohman K, Nordqvist K, Akusjarvi G. Two adenovirus proteins with redundant activities in virus growth facilitates tripartite leader mRNA accumulation. Virology. 1993;194:50–58. doi: 10.1006/viro.1993.1234. [DOI] [PubMed] [Google Scholar]
- 40.Orlando J S, Ornelles D A. An arginine-faced amphipathic alpha helix is required for adenovirus type 5 E4orf6 protein function. J Virol. 1999;73:4600–4610. doi: 10.1128/jvi.73.6.4600-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagano M, Pepperkok R, Lukas J, Baldin V, Ansorge W, Bartek J, Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 44.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55k transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNA. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 46.Poon R Y C, Jiang W, Toyoshima H, Hunter T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J Biol Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- 47.Qing K, Khuntirat B, Mah C, Kube D M, Wang X S, Ponnazhagan S, Zhou S, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qing K, Wang X S, Kube D M, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubenwolf S, Schutt H, Nevels M, Wolf H, Dobner T. Structural analysis of the adenovirus type 5 E1B 55-kilodalton-E4orf6 protein complex. J Virol. 1997;71:1115–1123. doi: 10.1128/jvi.71.2.1115-1123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell D W, Alexander I E, Miller A D. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell D W, Miller A D, Alexander I E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 55.Sanglioglu S, Duan D, Engelhardt J F. Two independent molecular pathways for recombinant adeno-associated virus genome conversion occur after UV-C and E4orf6 augmentation of transduction. Hum Gene Ther. 1999;10:591–602. doi: 10.1089/10430349950018661. [DOI] [PubMed] [Google Scholar]
- 56.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulman B A, Lindstrom D L, Harlow E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spitkovsky D, Schulze A, Boye B, Jansen-Durr P. Down-regulation of cyclin A gene expression upon genotoxic stress correlates with reduced binding of free E2F to the promoter. Cell Growth Differ. 1997;8:699–710. [PubMed] [Google Scholar]
- 59.Steegenga W T, Riteco N, Jochemsen A G, Fallaux F J, Bos J L. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 60.Teodoro J G, Halliday T, Whalen S G, Takayesu D, Graham F L, Branton P E. Phosphorylation at the carboxy terminus of the 55-kilodalton adenovirus type 5 E1B protein regulates transforming activity. J Virol. 1994;68:776–786. doi: 10.1128/jvi.68.2.776-786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai L H, Harlow E, Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991;353:174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- 61a.Wang L, Takabe K, Bidlingmaier S M, Ill C R, Verma I M. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ward P, Dean F B, O’Donnell M E, Berns K I. Role of the adenovirus DNA-binding protein in in vitro adeno-associated virus DNA replication. J Virol. 1998;72:420–427. doi: 10.1128/jvi.72.1.420-427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterman M J F, Waterman J L F, Halazonetis T D. An engineered four-stranded colied coil substitutes for the tetramerization domain of wild-type p53 and alleviates transdominant inhibition by tumor-derived p53 mutants. Cancer Res. 1996;56:158–163. [PubMed] [Google Scholar]
- 64.Weiden M D, Ginsberg H S. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc Natl Acad Sci USA. 1994;91:153–157. doi: 10.1073/pnas.91.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg D H, Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986;57:833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitzman M D, Fisher K J, Wilson J M. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J Virol. 1996;70:1845–1854. doi: 10.1128/jvi.70.3.1845-1854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66a.Weitzman, M. D. Unpublished data.
- 67.Wold M S. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 68.Xiao X, Li J, McCown T J, Samulski R J. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 69.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng Y, Forbes K C, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]