Abstract
The optimal treatment of adult-onset Wilms tumors (WTs) in elderly patients is a debated area, as pediatric protocols are thought to carry unacceptable toxicity. We treated a 62-year-old female with good performance status and Stage IV (T1b N1 M1) favorable histology WT using pediatric adjuvant and salvage chemoradiation protocols. Though she experienced nodal relapse and both adjuvant and salvage treatment were discontinued early due to toxicity, she obtained excellent oncologic outcomes, having remained disease-free for 32 months. We recommend considering pediatric protocols for elderly WT patients with good performance status, anticipating dose reductions and possible early chemotherapy termination.
1. Introduction
Adult Wilms tumor (WT), nephroblastoma, accounting for approximately 3 % of WTs and 0.5 % of adult renal neoplasms, presents a multitude of challenges to oncologists.1,2 Cases have been reported in the literature with ages ranging as high as 84.1,3, 4, 5, 6, 7, 8 The diagnosis is often made incidentally, frequently leading to treatment delays. Additionally, there is no consensus on the optimal therapy for adult WT patients. Likely due, in part, to these factors, adults have been reported to have a worse prognosis than their pediatric counterparts.3
We present a case of a 62-year-old female with WT and subsequent metastatic recurrence who remains disease-free after salvage therapy using pediatric regimens. There have been few reports of successful treatment of metastatic WTs in adult patients.
2. Case presentation
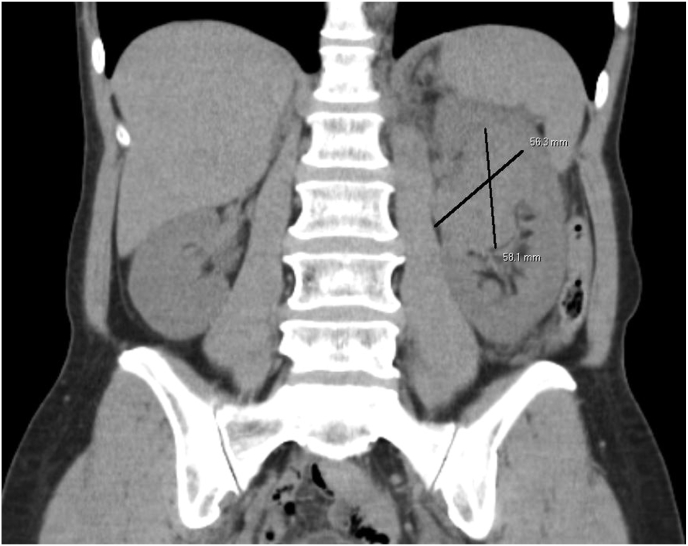
A 62-year-old previously healthy female with no familial cancer history presented to her primary care provider with a one-month complaint of left flank pain. She denied recent weight loss, fevers, chills, or gross hematuria. Urinalysis demonstrated microscopic hematuria, and subsequent Computer Assisted Tomography (CT) imaging of the abdomen and pelvis revealed a 5.6 x 5.6 × 5.8 cm mass within the left renal pelvis with a medial lymph node conglomerate along the renal vessels (Fig. 1). The mass contained scant calcifications on CT, and subsequent cystoscopy and retrograde pyeloureterogram heightened suspicion for a parenchymal renal mass. The leading differential diagnosis was renal cell carcinoma (RCC), given her age and imaging findings.
Fig. 1.
Coronal Computer Assisted Tomography (CT) image of initial finding of left-sided renal mass determined to be Wilms tumor.
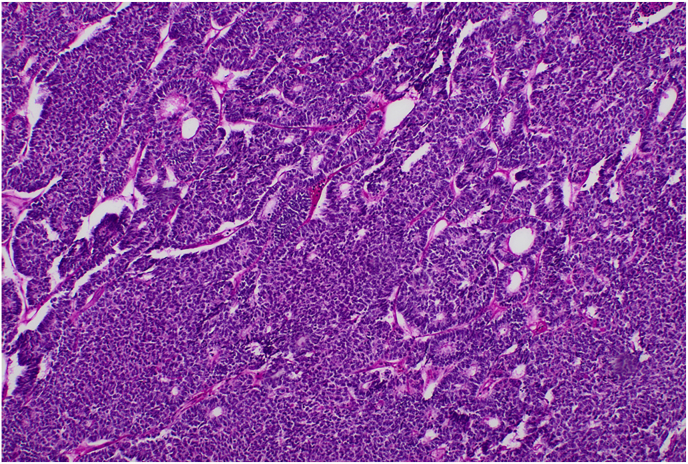
The patient was taken to the operating room for laparoscopic left radical nephrectomy and regional lymph node dissection. The specimen was removed en bloc without complication, containing a 5.2 x 4.5 × 4.5 cm tumor involving the mid-superior pole of the left kidney without capsular involvement. Upon pathologic examination, a nephroblastoma with epithelial predominance was identified. The cancer had primitive features, including a high nuclear-to-cytoplasmic ratio, increased mitoses with trabecular growth, and rosette-like tubules (Fig. 2). The tissue stained positively for WT1, CD99, PAX8, and CK7; no anaplasia was identified. Lymph node evaluation revealed a similar pathology involving 7 of 12 excised para-aortic lymph nodes. After the surgery, the patient developed clinically palpable left inguinal adenopathy, which demonstrated WT on biopsy. Based on these findings and given that the positive inguinal lymph node was not considered a draining regional node, the patient was diagnosed with a left-sided Stage IV (T1b N1 M1) favorable histology WT (FHWT). Subsequent cytogenetic testing of the specimen demonstrated copy-neutral loss-of-heterozygosity (LOH) of 11q but no abnormalities in 1p or 16q.
Fig. 2.
Hematoxylin and eosin stain of left renal mass sample demonstrating primitive appearing cells with high nuclear-to-cytoplasmic ratios, trabecular growth, and rossetting characteristic of Wilms Tumor.
The patient was evaluated by radiation and adult medical oncology with pediatric oncology consultation. After a collaborative discussion, she was initiated per the National WT Study (NWTS) approach on DD-4A chemotherapy (alternating dactinomycin [45 mcg/m2] and doxorubicin [45 mg/m2] with vincristine [2 mg on days 1, 8, and 15] in nine 21-day cycles) with concurrent radiation of 10.8 Gy in 6 fractions [10.8 Gy/6 Fx] to the surgical bed and para-aortic and L inguinal lymph nodes with an additional [9 Gy/5 Fx] boost to the undissected inguinal nodes. After developing grade 2 (G2) nausea and G3 peripheral neuropathy by cycle 3, vincristine was discontinued, and the remaining doses were reduced by 50 %. CT imaging three months after completing definitive therapy demonstrated a complete response with resolution of pelvic adenopathy and no evidence of disease.
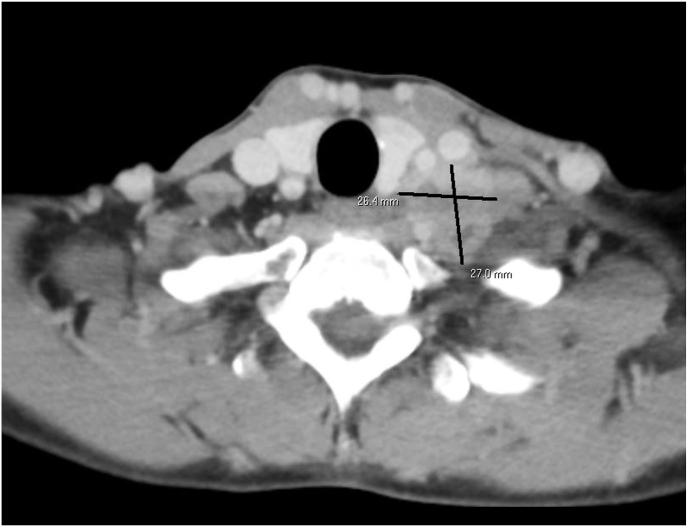
Approximately seven months later, the patient noted a palpable, tender mass in her left supraclavicular neck. Ultrasound, CT, and biopsy demonstrated a 2.6 x 2.6 × 2.5 cm lymph node conglomerate with recurrent, metastatic WT directly invading the anterior scalene muscle (Fig. 3). Systemic imaging demonstrated no other foci of disease. After careful interdisciplinary discussion, she underwent local radiation to [10.8 Gy/6 Fx] to the left supraclavicular region and axilla with a sequential boost of [9 Gy/5 Fx] to the undissected left supraclavicular nodes. She subsequently started salvage chemotherapy per the AREN1921 regimen of alternating ifosfamide [1800 mg/m2], carboplatin [370 mg], and etoposide [75 mg/m2] with cyclophosphamide [400 mg/m2] and topotecan [1.2 mg/m2] (ICE + CT). The patient experienced refractory nausea, visual changes, and neutropenia, requiring repeat hospital admissions. Due to this, doses were reduced by 20 % for cycles 4 and 5 and by 50 % for cycle 6. Chemotherapy was discontinued after 6 out of 10 planned cycles and transitioned to surveillance.
Fig. 3.
Coronal Computer Assisted Tomography (CT) image of left supraclavicular nodal recurrence.
After 32 months following treatment completion, the patient remains without evidence of disease.
3. Discussion
WT is the most common pediatric renal malignancy, making up approximately 6–7% of all childhood cancers.9 These tumors have highly specific imaging findings in the pediatric population; however, they are less distinct when found in adults. There is significant radiographic overlap with RCC, which is much more common in adults.9,10 Thus, many cases of adult WT in the United States are diagnosed unexpectedly following nephrectomy for presumed RCC. The subsequent time needed for diagnosis and referral to a specialized center often exceeds the 14-day surgery-to-radiation window recommended for optimal treatment outcomes in non-metastatic disease.11
There is no uniform consensus on optimal treatment for adults diagnosed with WT.12 Investigations utilizing multi-modal COG and SIOP pediatric regimens in adults have improved survival outcomes but demonstrated increased toxicity, particularly neuropathy and hepatotoxicity, compared to pediatric patients.13,14 We were conscious of this when considering this patient's therapy.
Our patient was initially diagnosed with Stage IV FHWT without 1p/16q LOH. Of note, she had no distant organ metastases but had involved inguinal lymph nodes, which some consider to be pelvic lymph nodes and would give a Stage III designation. Regarding treatment guidelines, NWTS 5 demonstrated a 4-year relapse-free survival of 83.0 % and overall survival (OS) of 91.9 % for combined stage III/IV pediatric patients without 1p/16q LOH treated with DD4A and radiation.15 During initial treatment discussions, we considered the potential toxicity of pediatric regimens for adult patients as well as poor OS (≤50 %) for those who relapse following treatment with 3-drug regimens.16,17 As our patient was otherwise healthy, had an excellent KPS score, and desired an aggressive treatment strategy, the pediatric regimen seemed the most appropriate offering. Although she experienced treatment-related peripheral neuropathy and stopped chemotherapy prematurely, she had a complete radiographic response.
Unfortunately, she experienced an early supraclavicular relapse. Per the rationale of AREN1921 for patients with FHWT who relapse following three-drug pretreatment, we offered ICE + CT.18, 19, 20 As can be seen even in the pediatric population, our patient tolerated the salvage therapy poorly, prompting discontinuation after six cycles. Despite this, she has remained disease-free for 32 months.
4. Conclusion
In summary, for this 62-year-old patient with relapsed metastatic WT, pediatric chemotherapy regimens were associated with treatment-limiting toxicity while providing an excellent oncologic response. Without alternative consensus guidelines for adult WT patients, we recommend following pediatric protocols for both systemic and radiation treatment in adult patients, with the expectation that toxicity may be more significant and possibly lead to early treatment termination.
Ethics approval and consent to participate
This case study is a systematic investigation designed to develop or contribute to generalizable knowledge using human subjects and de-identified data. Thus, this study is regarded as non-human subject research and does not require approval by the institutional IRB.
Consent for publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Availability of data and materials
Not applicable.
Funding
This work was produced without outside funding contributions.
CRediT authorship contribution statement
Jackson Howell: Conceptualization, Writing – original draft, Writing – review & editing. Benjamin Maughan: Conceptualization, Data curation, Writing – review & editing. Mark Fluchel: Conceptualization, Data curation, Writing – review & editing. Matthew Poppe: Conceptualization, Data curation, Writing – review & editing.
Declaration of competing interest
Dr. Maughan has received financial compensation as a paid consultant/advisor to Abbive, Pfizer, AVEO oncology, Janssen, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology, Lilly, Sanofi, Telix, Xencor and Peloton Therapeutics. The rest of the authors have no competing interests to declare.
Acknowledgements
The authors would like to thank Kiramey Gilleese, Research Manager for the Department of Radiation Oncology at the Huntsman Cancer Institute, for her assistance in editing and formatting for submission.
References
- 1.Varma A., Malukani K., Rihal P., Nandedkar S. Adult Wilms' tumor: a case report with review of literature. J Cancer Res Therapeut. 2015;11(4):934. doi: 10.4103/0973-1482.157331. [DOI] [PubMed] [Google Scholar]
- 2.Alapont J.M., Pontones J.L., Jimenez-Cruz J.F. Wilms' tumor in adults. Int Braz J Urol. 2003;29(1):40–42. doi: 10.1590/s1677-55382003000100008. [DOI] [PubMed] [Google Scholar]
- 3.Terenziani M., Spreafico F., Collini P., et al. Adult Wilms' tumor: a monoinstitutional experience and a review of the literature. Cancer. 2004;101(2):289–293. doi: 10.1002/cncr.20387. [DOI] [PubMed] [Google Scholar]
- 4.Ali E.M., Elnashar A.T. Adult Wilms' tumor: review of literature. J Oncol Pharm Pract. 2012;18(1):148–151. doi: 10.1177/1078155210396264. [DOI] [PubMed] [Google Scholar]
- 5.Kartsanis G., Douros K., Ravazoula P., Fokaefs E. Adult Wilms' tumor: a case report and review of literature. Int Urol Nephrol. 2007;39(1):3–6. doi: 10.1007/s11255-006-0029-y. [DOI] [PubMed] [Google Scholar]
- 6.Okasho K., Nishiyama H., Watanabe J., et al. Adult Wilms tumor in the renal pelvis: case report with review of the literature. Urology. 2008;72(5):1185.e5–1185.e7. doi: 10.1016/j.urology.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Gürsoy R., Akyol G., Tiras B., et al. Adult extrarenal Wilms' tumor: a case report. Gynecol Obstet Invest. 2010;40(2):141–144. doi: 10.1159/000292324. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj S., Gandhi D., Shah J., Serhal M., Sharma S. Adult Wilms tumor: an unusual case report with dedicated literature review. Clin Imag. 2022;83:138–143. doi: 10.1016/j.clinimag.2021.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Servaes S.E., Hoffer F.A., Smith E.A., Khanna G. Imaging of Wilms tumor: an update. Pediatr Radiol. 2019;49(11):1441–1452. doi: 10.1007/s00247-019-04423-3. [DOI] [PubMed] [Google Scholar]
- 10.Abou Elkassem A.M., Lo S.S., Gunn A.J., et al. Role of imaging in renal cell carcinoma: a multidisciplinary perspective. Radiographics. 2021;41(5):1387–1407. doi: 10.1148/rg.2021200202. [DOI] [PubMed] [Google Scholar]
- 11.Stokes C.L., Stokes W.A., Kalapurakal J.A., et al. Timing of radiation therapy in pediatric Wilms tumor: a report from the national cancer database. Int J Radiat Oncol. 2018;101(2):453–461. doi: 10.1016/j.ijrobp.2018.01.110. [DOI] [PubMed] [Google Scholar]
- 12.Sakthivel V., Adeeb I.Z., Vijayabalan D. Recent improvements in adult Wilms tumor diagnosis and management: review of literature. J Kidney Cancer VHL. 2023;10(3):32–36. doi: 10.15586/jkcvhl.v10i3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhard H., Aliani S., Ruebe C., Stöckle M., Leuschner I., Graf N. Wilms' tumor in adults: results of the society of pediatric oncology (SIOP) 93-01/society for pediatric oncology and hematology (GPOH) study. J Clin Oncol. 2004;22(22):4500–4506. doi: 10.1200/JCO.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 14.Kalapurakal J.A., Nan B., Norkool P., et al. Treatment outcomes in adults with favorable histologic type Wilms tumor—an update from the National Wilms Tumor Study Group. Int J Radiat Oncol. 2004;60(5):1379–1384. doi: 10.1016/j.ijrobp.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Grundy P.E., Breslow N.E., Li S., et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the national Wilms tumor study group. J Clin Oncol. 2005;23(29):7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 16.Malogolowkin M., Spreafico F., Dome J s, et al. Incidence and outcomes of patients with late recurrence of Wilms' tumor. Pediatr Blood Cancer. 2013;60(10):1612–1615. doi: 10.1002/pbc.24604. [DOI] [PubMed] [Google Scholar]
- 17.Malogolowkin M., Cotton C.A., Green D.M., et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2008;50(2):236–241. doi: 10.1002/pbc.21267. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz M.V., Koenig C., Armstrong A.E., et al. Advances in the clinical management of high-risk Wilms tumors. Pediatr Blood Cancer. 2023;70(S2) doi: 10.1002/pbc.30342. [DOI] [PubMed] [Google Scholar]
- 19.Metzger M.L., Stewart C.F., Freeman B.B., et al. Topotecan is active against Wilms' tumor: results of a multi-institutional phase II study. J Clin Oncol. 2007;25(21):3130–3136. doi: 10.1200/JCO.2007.10.9298. [DOI] [PubMed] [Google Scholar]
- 20.Saylors R.L., Stewart C.F., Zamboni W.C., et al. Phase I study of topotecan in combination with cyclophosphamide in pediatric patients with malignant solid tumors: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16(3):945–952. doi: 10.1200/JCO.1998.16.3.945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.