Abstract
Objective
Pyrotinib is a novel irreversible tyrosine kinase inhibitor that has shown efficacy for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). This study explored the efficacy and safety of pyrotinib in the treatment of HER2-positive MBC patients in the real world.
Methods
From September 2018 to February 2022, 137 female patients with HER2-positive MBC treated in this center were enrolled in this study. The follow-up period ended on January 12, 2023. The primary endpoint of this study was progression-free survival (PFS). Overall survival (OS), objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR), central nervous system (CNS)-PFS, CNS-ORR, CNS-CBR, CNS-DCR, and adverse event (AE) were the secondary endpoints.
Results
The ORR, DCR and CBR were 41.98 % (55/131), 87.79 % (115/131) and 44.27 % (58/131) in this cohort, respectively. The median PFS for this cohort was 10.37 months [95 % confidence interval (CI): 9.205-11.535] and the median OS was 37.53 months (not reached). Univariate and multivariate analyses showed that trastuzumab sensitivity was an independent predictor of improved PFS [hazard ratio (HR): 0.579 (0.371-0.904, p=0.016)] and improved OS [0.410 (0.213-0.790, p=0.008)]. Patients treated with a pyrotinib-based regimen as second-line and third-or-post-line therapy had poorer PFS [second-line: 3.315 (1.832-6.000, p<0.001); third-or-post-line: 3.304 (1.749-6.243, p<0.001)] and OS [second-line: 4.631 (1.033-20.771, p=0.045); third-or-post-line: 5.738 (1.212-27.174, p=0.028)]. There were 38 brain metastases (BM) patients in this study, the CNS-mPFS [14.37 months (7.815-20.925) vs. 7.83 months (7.047-8.613), p=0.375] and mOS [not reached vs. 36.40 months (18.551-54.249), p=0.034] were better in brain radiotherapy (BRT) group than NBRT group. 18.98 % (26/137) of patients experienced grade 3 or higher diarrhea. No AE-related death was reported.
Conclusion
This study confirms the promising antitumor activity and acceptable safety of real-world pyrotinib-based regimens for the treatment of HER2-positive MBC patients, particularly those who are trastuzumab-sensitive and who are receiving pyrotinib-based regimens as advanced first-line therapy. It has also been demonstrated that these regimens combined with BRT, provide better intracranial responses and long-term survival benefits for these patients with BM.
Keywords: Pyrotinib, Metastatic breast cancer, Human epidermal growth factor receptor 2, Brain metastases, Real-world study
Introduction
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor encoded by the oncogene ErbB2, a member of the epidermal growth factor receptor (EGFR) family [1]. It activates downstream signaling in the form of homo- and heterodimers to regulate tumor cell proliferation, invasion, and migration [1,2]. HER2 overexpression occurs in 15–20 % of breast cancer (BC) and is associated with high aggressiveness and a poor prognosis [3,4].
Trastuzumab is used throughout the neoadjuvant to advanced treatment of patients with HER2-positive BC, and its discovery revolutionized the treatment of HER2-positive BC patients as well as the prognosis and survival, thus paving the way for many other HER2-targeting drugs. Other HER2-targeting drugs currently on the market include: small-molecule tyrosine kinase receptor inhibitors (TKIs) such as lapatinib, neratinib, tucatinib, and pyrotinib; antibody-drug conjugates (ADCs) such as T-DM1 and T-DXd. TKIs have several advantages over large-molecule monoclonal antibodies, including easier oral administration, multi-target interaction, and lower cardiotoxicity [5,6]. In March 2023, T-DM1 was included in the Chinese medical insurance reimbursement list; neratinib and tucatinib were not approved for the treatment of HER2 MBC patients in China. In the case of low accessibility of these drugs before 2023, pyrotinib, which was independently developed in China, has demonstrated remarkable anticancer efficacy with tolerable side effects.
Pyrotinib is an oral, irreversible pan-HER TKI whose mechanism of action is to irreversibly bind covalently to the ATP-binding sites of the EGFR/HER1, HER2 and HER4 intracellular kinase regions to prevent the formation of homo- and heterodimers, thereby blocking the activation of downstream signaling pathways by inhibiting self-phosphorylation [7]. The phase II/III clinical trial showed that treatment with pyrotinib in combination with capecitabine significantly prolonged mPFS compared to lapatinib in combination with capecitabine (phase II: 18.1 months vs. 7.0 months, p<0.001 [8]; phase III: 12.5 months vs. 6.8 months, one-sided p<0.0001 [9]). The PHENIX trial [10] found that the pyrotinib plus capecitabine group had a significantly higher mPFS compared to the pyrotinib plus placebo group (HR: 0.18, 95 % CI: 0.13-0.26; p<0.001). Following unblinding, the placebo group received pyrotinib monotherapy, with an ORR of 38.0 %. In August 2018, the China National Medical Products Administration first authorized pyrotinib in combination with capecitabine for the treatment of HER2-positive recurrent or metastatic BC patients who had previously received anthracycline- or paclitaxel-based chemotherapy.
The aim of this study is to retrospectively analyze the efficacy, long-term survival, and safety of patients with HER2-positive MBC treated with pyrotinib in the real world, as well as the efficacy of intracranial lesions and survival prognosis in patients with brain metastases (BM).
Patients and Methods
Patient and date collection
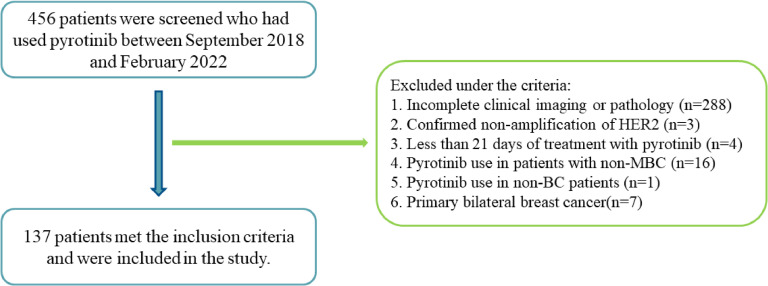
This is a single-center, retrospective real-world study. From September 2018 to February 2022, 456 patients treated with pyrotinib were screened. The last follow-up time was January 12, 2023. Finally, 137 female HER2-positive MBC patients were enrolled according to the inclusion and exclusion criteria, and excluded patients are shown in the study flow chart (Fig. 1).
Fig. 1.
Study flow chart.
Inclusion criteria
Female sex; age ≥18 years; pathologically confirmed HER2-positive BC, primary and/or metastatic tumor tissue immunohistochemistry category HER2 3+, and/or immunofluorescence in situ hybridization positive; Treatment with pyrotinib for at least 21 days, whether or not in combination with any chemotherapeutic agents, endocrine agents, and other HER2-targeting agents; and at least 1 measurable lesion, as defined by the criteria in the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) [11], was permitted to have no measurable lesions due to surgical resection within 1 month prior to pyrotinib treatment.
Exclusion criteria
Treatment regimens with pyrotinib in a non-metastatic setting; discontinued pyrotinib for treatment-unrelated reasons; primary bilateral BC; expected survival <1 month.
Pathologic and clinical data such as age at diagnosis, hormonal receptor (HR) status, Ki67 and HER2 status of the primary or metastatic tumor, histologic type, clinical stage at first diagnosis, number of metastatic sites, duration of pyrotinib administration, combination therapy regimen, time to progression, time to survival, and drug-related adverse events were collected from patients' medical record instruments, pathology and imaging reports, and follow-ups. HR-positive primary tumors were defined as estrogen receptor (ER) ≥1 % and/or progesterone receptor (PR) ≥1 %; both ER and PR <1 % were defined as HR-negative.
Treatment and dose modification
Depending on the indication, pyrotinib is taken orally at a standard dose of 400 mg/day. Dosage adjustments, combination, or non-combination of any antineoplastic agents are considered according to the physician's decision-making, the patient's previous treatment regimen, and the patient's own circumstances and wishes.
Study endpoint
The primary endpoint of this study was progression-free survival (PFS). Secondary endpoints were overall survival (OS), objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR), central nervous system (CNS)-PFS, CNS-ORR, CNS-DCR, CNS-CBR, and adverse event (AE).
Efficacy and safety assessments
The definitions of PFS, OS, ORR, DCR and CBR were as previously described [9]. Response Evaluation Criteria in Solid Tumors (RECIST1.1) [11] were used to assess tumor response. The efficacy evaluation of brain metastases (BM) was also performed according to RECIST 1.1, with the calculation of the response of the target lesion in the brain only, regardless of the response of other organs’ target lesion. The Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, was used for safety assessments.
Trastuzumab sensitivity is defined as that of trastuzumab-sensitive patients [12]: (1) patients with stage IV BC at first diagnosis; (2) patients with recurrent or metastatic BC who received (neo)adjuvant therapy but whose treatment regimen did not include trastuzumab; and (3) patients with early-stage BC who developed recurrence or metastasis more than 12 months after the end of (neo)adjuvant trastuzumab therapy. Trastuzumab-insensitive: (1) patients with MBC who had disease progression within 3 months of starting trastuzumab treatment; and (2) patients with early-stage BC who had recurrence or metastasis during or within 12 months of finishing (neo)adjuvant trastuzumab therapy.
Definition of the character of BM [13]: active BM was defined as BM that was newly detected and/or showed progression as confirmed by imaging (MRI/enhanced CT) prior to the use of the pyrotinib-containing regimen; stable BM was defined as BM that did not show progression as confirmed by imaging (MRI/enhanced CT) in BM patients who received CNS-directed therapy and had no CNS-related clinical symptoms.
Statistical analysis
Descriptive statistics (e.g., median, range, or percentage) were used to represent the clinicopathologic characteristics of patients. The Pearson χ2 test or Fisher exact test was used to compare differences in ORR, DCR, and CBR between subgroups. The Kaplan-Meier method was used to plot survival curves, and log-rank analysis was used to compare differences in PFS and OS between subgroups. Hazard ratio (HR) and 95 % confidence interval (CI) for time-to-event endpoints were calculated using the Cox multivariate regression model. The test level is α = 0.05 (two-tailed). SPSS 25 (https://www.ibm.com/products/spss-statistics) and GraphPad Prism 9.4.1 (https://www.graphpad.com/) were used for all statistical analyses and graphing.
Results
Baseline characteristics
From September 2018 to February 2022, a total of 137 patients met the inclusion and exclusion criteria and were enrolled in the cohort. The baseline characteristics of all patients are shown in Table 1. In this cohort, 67.88 % (93/137) of the patients were under the age of 50. The pathologic type of invasive ductal carcinoma was found in 97.08 % (133/137) of all patients, while the remaining four patients had invasive micropapillary carcinoma, occult breast carcinoma, inflammatory breast carcinoma, and infiltrating metaplastic carcinoma. HR status was positive in 53.28 % (73/137) patients and negative in 46.72 % (64/137) patients. 24.82 % (34/137) of the patients were stage IV at first diagnosis, and 75.18 % (103/137) were recurrent or metastatic BC patients. Among the recurrent or metastatic BC patients, 41.75 % (43/103) underwent neoadjuvant chemotherapy (NAC), 53.40 % (55/103) underwent adjuvant chemotherapy (AC), 2.91 % (3/103) developed metastasis during NAC, and another 1.94 % (2/103) did not undergo any treatment after surgery, respectively. In this cohort, 78.10 % (107/137) of patients had visceral metastases, with 35.77 % (49/137), 40.15 % (55/137), and 27.74 % (38/137) having metastases in the liver, lung, and brain, respectively. Only 28.47 % (39/137) of patients had only one metastasis, while 71.53 % (98/137) had more than two different visceral organs. 1.46 % (2/137), 2.19 % (3/137), 24.82 % (34/137), and 62.04 % (85/137) of the patients had received 4, 3, 2, and 1 other anti-HER2 treatment prior to pyrotinib, respectively, and 9.49 % (13/137) of the patients had not received any prior anti-HER2 therapy (Table 1).
Table 1.
Patient characteristics at baseline.
| Characteristic | No (%), n=137 |
|---|---|
| Age at diagnosis (years), median (range) | 48 (27-74) |
| Age at diagnosis (years) | |
| ≤50 | 93 (67.88) |
| >50 | 44 (32.12) |
| Pathological type | |
| Invasive ductal carcinoma | 133 (97.08) |
| Others | 4 (2.92) |
| Hormone receptor status | |
| Positive | 73 (53.28) |
| Negative | 64 (46.72) |
| Ki67 (%) | |
| <30 | 62 (45.26) |
| ≥30 | 75 (54.74) |
| Extent of disease at diagnosis | |
| Stage IV at first diagnosis | 34 (24.82) |
| Recurrence or metastasis | 103 (75.18) |
| Metastatic sites | |
| Bone | 58 (42.34) |
| Liver | 49 (35.77) |
| Lung | 55 (40.15) |
| Brain | 38 (27.74) |
| Lymph nodes | 62 (45.26) |
| Local recurrence | 31 (22.63) |
| Contralateral breast | 9 (6.57) |
| No. of metastatic sites | |
| 1 | 39 (28.47) |
| 2 | 45 (32.85) |
| 3 | 27 (19.71) |
| ≥4 | 26 (18.98) |
| Visceral metastases | |
| Yes | 107 (78.10) |
| No | 30 (21.90) |
| Trastuzumab sensitivity | |
| sensitivity | 92 (67.15) |
| insensitivity | 45 (32.85) |
| Prior HER2-targeted therapy | |
| Trastuzumab | 124 (90.51) |
| Pertuzumab | 23 (16.79) |
| Lapatinib | 19 (13.87) |
| T-DM1 | 4 (2.92) |
| Non-used | 13 (9.49) |
Pyrotinib-related schemes
Pyrotinib-based regimens were used as advanced first-line, second-line, and third-or-post-line therapy in 27.01 % (37/137), 35.77 % (49/137), and 37.23 % (51/137) of patients, respectively. Only 7.30 % (10/137) of patients received pyrotinib monotherapy, while the remaining 127 patients received pyrotinib in combination with other agents, of which 63.50 % (87/137) were combined with different chemotherapeutic durgs. The most frequently combined chemotherapeutic agent was capecitabine (52.55 %, 72/137). Chemotherapeutic, other HER2-targeted, and endocrine drugs were taken concurrently with pyrotinib. There are three ways to combine endocrine therapy: pyrotinib combined with endocrine therapy alone, pyrotinib combined with both endocrine therapy and HER2-targeted therapy, and pyrotinib combined with both endocrine therapy and chemotherapy. Table 2 shows the specific combinations between them. All the patients had a starting dose of pyrotinib of 400 mg/day, and 14 patients (10.22 %) had their dose adjusted to 320 mg/day due to adverse events, of which 3 (2.19 %) had their dose adjusted again to 240 mg/day (Table 2).
Table 2.
Pyrotinib-related treatment administration.
| Pyrotinib-related schemes | No (%), (n=137) | |
|---|---|---|
| Lines of systematic therapy of pyrotinib | ||
| 1 | 37 (27.01) | |
| 2 | 49 (35.77) | |
| ≥3 | 51 (37.23) | |
| Regimens | ||
| Pyrotinib single agent | 10 (7.30) | |
| Pyrotinib + chemotherapy | ||
| Pyrotinib + Capecitabine | 72 (52.55) | |
| Pyrotinib + Gemcitabine | 2 (1.46) | |
| Pyrotinib + Vinorelbine | 4 (2.92) | |
| Pyrotinib + Paclitaxel | 8 (5.84) | |
| Pyrotinib + Eribulin | 1 (0.73) | |
| Pyrotinib + HER2-targeted therapy | ||
| Pyrotinib + Trastuzumab | 5 (3.65) | |
| Pyrotinib + HER2-targeted therapy + chemotherapy | ||
| Pyrotinib + Trastuzumab + Capecitabine | 8 (5.84) | |
| Pyrotinib + Trastuzumab + Vinorelbine | 1 (0.73) | |
| Pyrotinib + Trastuzumab + Paclitaxel | 7 (5.11) | |
| Pyrotinib + Trastuzumab + Paclitaxel + Carboplatin | 2 (1.46) | |
| Pyrotinib + Trastuzumab + Vinorelbine + Cisplatin | 1 (0.73) | |
| Pyrotinib + Inetetamab + Capecitabine | 1 (0.73) | |
| Pyrotinib + Inetetamab + Paclitaxel | 1 (0.73) | |
| Pyrotinib + endocrine | ||
| Pyrotinib + Tamoxifen | 1 (0.73) | |
| Pyrotinib + Anastrozole | 2 (1.46) | |
| Pyrotinib + Fluvastatin | 2 (1.46) | |
| Pyrotinib + Anastrozole + Goserelin | 2 (1.46) | |
| Pyrotinib + endocrine + HER2-targeted therapy | ||
| Pyrotinib + Goserelin + Trastuzumab + Pertuzumab | 1 (0.73) | |
| Pyrotinib + Leuprorelin + Anastrozole + Trastuzumab | 1 (0.73) | |
| Pyrotinib + endocrine + chemotherapy | ||
| Pyrotinib + Anastrozole + capecitabine | 2 (1.46) | |
| Pyrotinib + Fluvastatin + capecitabine | 1 (0.73) | |
| Pyrotinib + Goserelin +capecitabine | 2 (1.46) | |
| Dosage | ||
| Starting dosage (mg/day) | ||
| 400 | 137 (100) | |
| Dose reduction (mg/day) | ||
| 400-320 | 14 (10.22) | |
| 400-320-240 | 3 (2.19) | |
Clinical efficacy
Six of the 137 patients were not included in the clinical efficacy analysis because they lacked measurable lesions due to surgical resection within 1 month prior to pyrotinib treatment. The ORR, DCR and CBR analyses included 131 patients with measurable lesions, of which 41.98 % (55/131) had ORR, 87.79 % (115/131) had DCR, and 44.27 % (58/131, Table 3) had CBR. Of the 9 (6.87 %) patients who obtained CR, 6 of them had brain metastasis, 2 had regional lymph node metastasis, and 1 had distant lymph node metastasis.
Table 3.
Tumor response with measurable lesions.
| Response | No (%), n=131a |
|---|---|
| Best response | |
| CR | 9 (6.87) |
| PR | 46 (35.11) |
| SD | 60 (45.80) |
| PD | 16 (12.21) |
| Objective response rate, n (%) | 55 (41.98) |
| Disease control rate, n (%) | 115 (87.79) |
| Clinical benefit rate, n (%) | 58 (44.27) |
6 patients lacked measurable lesions due to surgically resected lesions; CR: Complete Response; PR: Partial Response; SD: Stable Disease; PD: Progressive Disease.
Subgroup analyses of ORR and CBR (Table 4) showed that pyrotinib-based regimens as advanced first-line treatment patients had statistically higher ORR (63.64 % vs. 40.43 % vs. 29.41 %, p=0.008) and CBR (81.82 % vs. 27.66 % vs. 35.29 %, p<0.001) than advanced second-line and third-or-post-line treatment patients (Table 4). In the subgroup analysis of the ORR, the ORR of the patients without bone metastasis was significantly higher than that of those with bone metastasis (50.68 % vs. 31.03 %, p=0.024). And the ORR was significantly higher in patients with no prior lapatinib treatment than those with prior lapatinib treatment (46.02 % vs. 16.67 %, p=0.019). In the CBR subgroup analysis, patients with the number of metastasic sites ≤2 (52.56 % vs. 32.08 %, p=0.020) and patients without visceral metastases (73.08 % vs. 37.14 %, p=0.001) had significantly higher CBR (Table 4). There were no statistically significant differences in the distribution of ORR, DCR, and CBR with respect to age at diagnosis, HR status, Ki67, disease stage at diagnosis, BM, liver metastases, pyrotinib combined with or without capecitabine and trastuzumab sensitivity (supplementary Table 1).
Table 4.
Subgroup analyses of ORR and CBR.
| ORR, No (%) | p value | CBR, No (%) | p value | |
|---|---|---|---|---|
| Lung metastases | 0.787 | 0.007* | ||
| with (n=53) | 23 (43.40) | 16 (30.19) | ||
| without (n=78) | 32 (41.03) | 42 (53.85) | ||
| Bone metastases | 0.024* | 0.343 | ||
| with (n=58) | 18 (31.03) | 23 (39.66) | ||
| without (n=73) | 37 (50.68) | 35 (47.95) | ||
| No. of metastatic sites | 0.417 | 0.020* | ||
| ≤2 (n=78) | 35 (44.87) | 41 (52.56) | ||
| >2 (n=53) | 20 (37.74) | 17 (32.08) | ||
| Type of metastasis | 0.196 | 0.001* | ||
| non-visceral metastasis (n=26) | 8 (30.77) | 19 (73.08) | ||
| visceral metastasis (n=105) | 47 (44.76) | 39 (37.14) | ||
| Lines of pyrotinib | 0.008* | <0.001* | ||
| first-line (n=33) | 21 (63.64) | 27 (81.82) | ||
| second-line (n=47) | 19 (40.43) | 13 (27.66) | ||
| third-or-post-line (n=51) | 15 (29.41) | 18 (35.29) | ||
| Exposure to lapatinib | 0.019* | 0.599 | ||
| no prior exposure (n=113) | 52 (46.02) | 49 (43.36) | ||
| prior exposure (n=18) | 3 (16.67) | 9 (50.00) |
ORR: Objective response rate; CBR: Clinical Benefit Rate
Clinical efficacy and CNS survival analysis in BM patients
In this study, a total of 38 BM patients had measurable lesions. 15 (39.47 %) patients were treated with pytotinib in combination with brain radiotherapy (BRT), of which 11 patients were treated with whole brain radiotherapy and 4 patients with stereotactic radiotherapy. Twenty-three (60.53 %) patients were not treated with BRT. Multiple BMs were found in 86.67 % (13/15) and 65.22 % (15/23) of the patients in both groups, respectively. In the BRT group, all of them had active BM. While 73.91 % (17/23) of the patients in the NBRT group had active BM, another 6 patients had stable BM (Table 5).
Table 5.
CNS baseline and CNS efficacy and survival analysis of BM Patients.
| BRT group (n=15) | NBRT group (n=23) | p value | all patients (n=38) | |
|---|---|---|---|---|
| No (%) | No (%) | No (%) | ||
| No. of BM lesion | 0.259 | |||
| one | 2 (13.33) | 8 (34.78) | 10 (26.32) | |
| multiple | 13 (86.67) | 15 (65.22) | 28 (73.68) | |
| Characteristic of BM | 0.063 | |||
| stable | 0 (0) | 6 (26.09) | 6 (15.79) | |
| active | 15 (100) | 17 (73.91) | 32 (84.21) | |
| Best CNS response | 0.197 | |||
| CR | 6 (40.00) | 6 (26.09) | 12 (31.58) | |
| PR | 5 (33.33) | 3 (13.04) | 8 (21.05) | |
| SD | 2 (13.33) | 9 (39.13) | 11 (28.95) | |
| PD | 2 (13.33) | 5 (21.74) | 7 (18.42) | |
| CNS-ORR | 11 (73.33) | 9 (39.13) | 0.052 | 20 (52.63) |
| CNS-DCR | 13 (86.67) | 18 (78.26) | 0.681 | 31 (81.58) |
| CNS-CBR | 13 (86.67) | 10 (43.48) | 0.016* | 23 (60.53) |
| CNS-mPFS (95 %CI) | 14.37 (7.815-20.925) | 7.83 (7.047-8.613) | 0.375 | 9.77 (7.557-11.983) |
| mOS (95 %CI) a | not reached | 36.40 (18.551-54.249) | 0.034* | 36.40 (16.102-56.698) |
BM: Brain metastasis; CNS: Central nervous system; CNS-ORR: Objective response rate of the central nervous system; CNS-DCR: Central nervous system disease control rate; CNS-CBR: Clinical benefit rate of central nervous system; mPFS: Median progression free survival; mOS: Median overall survival; CI: confidence interval; a Two people were lost to follow-up in OS survival analysis.
Intracranial CR was achieved in 12 of 38 BM patients, with a CNS-ORR of 52.63 % (20/38), a CNS-DCR of 81.58 % (31/38), and a CNS-CBR of 60.53 % (23/38). The BRT group had higher CNS-ORR (73.33 % vs. 39.13 %), CNS-DCR (86.67 % vs. 78.26 %), and CNS-CBR (86.67 % vs. 43.48 %, p=0.016) than the NBRT group. 38 BM patients had a median CNS-PFS of 9.77 months (95 % CI: 7.557-11.983) and a mOS of 36.40 months (95 % CI: 16.102-56.698). The CNS-mPFS [14.37 months (95 % CI: 7.815-20.925) vs. 7.83 months (95 % CI: 7.047-8.613), p=0.375] and mOS [not achieved vs. 36.40 months (95 % CI: 18.551-54.249), p=0.034] in the BRT group were better than the NBRT group, where the difference in OS was statistically significant.
Outcomes
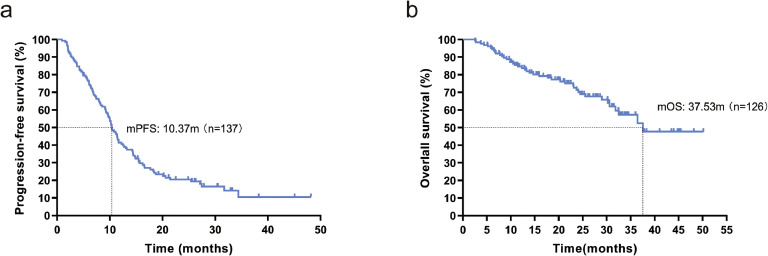
As of the last follow-up time, January 12, 2023, the number of PFS events was 108/137, and the number of OS events was 42/126 (11 lost to follow-up). The mPFS for this cohort was 10.37 months (95 % CI: 9.205-11.535, Fig. 2a), and the mOS was 37.53 months (95 % CI: not reached, Fig. 2b).
Fig. 2.
(a) Kaplan-Meier curve for PFS in 137 patients; (b) Kaplan-Meier curve for OS in 126 patients. mPFS-median progression free survival, mOS- median overall survival.
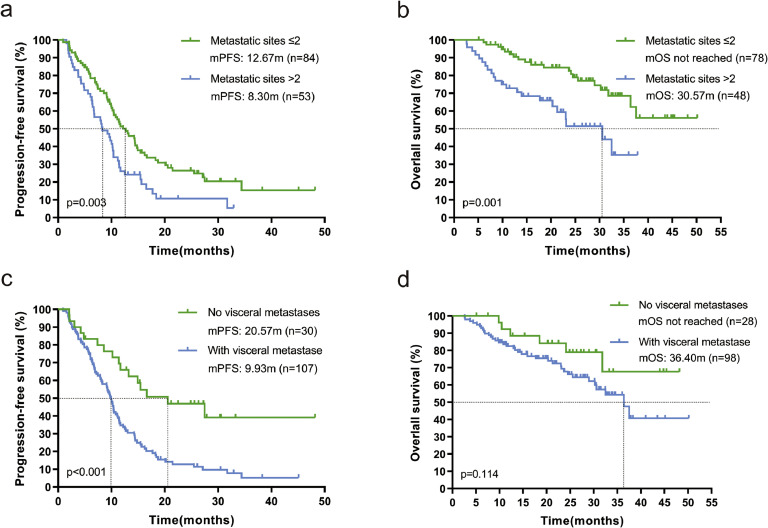
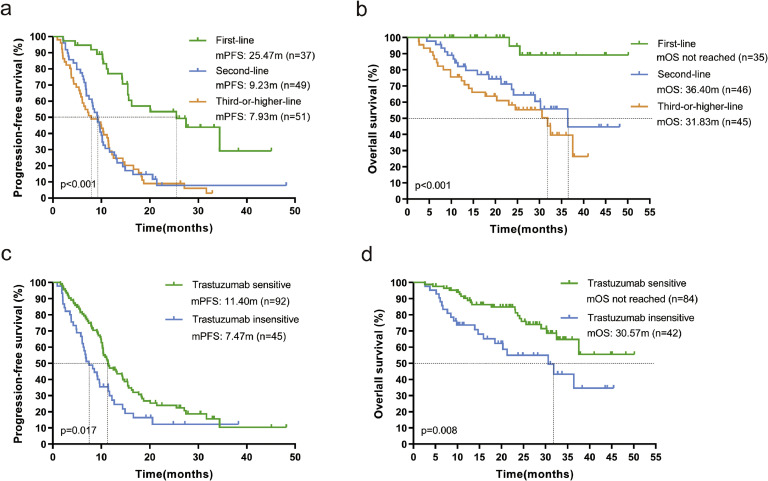
Subgroup analysis showed that mPFS was 12.67 months (95 % CI: 9.392-15.948) for patients with ≤2 metastases compared to 8.30 months (95 % CI: 5.27-11.327; p=0.005, Fig. 3a) for patients with >2 metastases, and also that mOS was significantly longer in patients with ≤2 metastases than in patients [not reached vs. 30.57 months (95 % CI: 17.586-43.554); p=0.001, Fig. 3b]. Patients without visceral metastases had significantly better mPFS than those with visceral metastases [20.57 months (95 % CI: 8.562-32.578) vs. 9.93 months (95 % CI: 8.985-10.875); p<0.001, Fig. 3c], and the same trend was observed for mOS in the two groups [not reached vs. 36.40 months (95 % CI: 29.363-43.437); p=0.114, Fig. 3d], but the difference was not statistically significant. Patients receiving pyrolitinib-based regimens as advanced first-line had significantly better mPFS than patients treated as second-line and third-or-post-line [25.47 months (95 % CI: 10.112-40.828) vs. 9.23 months (95 % CI: 7.447-11.013) vs. 7.93 months (95 % CI: 4.331-11.529); p < 0.001, Fig. 4a], and likewise mOS was significantly better [not reached vs. 36.40 months (95 % CI: 23.100-49.700) vs. 31.83 months (95 % CI: 22.804-40.856); p < 0.001, Fig. 4b]. Trastuzumab-sensitive patients had both significantly better mPFS [11.40 months (95 % CI: 8.999-13.801) versus 7.47 months (95 % CI: 4.972-9.968), p=0.017, Fig. 4c] and mOS [not reached versus 30.57 months (95 % CI: 17.008-44.132), p=0.008, Fig. 4d] than trastuzumab-insensitive patients.
Fig. 3.
Kaplan-Meier curves of PFS and OS containing log-rank test. (a) and (b) patients with ≤2 and >2 metastases; (c) and (d) patients with non-visceral metastases and those with visceral metastases. mPFS-median progression free survival, mOS- median overall survival.
Fig. 4.
Kaplan-Meier curves of PFS and OS containing log-rank test. (a), (b) advanced first-line, second-line and third-or-post-line patients; (c), (d) Trastuzumab-sensitive and Trastuzumab-insensitive patients. mPFS-median progression free survival, mOS- median overall survival.
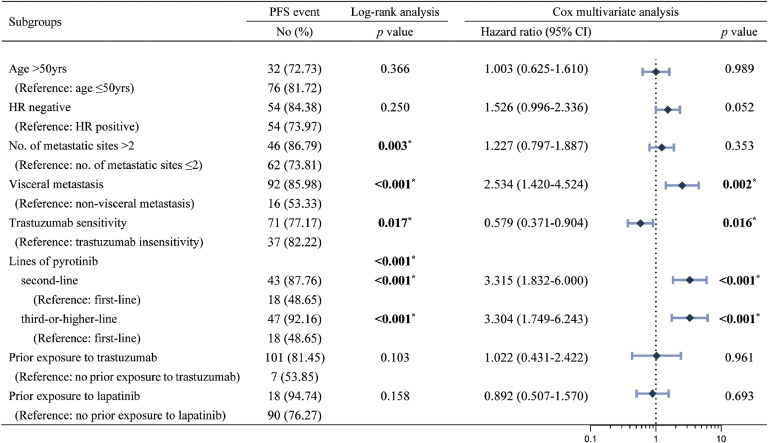
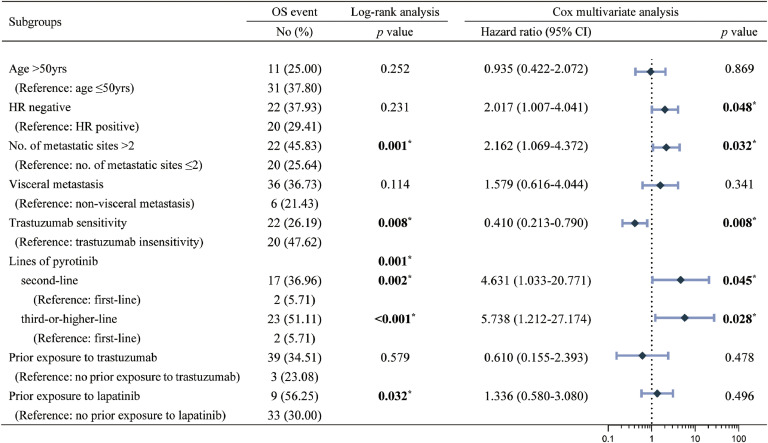
Cox multivariate analysis showed (Fig. 5, Fig. 6) that trastuzumab-sensitivity was an independent predictor of improved PFS (HR: 0.579, 95 % CI: 0.371-0.904, p=0.016) and OS (HR: 0.410, 95 % CI: 0.213-0.790, p=0.008), and that the use of pyrotinib-based regimens as advanced second-line and third-or-post-line therapy resulted in worse PFS (second-line: HR=3.315, 95 % CI: 1.832-6.000, p<0.001; third-or-post-line: HR=3.304, 95 % CI: 1.749-6.243, p<0.001) and OS (lHR=4.631, 95 % CI: 1.033- 20.771, p=0.045; HR=5.738, 95 % CI: 1.212-27.174, p=0.028). Also, visceral metastasis was a poor predictor of PFS (HR: 2.534, 95 % CI: 1.420-4.524, p=0.002), and the number of metastasic sites >2 was a poor predictor of OS (HR: 2.162, 95 % CI: 1.069-4.372, p=0.032).
Fig. 5.
Log-rank univariate and Cox multivariate analyses and forest plots of PFS. PFS-progression free survival, HR-hormone receptor.
Fig. 6.
Log-rank univariate and Cox multivariate analyses and forest plots of OS. OS-overall survival, HR-hormone receptor.
Safety
Due to the retrospective nature of this study and the fact that we collected data on adverse events (AE) based on the patient's recollection and medical documentation, omissions in the reporting of AE are unavoidable. The incidence of any grade AE was 93.43 % for diarrhea, 45.99 % for hand-foot syndrome, 40.88 % for nausea, 30.66 % for malaise, 28.47 % for leukopenia, 21.90 % for vomiting, 18.98 % for ALT/AST elevation, 18.25 % for thrombocytopenia, 9.49 % for oral mucositis, and 6.57 % for rash. Diarrhea was the most common AE of the EGFR-TKI, reported in almost all patients, with 18.98 % (26/137) of patients experiencing grade≥3 diarrhea. Other grade≥3 AEs were hand-foot syndrome (3.65 %), leukopenia (2.19 %), and elevated ALT/AST (1.46 %). One patient discontinued the drug due to an intolerance of diarrhea. There were no AE-related deaths reported, as detailed in Table 6.
Table 6.
Pyrotinib-related adverse events.
| Any events, No (%) | Grade 3, No (%) | |
|---|---|---|
| Diarrhea | 128 (93.43) | 26 (18.98) |
| Hand-foot syndrome | 63 (45.99) | 5 (3.65) |
| Nausea | 56 (40.88) | 0 (0) |
| Asthenia | 42 (30.66) | 0 (0) |
| Leukopenia | 39 (28.47) | 3 (2.19) |
| Vomiting | 30 (21.90) | 0 (0) |
| ALT/AST increased | 26 (18.98) | 2 (1.46) |
| Thrombocytopenia | 25 (18.25) | 0 (0) |
| Stomatitis | 13 (9.49) | 0 (0) |
| Rash | 9 (6.57) | 0 (0) |
Discussion
HER2-overexpression contributes to the poor prognosis of HER2-positive BC patients, and at the same time this has stimulated the acceleration of the development of anti-HER2 drugs, which has led to a significant improvement in the survival of such patients [1]. This retrospective RWS found that the ORR of the cohort's pyrotinib-based regimen for patients with HER2-positive MBC was 41.98 %, the DCR was 87.79 %, the CBR was 44.27 %, the mPFS was 10.37 months, and the mOS was 37.53 months. In the phase II/III studies of pyrotinib [8,9], the mPFS was 18.1 and 12.5 months with ORRs of 78.5 % and 67 %, respectively. Our mPFS was similar to the results of the phase III clinical study, but the ORR was worse. This study was a retrospective single-center study, there are two main reasons for this situation: first, only 27.01 % of patients in our cohort used pyrotinib as advanced first-line treatment, and 37.23 % of the patients used it as advanced third-or-post-line treatment. However, in the pyrotinib phase II/III trial, nearly half of the patients in the pyrotinib arm (42.5 %/50.8 %) used it as first-line therapy. Second, 28.47 % (39/137) of patients in our cohort had prior use of two or more HER2-targeted agents, which will somewhat affect the sensitivity of pyrotinib. In the subgroup analysis of ORR, patients with no prior exposure to lapatinib had a significantly better ORR (46.02 % vs. 16.67 %, p=0.019), as only 18 patients (13.14 %) in this cohort had received prior treatment with lapatinib, leaving the ORR advantage untransformed into a statistically significant survival benefit. Several studies have come to a similar conclusion [[14], [15], [16], [17], [18]] that pyrotinib leads to a better survival prognosis in lapatinib-naïve patients compared to lapatinib-treated patients.
The CSCO and NCCN BC Guidelines [19,20] grade I recommendation for HER2-positive MBC is paclitaxel in combination with trastuzumab and pertuzumab, whereas the NCCN Guideline recommends T-DM1 as a second-line treatment after trastuzumab failure, and the CSCO Guideline recommends pyrotinib in combination with capecitabine as an advanced second-line treatment after trastuzumab failure. The cox multivariate analysis of PFS and OS in this study showed that pyrotinib-based regimens as advanced first-line therapy provided better survival benefits for patients compared with those used as second-line and third-or-post-line therapy patients (mPFS: 25.47 vs. 9.23 vs. 7.93 months; p < 0.001; mOS: not reached vs. 36.40 vs. 31.83 months; p < 0.001), as well as better ORR (63.64 %), DCR (96.97 %), and CBR (81.82 %) with advanced first-line therapy. T-DM1 monotherapy is a standard second-line regimen for HER2-positive MBC, as the EMILIA study showed [21] that T-DM1 monotherapy resulted in significant PFS (6.4 vs. 9.6 months) and OS (25.1 vs. 30.9 months) benefits compared to lapatinib combined with capecitabine. A RWS [22] initially demonstrated the efficacy of T-DM1 monotherapy versus pyrotinib combined with capecitabine in the treatment of HER2-positive MBC, which showed an mPFS of 6.0 months in the pyrotinib group and 4.2 months in the T-DM1 group (p=0.044). Meanwhile, a meta-analysis that included 12 RCTs [23] showed that the PFS of the pyrotinib combined with capecitabine group was superior to that of the T-DM1 group (HR: 0.77, 95 % CI: 0.70-0.86). Taken together, this preliminary confirms that the use of pyrotinib in combination with capecitabine is feasible as an advanced second-line treatment in the context of low T-DM1 accessibility in our country.
Our study also found that trastuzumab sensitivity was an independent predictor of improved PFS (HR: 0.579, 95 % CI: 0.371-0.904, p=0.016) and improved OS (HR: 0.410, 95 % CI: 0.213-0.790, p=0.008). HER2-targeted drug resistance is the result of multiple downstream signaling pathways working together, and it is unclear whether there is cross-resistance between trastuzumab and pyrotinib. The Cox multivariate analysis also showed that visceral metastasis resulted in worse PFS (HR: 2.534, 95 % CI: 1.420-4.524, p=0.002) and the number of metastases more than two resulted in worse OS (HR: 2.162, 95 % CI: 1.069-4.372, p=0.032), which is also similar to the results of previous studies [24,25].
The incidence of BM in HER2-positive BC is approximately 30 % to 50 % [26,27]. Large-molecule regiments such as trastuzumab are ineffective against BM due to the blood-brain barrier (BBB), and TKIs can penetrate the BBB and target BM due to their small-molecule nature [15,27]. Renata D. et al. showed that the combination of TKIs such as lapatinib and neratinib with cytotoxic drugs, especially capecitabine, can improve the DCR and the long-term survival of patients [28]. For HER2-positive BC patients with active BM, the combination regimens of lapatinib plus capecitabine [29,30], neratinib plus capecitabine [31], and tucatinib plus capecitabine [13] produced CNS-ORRs of 29.2-65.9 %, 33–49 %, and 47.3 % and CNS-mPFSs of 5.5, 3.1-5.5, and 9.9 months, respectively. Meanwhile, in the PERMEATE study of pyrotinib in combination with capecitabine [32], the 59 patients who had not received radiotherapy (Cohort A) had a CNS-ORR of 74.6 % and a CNS-DCR of 93.2 %, and the 19 patients who had received radiotherapy and then had re-progressed (Cohort B) had a CNS-ORR of 42.1 % and a CNS-DCR of 63.2 %. The mPFS for Cohort A and Cohort B were 11.3 and 5.6 months, respectively. The CNS-ORR of the 38 BM patients in our study was 52.63 %, CNS-DCR was 81.58 %, CNS-CBR was 60.53 %, mCNS-PFS was 9.77 months, and mOS was 36.40 months. This data is close to the results of the PERMEATE study, which had bias in the cohort due to the small sample size of BM patients, and 15.79 % (6/38) of BM patients in this study had stable lesions, unlike the PERMEATE study, which included all patients with active BM. However, the results of this study can provide evidence in the real world that pyrotinib can benefit HER2-positive BM patients. Meanwhile, this study found that pyrotinib combined with BRT could improve CNS-ORR (BRT group: 73.33 %, NBRT group: 39.13 %, p=0.052) and CNS-CBR (86.67 % vs. 43.48 %, p=0.016). Also, it brings prolonged CNS-mPFS (14.37 months vs. 7.83 months, p=0.375) and OS (not reached vs. 36.40 months, p=0.034). With only 15 patients treated with combined BRT in this study, the insufficient sample size prevented the patients' benefit in intracranial lesion response from translating into a statistical benefit in CNS-PFS. Other RWSs have found similar results, with better long-term survival in patients who experienced BRT or brain surgery during pyrotinib treatment [15,33]. NCT04582968 and NCT05042791 are two clinical trials demonstrating the efficacy of pyrotinib in combination with capecitabine and BRT in HER2-positive BM patients. Patient enrollment has been completed in one of these trials, pending further evidence of whether pyrotalinib in combination with BRT is a better option for these patients.
Pyrotinib was well tolerated. The starting dose of pyrotinib was 400 mg/day in all patients, and 14 (10.22 %) patients experienced one dose adjustment, of which 3 (2.19 %) patients experienced two dose adjustments. The most common AEs in this study were diarrhea (93.43 %), hand-foot syndrome (45.99 %), and nausea (40.88 %). Grade 3 or higher AEs were diarrhea (18.98 %), hand-foot syndrome (3.65 %), leukopenia (2.19 %), and elevated ALT/AST (1.46 %). Diarrhea is the most common AE associated with TKI therapy [5,34], and the incidence of grade 3 to 4 diarrhea ranged from 15.4 % to 31 % in pyrotinib phase II/III clinical trials [[8], [9], [10],32,35]. In this study, one patient discontinued pyrotinib in the first cycle of a pyrotinib-based regiment due to intolerance of diarrhea; she progressed 2 months later, and a pyrotinib-based regimen was reintroduced to give her a PFS of 11.50 months. In this study, AEs were reported through medical records and follow-up data, and recollection bias resulted in missing reports of AEs.
The efficacy and tolerability of pyrotinib combined with capecitabine in the treatment of HER2-positive MBC have been confirmed by clinical trials [[8], [9], [10],24,32], and this regimen was used in 52.55 % (72/137) of patients in this study. We find the ORR (45.31 % vs. 38.81 %), CBR (50.00 % vs. 38.81 %) and DCR (89.06 % vs. 86.57 %) were slightly higher in the pyrotinib combination without capecitabine group than in the pyrotinib combination with capecitabine group, although there was no statistically significant difference (supplementary Table 1). We believe that this occurred because patients in the pyrotinib not combined with capecitabine group were on regimens that included other chemotherapeutic agents (e.g., Paclitaxel, Carboplatin), HER2-targeting agents (e.g., Trastuzumab, Pertuzumab, etc.), and endocrine drugs (e.g., Anastrozole, Fluvastatin, etc.). This resulted in a clinical efficacy and survival benefit for 47.45 % (65/137) of the patients in our cohort from more than just pyrotinib combined with capecitabine.
There are limitations to this study. First, it was a single-center retrospective study, resulting in selection bias. The sample size was small, and the study design was less rigorous than in prospective studies. Second, we didn't obtain all patients's pathology reports for re-biopsy of recurrent or metastatic tumors. Some patients were unable to re-biopsy, probably due to tumor's heterogeneity or HER2 addiction, which might have caused changes in their HER2 expression. Despite the limitations, this study confirms the efficacy as well as the safety of real-world pyrotinib in the treatment of HER2-positive MBC patients, especially in the trastuzumab-sensitive and pyrotinib-based regimens used as first-line treatment in advanced stages. It was also demonstrated that pyrotinib-based regimens in combination with BRT resulted in better survival benefits for BM patients.
Although pyrotinib has shown remarkable clinical efficacy, it is used only in China nowadays, where the major study objective is Asian people. More RCTs in different nations and among diverse ethnic groups are necessary for verifying its superiority. TDX-d, a novel ADC drug, has demonstrated outstanding efficacy in HER2-positive and HER2 low-expression MBC. Due to its unique structure and complex action mechanisms, the resistance mechanisms show more diversity. TKIs’ underline mechanism is binding to the intracellular structural domain of HER, potentially overcoming the resistance resulting from HER2 shedding by ADC drugs [36]. The combination of neratinib with T-DM1, demonstrated beneficial effects in HER2-positive MBC patients [37]. Currently, the clinical trial HER2CLIMB-04 (NCT02614794) is underway, which is investigating the use of tucatinib in combination with T-DXd in patients with HER2 positive MBC or unresectable locally advanced BC. We can expect the combination of pyrotinib with T-DXd in the future to explore the novel prospects of pyrotinib. BC has now entered the era of precision therapy, and many scholars are exploring the optimal regiment and management of AE of pyrotinib in HER2-positive MBC patients with different phenotypes, different treatment histories, and different ethnicities (NCT04367090, NCT03876587, NCT05346861). It is believed that with further clinical trials, pyrotinib can bring new choices and hope to HER2-positive MBC patients.
Funding
This work was supported by the National Natural Scientific Foundation of China (82160532 to S.H).
Ethics approval
Our study was approved by The Third Affiliated Hospital of Kunming Medical University Institution Review Board (approval no. KYLX2023-050) on Jan, 2023. This is a retrospective study and does not require informed consent. Patient data will not be shared with outside parties.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Lanyi Dai: Writing – original draft, Methodology, Investigation, Formal analysis. Ting Gao: Writing – original draft, Investigation, Formal analysis, Data curation. Rong Guo: Resources, Methodology, Conceptualization. Yuyuan Chen: Investigation, Formal analysis, Data curation. Jiankui Wang: Resources, Data curation. Shaoqiang Zhou: Writing – original draft, Visualization. Yiyin Tang: Writing – review & editing, Resources, Investigation. Dedian Chen: Supervision, Conceptualization. Sheng Huang: Writing – review & editing, Supervision, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2024.101029.
Contributor Information
Dedian Chen, Email: chendedian2006@126.com.
Sheng Huang, Email: sammer312@126.com.
Appendix. Supplementary materials
References
- 1.Hamilton E., Shastry M., Shiller S.M., Ren R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat. Rev. 2021;100 doi: 10.1016/j.ctrv.2021.102286. [DOI] [PubMed] [Google Scholar]
- 2.Marchio C., Annaratone L., Marques A., Casorzo L., Berrino E., Sapino A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021;72:123–135. doi: 10.1016/j.semcancer.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 4.Wolff A.C., Hammond M.E., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M., Bilous M., Fitzgibbons P., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 5.Xuhong J.C., Qi X.W., Zhang Y., Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am. J. Cancer Res. 2019;9(10):2103–2119. [PMC free article] [PubMed] [Google Scholar]
- 6.Le Du F., Diéras V., Curigliano G. The role of tyrosine kinase inhibitors in the treatment of HER2+ metastatic breast cancer. Eur. J. Cancer. 2021;154:175–189. doi: 10.1016/j.ejca.2021.06.026. (Oxford, England: 1990) [DOI] [PubMed] [Google Scholar]
- 7.Ma F., Li Q., Chen S., Zhu W., Fan Y., Wang J., Luo Y., Xing P., Lan B., Li M., et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2–Positive metastatic breast cancer. J. Clin. Oncol. 2017;35(27):3105–3112. doi: 10.1200/JCO.2016.69.6179. [DOI] [PubMed] [Google Scholar]
- 8.Ma F., Ouyang Q., Li W., Jiang Z., Tong Z., Liu Y., Li H., Yu S., Feng J., Wang S., et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: A randomized, phase ii study. J. Clin. Oncol. 2019;37(29):2610–2619. doi: 10.1200/JCO.19.00108. [DOI] [PubMed] [Google Scholar]
- 9.Xu B., Yan M., Ma F., Hu X., Feng J., Ouyang Q., Tong Z., Li H., Zhang Q., Sun T., et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–360. doi: 10.1016/S1470-2045(20)30702-6. [DOI] [PubMed] [Google Scholar]
- 10.Yan M., Bian L., Hu X., Zhang Q., Ouyang Q., Feng J., Yin Y., Sun T., Tong Z., Wang X., et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl. Breast Cancer Res. 2020;1 13-13. [Google Scholar]
- 11.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Wong H., Leung R., Kwong A., Chiu J., Liang R., Swanton C., Yau T. Integrating molecular mechanisms and clinical evidence in the management of trastuzumab resistant or refractory HER-2⁺ metastatic breast cancer. Oncologist. 2011;16(11):1535–1546. doi: 10.1634/theoncologist.2011-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin N.U., Borges V., Anders C., Murthy R.K., Paplomata E., Hamilton E., Hurvitz S., Loi S., Okines A., Abramson V., et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775. Official Journal of the American Society of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua Y., Li W., Jin N., Cai D., Sun J., Sun C., Yang F., Wu X., Huang X., Wang B., et al. Treatment with pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer: a multicenter real-world study. Ther. Adv. Med. Oncol. 2022;14 doi: 10.1177/17588359221085232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y., Lin M., Zhang J., Wang B., Tao Z., Du Y., Zhang S., Cao J., Wang L., Hu X. Real-world data of pyrotinib-based therapy in metastatic HER2-positive breast cancer: promising efficacy in lapatinib-treated patients and in brain metastasis. Cancer Res. Treat. 2020;52(4):1059–1066. doi: 10.4143/crt.2019.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang D.J., Chen Q.T., Anwar M., Xie N., Ouyang Q.C., Fan P.Z., Qian L.Y., Chen G.N., Zhou E.X., Guo L., et al. The efficacy of pyrotinib as a third- or higher-line treatment in HER2-positive metastatic breast cancer patients exposed to lapatinib compared to lapatinib-naive patients: a real-world study. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.682568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Wu X., Zhou J., Zhu M., Yu H., Zhang Y., Zhao Y., Han Z., Guo Y., Guan X., et al. Pyrotinib in the treatment of women with HER2-positive advanced breast cancer: a multicenter, prospective, real-world study. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.699323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin S., Chi Y., Du Y., Wang J., Shan C., Yi W., Shang M., Man X., Tan Q., Li H. Efficacy and safety of pyrotinib-containing regimen in the patients with HER2-positive metastatic breast cancer: A multicenter real-world study. Cancer Med. 2022 doi: 10.1002/cam4.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J., Jiang Z. Chinese society of clinical oncology breast cancer (CSCO BC) guidelines in 2022: stratification and classification. Cancer Biol. Med. 2022;19(6):769–773. doi: 10.20892/j.issn.2095-3941.2022.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., Anderson B., Burstein H.J., Chew H., Dang C., et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 21.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.Y., Diéras V., Guardino E., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F., Xu F., Li J., Wang T., Bian L., Zhang S., Jiang Z. Pyrotinib versus trastuzumab emtansine for HER2-positive metastatic breast cancer after previous trastuzumab and lapatinib treatment: a real-world study. Ann. Transl. Med. 2021;9(2):103. doi: 10.21037/atm-20-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao H., Huang W., Liu Y., Pei W., Li H. Efficacy and safety of pyrotinib versus T-DM1 in HER2+ metastatic breast cancer patients pre-treated with trastuzumab and a taxane: a bayesian network meta-analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.608781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Guan X., Chen S., Yi Z., Lan B., Xing P., Fan Y., Wang J., Luo Y., Yuan P., et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin. Cancer Res. 2019;25(17):5212–5220. doi: 10.1158/1078-0432.CCR-18-4173. : an Official Journal of the American Association For Cancer Research. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Li Z., Han L., Lv Z., Teng Y., Cui X., Zhou C., Wu H., Fang W., Xu L., et al. Efficacy and safety of pyrotinib in human epidermal growth factor receptor 2-positive advanced breast cancer: a multicenter, retrospective, real-world study. OncoTargets Ther. 2022;15:1067–1078. doi: 10.2147/OTT.S379591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J. Clin. Oncol. 2009;27(31):5278–5286. doi: 10.1200/JCO.2008.19.8481. : Official Journal of the American Society of Clinical Oncology. [DOI] [PubMed] [Google Scholar]
- 27.Lin N.U., Amiri-Kordestani L., Palmieri D., Liewehr D.J., Steeg P.S. CNS metastases in breast cancer: old challenge, new frontiers. Clin. Cancer Res. 2013;19(23):6404–6418. doi: 10.1158/1078-0432.CCR-13-0790. : an Official Journal of the American Association For Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duchnowska R., Loibl S., Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev. 2018;67:71–77. doi: 10.1016/j.ctrv.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Bachelot T., Romieu G., Campone M., Diéras V., Cropet C., Dalenc F., Jimenez M., Le Rhun E., Pierga J.Y., Gonçalves A., et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 30.Petrelli F., Ghidini M., Lonati V., Tomasello G., Borgonovo K., Ghilardi M., Cabiddu M., Barni S. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur. J. Cancer. 2017;84:141–148. doi: 10.1016/j.ejca.2017.07.024. (Oxford, England: 1990) [DOI] [PubMed] [Google Scholar]
- 31.Freedman R.A., Gelman R.S., Anders C.K., Melisko M.E., Parsons H.A., Cropp A.M., Silvestri K., Cotter C.M., Componeschi K.P., Marte J.M., et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511. : Official Journal of the American Society of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan M., Ouyang Q., Sun T., Niu L., Yang J., Li L., Song Y., Hao C., Chen Z., Orlandi A., et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–361. doi: 10.1016/S1470-2045(21)00716-6. [DOI] [PubMed] [Google Scholar]
- 33.Anwar M., Chen Q., Ouyang D., Wang S., Xie N., Ouyang Q., Fan P., Qian L., Chen G., Zhou E., et al. Pyrotinib treatment in patients with HER2-positive metastatic breast cancer and brain metastasis: Exploratory final analysis of real-world, multicenter data. Clin. Cancer Res. 2021;27(16):4634–4641. doi: 10.1158/1078-0432.CCR-21-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan A. Neratinib in HER-2-positive breast cancer: results to date and clinical usefulness. Ther. Adv. Med Oncol. 2016;8(5):339–350. doi: 10.1177/1758834016656494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie X.F., Zhang Q.Y., Huang J.Y., Chen L.P., Lan X.F., Bai X., Song L., Xiong S.L., Guo S.J., Du C.W. Pyrotinib combined with trastuzumab and chemotherapy for the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer: a single-arm exploratory phase II trial. Breast Cancer Res. Treat. 2023;197(1) doi: 10.1007/s10549-022-06770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Arx C., De Placido P., Caltavituro A., Di Rienzo R., Buonaiuto R., De Laurentiis M., Arpino G., Puglisi F., Giuliano M., Del Mastro L. The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer. Cancer Treat. Rev. 2023;113 doi: 10.1016/j.ctrv.2022.102500. [DOI] [PubMed] [Google Scholar]
- 37.Abraham J., Montero A.J., Jankowitz R.C., Salkeni M.A., Beumer J.H., Kiesel B.F., Piette F., Adamson L.M., Nagy R.J., Lanman R.B., et al. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. J. Clin. Oncol. 2019;37(29):2601–2609. doi: 10.1200/JCO.19.00858. : Official Journal of the American Society of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.