Abstract
This experiment was conducted to investigate the effects of mulberry leaf extract (MLE) on alleviating fatty liver hemorrhagic syndrome (FLHS) in laying hens. The 576 Jing Fen laying hens of 56 weeks of age with good health and similar weights (1.76 ± 0.17 kg) were randomly divided into 6 groups, with 8 replicates in each group and 12 chickens in each replicate. The experiment lasted 56 d. The control group was fed a corn-soybean meal diet. The FLHS group was fed a high energy-low protein (HELP) diet, and the other four experimental groups were fed HELP diets supplemented with 0.04, 0.40, 0.80, and 1.20% MLE, respectively. The results showed that HELP treatment significantly induced liver injury, which indicated that the FLHS model was successfully established. MLE supplementation could alleviate the FLHS by reducing the liver index, abdominal fat percentage, total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) in the serum (P < 0.05), and subsequently increase the egg production rate (P < 0.05). The laying hens fed 0.8% MLE exhibited the greatest production performance (P < 0.05) and could improve serum lipid levels. In addition, the genes associated with fatty acid synthesis (ACC, HMGR and SREBP-1C) were downregulated (P < 0.05), and genes related to fatty acid oxidation (CPT1A, AMPK, and ATGL) were found to be upregulated (P < 0.05). Supplementation with 1.2% MLE significantly reduced the relative abundance of Firmicutes and Desulfurized Bacillus (P < 0.05) and significantly increased the relative abundance of Fecal Bacillus (P < 0.05). In conclusion, MLE may regulate the mRNA expression of lipid metabolism-related genes through the AMPK signaling pathway and improve cecal microbiota balance and serum lipid levels to alleviate FLHS in laying hens and subsequently improve egg production performance.
Key words: mulberry leaf extract, fatty liver hemorrhagic syndrome, laying hen, lipid metabolism
INTRODUCTION
Fatty liver hemorrhagic syndrome (FLHS) in laying hens is a nutritional metabolic disease characterized by an imbalance in fat synthesis and oxidation (Yang et al., 2017), leading to excessive fat deposition in the liver and abdominal fat and hepatic cell steatosis (Peng et al., 2019), hemorrhagic necrosis and other pathological features (Yao et al., 2022). The egg production rate of laying hens with FLHS decreased, and the mortality rate increased sharply (Feng et al., 2023). The incidence rate of this disease is approximately 5% worldwide, which seriously constrains on the economic benefits and development of the laying hen breeding industry (You et al., 2023). Therefore, exploring methods for preventing, alleviating and treating FLHS in laying hens has become an important research directions in the poultry industry. At present, one of the effective solutions for alleviating FLHS in laying hens was to supplement plant extracts in feed (Li et al., 2023; Yang et al., 2023; Dansou et al., 2024).
Mulberry leaf extract (MLE) is a natural plant extract containing various bioactive substances, including polysaccharides, flavonoids, 1-deoxynojirimycin (1-DNJ) and alkaloids (Ding et al., 2021). It has various biological activities such as regulating lipid metabolism, lowering blood sugar, and exerting anti-inflammatory, antibacterial, antioxidant and immune regulatory effects (Wang et al., 2022). Feeding 10% MLE could increase serum antioxidant enzyme activity, reduce oxidative stress, and improve the production performance of 3-wk-old broiler chickens (So-In et al., 2022). Adding 9% mulberry leaf powder to the diet improved the serum immunity and meat quality of Duroc fattening pigs (Ma et al., 2022). Adding 6% mulberry leaf powder to the diet improved the low-density lipoprotein cholesterol (LDL-C) and immunoglobulin A (IgA) levels in the serum, and altered the gut microbiota composition of 2-mo-old Chinese pastoral dogs (Yu et al., 2023). A previous report indicated that MLE could inhibit the secretion and accumulation of triglyceride (TG) in primary chicken liver cells (Luo et al., 2023). MLE significantly reduced the protein levels of peroxisome proliferator-activated receptor gamma (PPAR-γ), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), fatty acid synthase (FAS) and adiponectin and fat accumulation in differentiated adipocytes (Yang et al., 2014). The addition of mulberry leaf flavonoids could improve the antioxidant capacity of the ovaries and enhance fatty acid β-oxidative expression in the liver of aged laying hens (Huang et al., 2021). Mulberry leaf flavonoids could reduce the body fat percentage of hybrid pigs (Liu et al., 2022). The mRNA expression levels of PPARγ-LXRα-ABCA1 pathway genes and related protein abundance could be regulated by mulberry leaf flavonoid administration (Liu et al., 2024). Fermented mulberry leaves could significantly reduce the concentrations of LDL-C, TG, total cholesterol (TC) and glucose in the serum, the size of adipocytes and the number of lipid droplets of liver tissue in mice (Lee et al., 2019). Therefore, MLE is widely considered to have potential effects on regulating lipid metabolism or alleviating FLHS, but there has been relatively little research on its application in animal husbandry, especially in laying hens.
Therefore, the aim of this study was to construct an FLHS model in laying hens using a high energy-low protein (HELP) diet, and then to determine whether and how MLE alleviated FLHS in laying hens by measuring production performance, blood lipid metabolism indicators, gene expression related to lipid metabolism and microbial communities. This research can provide an important theoretical basis and data support for the prevention and mitigation of FLHS in laying hens.
MATERIALS AND METHODS
Ethics Statement
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Hebei Agricultural University (Protocol 2023001), China.
Chemical Analysis
All diets were analyzed to estimate the content of crude protein (CP, method 984.13), calcium (Ca, method 968.08) and total phosphorus (method 946.06) according to AOAC (2006).
Experimental Design and Animal Management
A total of 576 fifty-six-wk-old Jing Fen laying hens with good health and similar body weights (1.76 ± 0.17 kg) were selected and randomly divided into 6 groups, with 8 replicates per treatment and 12 laying hens in each replicate. The Jing Fen laying hens used in this experiment were high-yield laying hens that were independently cultivated by Beijing Yukou Poultry Industry Co., Ltd. The 6 treatments used in the experiment are shown in Table 1. The 3 supplement levels of MLE including 0.4, 0.8, and 1.2% were determined based on our previous research (Zhang et al., 2022). The level of 0.04% was to investigate whether the addition of trace amounts of MLE could exert its effect under the pathological conditions of laying hens. Meanwhile, 0.4, 0.8, and 1.2% were 10, 20 and 30 times higher than 0.04%, respectively. The formulas and nutritional levels of the diets are shown in Table 2. The extraction method of MLE was hot-water extraction, and the main components were mulberry leaf polysaccharides (20%), mulberry leaf flavonoids (3%) and alkaloids (2%). The experimental diets were formulated according to the NRC (1994) recommendations for laying hens. The light/dark cycle was 16/8 h, and the temperature ranged from 20℃ to 22℃. The experiment lasted 8 wk. Feed (110 g) was provided twice a day and water was provided ad libitum.
Table 1.
Experimental groups and diet treatments.
| Items | Treatment method |
|---|---|
| Control group | Basic diet |
| HELP group | High energy-low protein diet |
| HELP with 0.04% MLE Group | High energy-low protein diet+0.04% MLE |
| HELP with 0.40% MLE Group | High energy-low protein diet+0.40% MLE |
| HELP with 0.80% MLE Group | High energy-low protein diet+0.80% MLE |
| HELP with 1.20% MLE Group | High energy-low protein diet+1.20% MLE |
Table 2.
Composition of the basic diet and high energy-low protein diet.
| Items | Control diet | High-energy low-protein diet |
|---|---|---|
| Corn | 63.53 | 65.27 |
| Soybean meal (43%) | 25.60 | 17.50 |
| Limestone (powder) | 8.52 | 8.43 |
| Chicken fat | - | 6.00 |
| Calcium hydrogen phosphate (21/18) | 1.49 | 1.75 |
| Premix1 (0.5%) | 0.50 | 0.50 |
| Salt | 0.30 | 0.30 |
| DL-Methionine | 0.06 | 0.10 |
| L-lysine hydrochloride HCL (98.5%) | - | 0.11 |
| L-Threonine (98.5%) | - | 0.04 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| ME (MJ/kg) | 11.03 | 12.75 |
| Crude protein (%) | 16.21 | 13.03 |
| Ca (%) | 3.47 | 3.52 |
| Total P (%) | 0.53 | 0.51 |
| Available P (%) | 0.36 | 0.34 |
| Lys (%) | 0.79 | 0.74 |
| Met (%) | 0.36 | 0.35 |
Provided per kilogram of diets: vitamin A, 12,500 IU; vitamin D3, 2,500 IU; vitamin E, 30 IU; vitamin K3, 1.70 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B12, 0.025 mg; niacin, 36 mg; biotin, 0.2 mg; folic acid, 1.25 mg; calcium pantothenate, 13 mg; iron, 80 mg; copper, 8 mg; manganese, 100 mg; zinc, 75 mg; iodine, 1.0 mg and selenium, 0.15 mg.
Crude protein, Ca, and total P are analyzed values, while the other values are calculated values.
Sample Collection
On the 56th d of the experiment, one laying hen from each replicate was randomly selected, and 48 laying hens in total were fasted for 12 h. Blood was collected from the wing vein and centrifuged at 3,000 rpm for 15 min, after which the supernatant was stored at -20℃. The laying hens were weighed and then slaughtered. Fresh liver and abdominal fat were weighed to calculate the liver index and abdominal fat percentage:
The complete liver of each laying hens was photographed and recorded. Liver samples was stored at -80℃ to measure gene expression, and liver samples were fixed in 4% paraformaldehyde to observe pathological status. The cecal contents of each chicken were collected aseptically and were stored at -80℃.
Productive Performance
The number of egg and egg weight were recorded every day. The egg production rate was calculated based on the number of egg daily divided by the number of laying hens on the same day. The average weight of eggs in each group were calculated weekly. The feed consumption was recorded to determine the average feed intake every week. The egg mass was calculated by multiplying the average egg weight by the egg production rate. The ratio of feed to egg was calculated by dividing the average feed intake by the egg mass.
Egg Quality Characteristics
On d 28 and 56, 2 eggs were randomly selected from each replicate for quality determination. Eggshell strength was measured using an egg force reader (EFR-01, ORKA Technology Co., Ltd., Herzliya, Israel). Egg yolk color was measured using a yolk color chart (Robotmation, Co., Ltd., Tokyo, Japan). We measured the weights of the egg, eggshell and yolk using a digital scale (Huachaoice Electrical Appliances, Shanghai, China). An echo-meter (ESTG-1; ORKA, Israel) was used to measure the thickness of the eggshell. The long and short diameters of the eggs were measured using an egg form coefficient measuring instrument (NFN385, FHK Corp., Tokyo, Japan). The egg shape index was calculated by the ratio of long diameter to short diameter. The albumen height and Haugh unit were measured using an egg multimeter (model EA-01, ORKA Technology Co., Ltd., Herzliya, Israel).
Hematoxylin-Eosin and Oil Red O Staining
Liver tissue in a 4% paraformaldehyde solution was embedded in paraffin, and then subjected to standard hematoxylin-eosin (H&E) staining. For determination of hepatic fat accumulation, liver tissue was cut into frozen sections and sequentially stained with Oil Red O.
Serum Biochemical Parameters
The serum concentrations of aspartate transaminase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), very-low-density lipoprotein cholesterol (VLDL-C), high-density lipoprotein cholesterol (HDL-C), nonesterified fatty acid (FFA), adiponectin (ADP) and leptin (LEP) were determined by an enzyme labeling instrument (Bio Tek Instruments, Inc., Vermont, VT), and commercial kits were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). All procedures were conducted according to the manufacturer's instructions.
Real-Time Quantitative PCR
The RNA of the liver was isolated with TRIzol reagent (Takara Bio Inc., Beijing, China). The concentration and purity of the RNA were determined with a NanoDrop 2000C spectrophotometer (Thermo, San Jose, CA). Then, the optimal RNA was reverse-transcribed into cDNA, and qRT-PCR was carried out on an ABI Quant Studio 7 Flex Real-Time Detection System (Life Technologies Holdings, Pte. Ltd., Wuhan, China) using a KAPA SYBR Fast universal qRT-PCR kit (TaKaRa, Dalian, China) according to the manufacturer's instructions. The primer sequences and related information are listed in Table 3 (synthesized by Shenggong Biological Engineering Co., Ltd., Shanghai, China). The relative expression levels of the six genes were measured using the 2−ΔΔCt method, with β-actin serving as a reference.
Table 3.
List of gene primer sequences.
| Gene | GenBank ID | PCR primers sequence (5′-3′) | PCR products(bp) |
|---|---|---|---|
| ACC | NM_205505.2 | F-ACTCCATCGCATCTTCCATTATGTC R-TCAGTGTCTTCATTAGTCGCTCAAC |
125 |
| HMGR | NM_204485.3 | F-GAAGCAGTCATTCCAGCCAAGG R-AGCCATAGCAGAACCCACCAG |
125 |
| ATGL | NM_001113291.2 | F-TTCTGACAACTTGCCACGATATGAG R-TTGGATGCTTGTATTGGTGACTCTC |
105 |
| CPT1A | NM_001012898.1 | F-GAGTCAGACACCACAGCAACAC R-CCGTAACCATCATCAGCCACAG |
105 |
| SREBP-1C | NM_204126.3 | F-AAGGCGGAGGTGATGGAGATG R-GGCTGTCAGGCTCGGAGTC |
133 |
| AMPKα | NM_001039603.2 | F-CCATCTGTCTCGCCCTCATCC R-AATGCCACTTCGCTCTTCTTACAC |
133 |
| β-actin | NM_205518.2 | F-CAGCCATGTATGTAGCCATCCAG R-GTAACACCATCACCAGAGTCCATC |
96 |
Abbreviations: ACC, Acetyl-CoA carboxylase; HMGR, 3-hydroxy-3-methyl glutaryl coenzyme A reductase; ATGL, Adipose triglyceride lipase; CPT1A, Carnitine palmitoyltransferase1; SREBP-1C, sterol regulatory element-binding protein-1c; AMPKα, Adenosine 5′-monophosphate (AMP)-activated protein kinase.
Intestinal Microbiota Characterization
Total microbial DNA was extracted from the cecal contents using the CTAB method. Then, the sequencing libraries were prepared according to the 16S Metagenomic Sequencing Library protocol to amplify the V3 and V4 regions. PCR amplification was carried out in a 25 µL reaction system, and the PCR amplification conditions were as follows: initial predenaturation at 94℃ for 4 min, denaturation at 98℃ for 10 s, renaturation at 58℃ for 30 s, elongation at 72℃ for 2 min, 30 cycles and a final elongation step at 72℃ for 10 min. After amplification and purification, equal molar amounts of amplicons were collected and sequenced. PCR amplicon sequence and analysis of relevant indicators were subsequently conducted using the Biomarker Cloud platform (Beijing, China).
Statistical Analysis
The data were analyzed using one-way ANOVA with Tukey's method for multiple comparisons between groups. Orthogonal polynomial contrasts were used to estimate the linear and quadratic effects of the five treatment groups, including HELP with 0, 0.04, 0.4, 0.8 and 1.2% MLE groups. The contrast coefficients were computed first in polynomial contrast using the following code in SPSS: CONTRAST (Factor) = POLYNOMIAL (0, 4, 40, 80, 120).
All the data were analyzed using SPSS (version 25.0; IBM Inc., New York, NY), and images were created using GraphPad Prism version 8.0.2 (GraphPad Software, La Jolla, CA). The results are presented as the means and standard error of the mean (SEM), and differences were considered significant at P values less than 0.05.
RESULTS
Production Performance
The effects of MLE on the production performance of laying hens are summarized in Table 4. Compared with the control group, the egg production rate of laying hens in HELP with 0.4 and 0.8% MLE group were not different, but was higher than the HELP with 0, 0.04 and 1.2% MLE group (P < 0.05). Compared with the HELP group, the feed-to-egg ratio of laying hens in HELP with 0.4 and 0.8% MLE group had decreased significantly (P < 0.05), and they had no difference with the control group.
Table 4.
Effects of MLE on production performance.
| Items | Control group | HELP groups with MLE levels (%) |
SEM |
P-Value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.04 | 0.40 | 0.80 | 1.20 | A | L | Q | |||
| Egg production rate (g) | 86.02c | 78.14a | 81.20b | 84.41c | 84.09c | 80.86b | 0.39 | <0.001 | 0.219 | <0.001 |
| Egg mass (g) | 53.41b | 48.55a | 50.51ab | 53.14b | 52.46b | 50.25ab | 0.51 | 0.007 | 0.401 | 0.012 |
| Average egg weight (g) | 62.09a | 62.13a | 62.21a | 62.95b | 62.39ab | 62.15a | 0.07 | 0.004 | 0.957 | 0.004 |
| Average daily feed intake (g) | 109.71 | 109.04 | 109.08 | 109.07 | 109.58 | 109.72 | 0.52 | 0.994 | 0.387 | 0.556 |
| Feed/egg | 2.05a | 2.25b | 2.16ab | 2.05a | 2.09a | 2.18ab | 0.01 | <0.001 | 0.440 | 0.005 |
Abbreviations: A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
In the same row, different superscript lowercase letters indicate significant differences (P < 0.05).
Egg Quality
The effects of MLE on the egg quality of laying hens are summarized in Table 5. The egg weight of laying hens in the control group and the HELP with 0.4 and 0.8% MLE groups were not different, but was higher than those in the HELP with 0, 0.04 and 1.2% MLE groups (P < 0.05). The egg yolk weight of laying hens in the control group and the HELP with 0.4, 0.8 and 1.2% MLE group had no difference (P > 0.05), but was higher than the HELP with 0 and 0.04% MLE groups (P < 0.05). There was a linear significance (P < 0.05) in egg shape index and quadratic significance (P < 0.05) in albumen height. The yolk color of the HLEP with 0.4%, 0.8% and 1.2% MLE group were significantly higher than that of the control group (P < 0.05).
Table 5.
Effects of MLE on egg quality.
| Items | Control group | HELP groups with MLE levels (%) |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.04 | 0.40 | 0.80 | 1.20 | A | L | Q | |||
| Eggshell thickness (mm) | 0.33 | 0.35 | 0.38 | 0.37 | 0.33 | 0.37 | 0.01 | 0.298 | 0.644 | 0.718 |
| Egg weight (g) | 65.11b | 57.62a | 59.86a | 65.99b | 65.64b | 61.87a | 0.87 | <0.001 | 0.073 | <0.001 |
| Egg shape index | 1.30 | 1.29 | 1.31 | 1.34 | 1.32 | 1.33 | 0.01 | 0.250 | 0.043 | 0.106 |
| Eggshell weight (g) | 10.24 | 9.36 | 9.50 | 10.07 | 10.64 | 9.91 | 0.17 | 0.271 | 0.134 | 0.073 |
| Yolk color | 7.86a | 8.57ab | 8.63ab | 8.88b | 8.88b | 9.38b | 0.13 | 0.019 | 0.192 | 0.111 |
| Egg yolk weight (g) | 17.70ab | 16.27a | 16.36a | 18.58b | 18.16b | 17.61ab | 0.19 | <0.001 | 0.514 | <0.001 |
| Albumen height (mm) | 8.77 | 8.75 | 9.03 | 9.27 | 9.57 | 9.21 | 0.12 | 0.304 | 0.142 | 0.034 |
| Haugh unit | 91.60 | 94.38 | 94.92 | 95.62 | 95.87 | 95.86 | 0.69 | 0.483 | 0.361 | 0.597 |
| Eggshell strength (N) | 32.36 | 33.82 | 32.26 | 36.01 | 36.81 | 35.67 | 0.66 | 0.234 | 0.457 | 0.609 |
Abbreviations: A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
In the same row, different superscript lowercase letters indicate significant differences (P < 0.05).
Pathological Changes of Liver
The effects of MLE on the pathological changes in the liver of laying hens are summarized in Figure 1. Figure 1A showed that compared with the control group, the livers of the HELP group were swollen, yellow and greasy in color with obvious bleeding points. However, as the supplement concentration of MLE increased, the pathological changes in the liver were alleviated, and the color gradually became ruddy and shiny gradually. As shown in Figure 1B, the liver cells of laying hens in the control group had a complete structure and clear boundaries. The liver cells of laying hens in the HELP group exhibited significant vacuolar degeneration, extensive necrosis and structural dissolution. With MLE supplementation, the vacuolar degeneration of liver cells was alleviated, and the liver cells gradually returned to a normal physiological morphology. Figure 1C showed that compared with the control group, a large number of red lipid droplets in hepatic cells appeared in the HELP group. As the concentration of MLE in the diets increased, the area and number of red lipid droplets had decreased in hepatic cells, and there were no obvious red lipid droplets in the HELP with 1.2% MLE group, which indicated that MLE supplementation contributed to reduced lipid accumulation.
Figure 1.
The effect of MLE on pathological changes of liver. Figure 1. Pathological observation results of the liver. (A) Histopathological observation. (B) H&E staining × 400. (C) Oil Red O staining × 400.
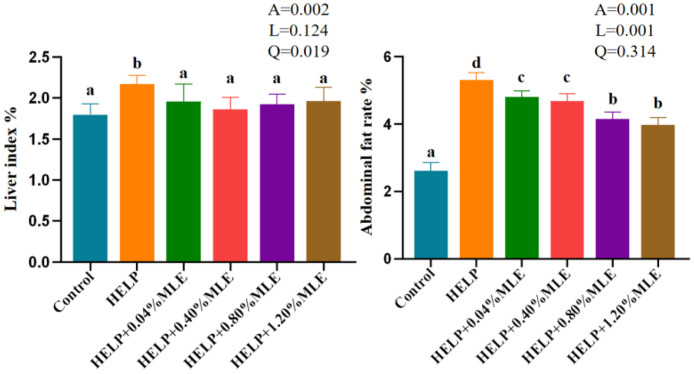
Liver Index and the Rate of Abdominal Fat
The effects of MLE on the liver index and the percentage of abdominal fat in laying hens are summarized in Figure 2. Compared with the control group, the liver indices and abdominal fat percentage of the laying hens in the HELP group were significantly increased (P < 0.05). Compared with the HELP group, the liver indices and abdominal fat percentage of laying hens in the HELP with MLE groups were significantly lower (P < 0.05). There were no significant difference in the liver indices of laying hens between the control group and the HELP with MLE addition groups (P > 0.05). The abdominal fat percentage of laying hens in HELP with 0.8 and 1.2% MLE groups were the lowest among all HELP groups (P < 0.05).
Figure 2.
Effects of MLE on the liver index and the rate of abdominal fat. Figure 2. Liver index (%) = liver weight/body weight × 100%; Abdominal fat percentage (%) = abdominal fat/body weight × 100%. A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Serum Biochemical Indices
The effects of MLE on the serum biochemical indices of laying hens are summarized in Table 6. Compared with the control group, the levels of TC, TG, FFA, LDL, VLDL, ADP, LEP, AST, and ALT in the HELP group were significantly higher (P < 0.01). Compared with the HELP group, the content of FFA, LDL, VLDL, ADP, LEP and AST of serum in laying hens had significantly reduced in the HLEP with 0.04, 0.4, 0.8, and 1.2% groups (P < 0.01). Compared with the HELP group, the content of TC and LDL of serum in laying hens had the lowest in HELP with 0.4% MLE group, which was no significant difference with the control group (P < 0.01). The serum content of TG of laying hens was the lowest in control group and the HELP with 0.8 and 1.2% MLE group (P < 0.01), and they were no significant difference. The ALT content of serum was the lowest in laying hens of control group and the HLEP with 0.8 and 1.2% MLE groups (P < 0.01).
Table 6.
Effects of MLE on serum biochemical indices.
| Items | Control group | HELP groups with MLE levels (%) |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.04 | 0.40 | 0.80 | 1.20 | A | L | Q | |||
| TC (mmol/L) | 3.72a | 4.87bc | 5.19c | 3.67a | 4.32ab | 4.12ab | 0.09 | <0.001 | 0.001 | <0.001 |
| TG (mmol/L) | 19.81a | 26.59c | 27.59c | 21.69b | 19.86a | 18.48a | 0.44 | <0.001 | <0.001 | <0.001 |
| FFA (μmol/L) | 361.54a | 598.86d | 549.34c | 568.03cd | 466.06b | 431.56b | 13.73 | <0.001 | 0.020 | 0.043 |
| HDL (mmol/L) | 0.92 | 0.87 | 0.90 | 0.89 | 0.93 | 0.92 | 0.02 | 0.656 | 0.468 | 0.659 |
| LDL (mmol/L) | 0.73b | 1.01d | 0.84c | 0.75b | 0.69ab | 0.66a | 0.02 | <0.001 | <0.001 | <0.001 |
| VLDL (μmol/L) | 409.03a | 720.46d | 685.87c | 663.33c | 641.37c | 475.59b | 18.55 | <0.001 | 0.011 | 0.006 |
| ADP (ng/mL) | 846.53a | 1646.03d | 1244.71c | 1362.60c | 1370.13c | 1025.33b | 44.47 | <0.001 | 0.063 | 0.063 |
| LEP (ng/mL) | 5.62a | |||||||||
| 14.40d | 11.98c | 12.47c | 9.35b | 11.73c | 0.48 | <0.001 | 0.224 | 0.281 | ||
| AST (U/L) | 50.92a | 80.32d | 70.22c | 66.92bc | 63.39b | 64.75b | 1.46 | <0.001 | 0.264 | 0.480 |
| ALT (U/L) | 9.39ab | 12.76c | 11.99c | 9.92b | 8.56a | 9.62ab | 0.25 | <0.001 | <0.001 | <0.001 |
Different letters in each row indicate a significant difference (P < 0.05).
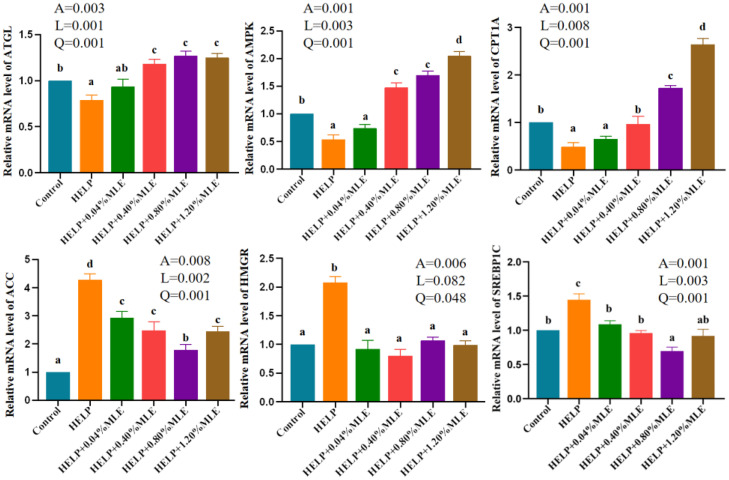
Expression Levels of Genes Related to Lipid Metabolism
The effects of MLE on the genetic expression of laying hens are summarized in Figure 3. Compared with the control group, the relative expression levels of ATGL, AMPK, and CPT1A in the HELP group were significantly lower (P < 0.05), while the relative mRNA expression levels of ACC, SREBP-1C, and HMGR in the HELP group were significantly increased (P < 0.05). As MLE supplementation increased, the relative expression levels of ATGL, AMPK, and CPT1A had increased (P < 0.05), and the relative mRNA expression levels of ACC, SREBP-1C, and HMGR decreased compared with those in the HELP group (P < 0.05). The relative expression level of ATGL of laying hens was the highest in the HELP with 0.4, 0.8, and 1.2% MLE groups (P < 0.05). The relative expression levels of AMPK and CPT1A of laying hens were the highest in the HELP with 1.2% MLE group (P < 0.05). In all the HELP groups, the relative expression levels of ACC and SREBP-1C of laying hens were the lowest in the HELP with 0.8% MLE group (P < 0.05). Compared with the HELP group, the relative expression level of HMGR of laying hens had decreased in the HELP with 0.04, 0.4, 0.8, and 1.2% MLE group (P < 0.05). The relative expression level of SREBP-1C of laying hens in the HELP with 0.8% MLE group was lower than that in the control group (P < 0.05).
Figure 3.
Effects of MLE on genetic expression. Figure 3. Abbreviations: ATGL, Adipose triglyceride lipase; AMPKα, Adenosine 5′-monophosphate (AMP)-activated protein kinase; CPT1A, Carnitine palmitoyltransferase1; ACC, Acetyl-CoA carboxylase; HMGR, 3-hydroxy-3-methyl glutaryl coenzyme A reductase; SREBP-1C, sterol regulatory element-binding protein-1c. A, P value of one-way ANOVA; L, P value of linear analysis; Q, P value of quadratic analysis.
Microbial Diversity
The effects of MLE on the cecal microbiota of laying hens are summarized in Figures 4A–4E and Table 7. A Venn diagram of the cecal microbiota in each group of laying hens at the OTU classification level is shown in Figure 4A. The results showed that the six experimental groups of laying hens had 199 common microorganisms at the OTU classification level of the cecal microbiota.
Figure 4.
Effects of MLE on the cecal microbiota. Figure 4. Analysis results of cecal microbiota diversity. (A) The Venn plot of cecal microbiota in each group. (B) The α diversity of cecal microbiota. (C) principal component analysis (PCA) conducted on the OTU components. (D) The compositions of cecal microbiota at the phylum levels. (E) The compositions of cecal microbiota at the genus levels. Abbreviations: CON, Control group; HELP, High energy-low protein group; MLE1, HELP+0.04% MLE; MLE2, HELP+0.4% MLE; MLE3, HELP+0.8% MLE; MLE4, HELP+1.2% MLE.
Table 7.
Effects of MLE on relative abundances of cecal microflora at phylum and genus levels of laying hens.
| Items | Control group | HELP groups with MLE levels (%) |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.04 | 0.40 | 0.80 | 1.20 | ||||
| Phylum level | ||||||||
| Firmicutes | 55.49c | 49.74a | 50.25a | 52.25b | 53.62b | 50.63a | 0.74 | <0.001 |
| Bacteroidota | 35.98 | 36.94 | 37.20 | 36.57 | 37.07 | 39.05 | 0.78 | 0.151 |
| Desulfobacterota | 3.92c | 5.78d | 4.66c | 4.52c | 2.59b | 1.57a | 0.33 | <0.001 |
| Actinobacteriota | 2.76 | 2.11 | 2.42 | 2.29 | 2.51 | 2.67 | 0.23 | 0.742 |
| Campylobacterota | 0.65 | 0.61 | 0.94 | 1.05 | 0.99 | 0.87 | 0.17 | 0.851 |
| Genus level | ||||||||
| Faecalibacterium | 7.64ab | 3.73a | 6.21a | 7.58ab | 11.29bc | 12.67c | 0.79 | <0.001 |
| Rikenellaceae_RC9_gut_ | ||||||||
| Group | 9.49a | 12.48b | 12.69b | 8.58a | 8.63a | 8.00a | 0.45 | <0.001 |
| Bacteroides | 10.03 | 9.76 | 9.80 | 10.50 | 10.31 | 9.07 | 0.27 | 0.720 |
| Phascolarctobacterium | 8.96 | 7.08 | 7.84 | 9.53 | 7.93 | 7.86 | 0.43 | 0.709 |
| Prevotellaceae_UCG_001 | 4.16 | 3.84 | 3.79 | 4.76 | 4.83 | 4.89 | 0.16 | 0.102 |
Different letters in each row indicate a significant difference (P < 0.05).
As shown in Figure 4B, principal component analysis (PCA) was conducted on the OTU components, and the contribution rates of the first and second principal components were 10.77% and 5.89%, respectively. There were fewer overlapping parts in the control group and HELP group, indicating that FLHS significantly altered the structure of the cecal microbiota. However, there were fewer overlapping parts in the 0.4, 0.8, and 1.2% MLE groups and the HELP group, indicating that the addition of MLE changed the composition of the cecal microbiota.
The α diversity of the cecal microbiota of laying hens at the OTU classification level is shown in Figure 4C. We utilized the ACE index and CHAO1 index to represent richness, and the Simpson index and Shannon index to represent diversity. The sequencing coverage of the cecal microbiota was above 99.95% in all groups, indicating that the sequencing depth basically covered all species in the sample. Compared with the HELP group, the Shannon index, ACE index and CHAO1 index of laying hens in the HELP with MLE group were significantly lower (P < 0.01).
The compositions of the cecal microbiota at the phylum and genus levels are summarized in Figures 4D–4E and Table 7. The relative abundances of Firmicutes, Bacteroidota, Desulfobacterota, Actinobacteriota, Campylobacterota and Fusobacteriota in the cecal microbiota of the experimental and control groups were greater than 1%, and these bacteria were the dominant bacterial genera. At the phylum level, compared with the HELP group, the relative abundance of Firmicutes in the HELP with 0.4 and 0.8% MLE groups was significantly higher, the relative abundance of Desulfobacterota bacteria of laying hens in the HELP with MLE group was significantly lower, and the relative abundance in the HELP with 1.2% MLE group was the lowest . At the genus level, the relative abundance of Faecalibacterium of laying hens in the HELP with 0.8 and 1.2% MLE groups was significantly higher than that in HELP group. The relative abundance of Rikenellaceae_RC9_gut_group was not significantly different between the control group and the 0.4, 0.8, and 1.2% MLE groups, but was ignificantly lower than that in the HELP group.
DISCUSSION
Serum biochemical indicators can reflect the metabolic level, nutritional status, and physiological and pathological changes of animals. Numerous studies have shown that MLE can decrease blood sugar and restrain the increase in blood lipids. Feeding fermented 50 mg/ml MLE for 12 wk could reduce the TG and TC concentrations of serum in mice, and significantly decrease the abdominal fat and the number of lipid droplets in liver cells (Lee et al., 2019). MLE significantly improved the pathological state of liver tissue in rats treated with lipopolysaccharide, and reduced the levels of AST, ALT, TNF-α, IL-1β, and IL-6 (Yu et al., 2022). Supplementation 100 mg/kg/d mulberry leaf for 15 wk decreased the relative weight of epididymal fat, liver, and kidney in mice with HELP caused by high-fat diet (He et al., 2022) The results of the present study showed that the FLHS severely led to liver damage and disrupted lipid metabolism in laying hens, and 0.8 and 1.2% MLE in the diet significantly relieved the FLHS of laying hens. These results indicated that MLE may improve lipid metabolism, and subsequently effectively alleviate the FLHS of laying hens.
The formation of FLHS in laying hens is closely related to the AMPK signaling pathway (Yao et al., 2022). AMPK is a master regulator of energy and lipid metabolism, and a therapeutic target for metabolic diseases (Neopane et al., 2022). There is a functional AMPK signaling pathway in chickens, which may alter the energy balance in FLHS induced by a high energy-low protein diet. When this pathway was activated, the mRNA expression of ACC was decreased, and fat deposition was subsequently blocked (Gao et al., 2019). The AMPK-ACC-CPT1A pathway was a key signaling pathway that regulated lipid metabolism, which could improve obesity and hepatic steatosis induced by diet (Yao et al., 2020). Moreover, mulberry leaf flavonoids could increase the phosphorylation of AMPK (Meng et al., 2020). Mulberry leaf at 4g/kg for 4 weeks alleviated the glucose and lipid metabolism disorders by activating the AMPK-PGC1α-CPT-1 signaling pathway in type Ⅱ diabetic rats (Cheng et al., 2022). The activated AMPK signaling pathway could increase the fatty acid oxidation and reduce fat synthesis in the liver cells of laying hens, thereby improving steatosis of liver (Zhang et al., 2021). The 50 and 100 μg/ml ethanol extracts of mulberry leaves significantly reduced the protein levels of PPAR-γ, PGC-1α, FAS and adiponectin in differentiated adipocytes (Yang et al., 2014). In the present study, the addition of MLE significantly upregulated the mRNA expression of lipid oxidation genes such as AMPK, ATGL and CPT1A in the liver of laying hens, and significantly downregulated the mRNA expression of lipid synthesis genes such as SREBP-1c, ACC and HMGR. The above results showed that MLE may improve lipid metabolism and relieve FLHS by regulating genes involved in the AMPK signaling pathways.
The gut microbiota plays a crucial role in energy homeostasis, lipid metabolism and intestinal health to ensure the balanced operation of the gut-liver axis, and imbalances in the gut microbiota were closely related to liver damage, the formation of FLHS and obesity (Dansou et al., 2024). The imbalance of the gut microbiota was closely related to the severity of liver fibrosis and FLHS in different stages of laying hens. Laying hens with a high degree of liver fibrosis and FLHS had a decrease in the abundance of Firmicutes in the cecum, with a significant decrease in the abundance of Lactobacillus and a higher abundance of Rhodococcus and Pseudomonas (Hamid et al., 2019). Supplementing 50 mg/kg/d of mulberry leaves to the diet in obese mice induced by high-fat diet for 20 wk could improve metabolic disorders, and modify the gut microbiota and lipid metabolism (Zhao et al., 2021). The addition of 45 g/d mulberry leaf flavonoids to water buffaloes for 35 d could significantly increase the abundance of Proteobacteria in the rumen of water buffaloes, and reduce the relative abundance of Actinobacteria, Acidobacteria and Chlorocurvata (Li et al., 2022). MLE could also reduce the relative abundance of Thermodesulfurization bacteria, which had been shown to have a proinflammatory effect and lead to pathology in the gut and are usually considered a pathogenic bacterium (Xu et al., 2023). Gavage with 200 mg/kg MLE for 7 weeks significantly reduced the relative abundance of Actinobacteria, significantly increased the relative abundance of Bacteroides in the cecal microbiota of rats with type II diabetes, and subsequently regulated lipid metabolism (Liu et al., 2021). MLE could decrease the abundance of Rikenellaceae in the HELP group, which was associated with sugar and lipid metabolism (Zhao et al., 2024). The results of the current study showed that compared with the HELP group, MLE supplement could significantly reduce the relative abundance of Desulfurization bacteria at the phylum level, and significantly increased the relative abundance of Fecal Bacillus genus at the genus level, which is consistent with previous research results. Therefore, MLE may regulate lipid metabolism and relieve FLHS by affecting the structure of the cecal microbiota in laying hens.
The current study revealed that the egg production rate of laying hens with FLHS significantly decreased, and the feed-to-egg ratio significantly increased. Studies had shown that feeding HELP diets could induce FLHS in laying hens, and the egg production rate of laying hens with FLHS significantly decreased (Li et al., 2024; Yao et al., 2022), which indicated that the FLHS was closely related to the production performance of laying hens. Improving FLHS in laying hens could significantly enhance production performance. Feeding 1200 mg/kg ginkgo biloba extract for 16 weeks could alleviate FLHS and then improve the egg production rate by reshaping the gut microbiota in laying hens (Yang et al., 2023). Supplement with 800 and 1,200 mg/kg MLE for 56 days could reduce abdominal fat deposition in AA broilers by decreasing the mRNA expression of FAS, ACC and associated fatty acid synthesis enzymes through the pathway of AMPK/SREBP-1c/ACC pathway in the liver (Qin et al., 2023). Addition with 0.8% MLE for 14 weeks could regulate lipid metabolism and reduce fat deposition in rats (Ko et al., 2022). The current study revealed that MLE could improve liver pathological status and the gut microbiota, regulate the expression of genes related to the AMPK signaling pathway with lipid metabolism, and enhance the production performance of laying hens. This may be due to the fact that MLE could regulate the gut-liver axis by improving the structure of the gut microbiota and the gene expression related to lipid metabolism to relieve FLHS in laying hens, which would enhance production performance. In addition, the color depth of egg yolks was mainly determined by the amount of exogenous carotenoids. The results showed that MLE could significantly improve egg yolk color (Zhang et al., 2022). In the present experiment, the addition of MLE to the diet significantly increased the egg yolk color. The reason may be that MLE could regulate lipid metabolism in laying hens, and subsequently promote the absorption of lipophilic carotenoids and their deposition in the egg yolk.
CONCLUSIONS
In summary, MLE supplementation in the diet may reduce liver damage, relieve the FLHS in laying hens and then significantly improve production performance and egg quality by improving the structure of the cecal microbiota, activating the AMPK signaling pathway and regulating the expression of genes related to lipid metabolism. Under the conditions of this experiment, the optimal addition level of MLE was 0.8%. The current study provides a theoretical basis for the prevention and relief of FLHS using MLE in laying hens.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The current research was supported by the National Natural Science Foundation of China (32302808) and the Key Research and Development Program Projects in Hebei Province (22327506D).
REFERENCES
- AOAC . 18th ed. AOAC Int.; Gaithersburg, MD: 2006. Official methods of analysis, in Association of Official Analytical Chemists. [Google Scholar]
- Cheng L., Wang J., An Y., Dai H., Duan Y., Shi L., Lv Y., Li H., Wang C., Du H., Zhong X., Zhao B. Mulberry leaf activates brown adipose tissue and induces browning of inguinal white adipose tissue in type 2 diabetic rats through regulating AMP-activated protein kinase signalling pathway. Br J Nutr. 2022;127:810–822. doi: 10.1017/S0007114521001537. [DOI] [PubMed] [Google Scholar]
- Dansou D.M., Chen H., Yu Y.N., Yang Y.Y., Tchana I.N., Zhao L.Y., Tang C.H., Zhao Q.Y., Qin Y.C., Zhang J.M. Enrichment efficiency of lutein in eggs and its function in improving fatty liver hemorrhagic syndrome in aged laying hens. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.N., Jiang X.D., Yao X.F., Zhang H.H., Song Z.H., He X., Cao R. Effects of feeding fermented mulberry leaf powder on growth performance, slaughter performance, and meat quality in chicken broilers. Animals. 2021;11:3294. doi: 10.3390/ani11113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Ma H., Yue Y.R., Wang L.J., Hao K.Y., Zhang Y.N., Li J.H., Xiang Y.J., Min Y.N. Saikosaponin a ameliorates diet-induced fatty liver via regulating intestinal microbiota and bile acid profile in laying hens. Poult. Sci. 2023;102:103155. doi: 10.1016/j.psj.2023.103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.N., Liu P., Wu C., Wang T.C., Liu G.H., Cao H.B., Zhang C.Y., Hu G.L., Guo X.Q. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019;98:2201–2210. doi: 10.3382/ps/pey586. [DOI] [PubMed] [Google Scholar]
- Hamid H., Zhang J.Y., Li W.X., Liu C., Li M.L., Zhao L.H., Ji C., Ma Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult Sci. 2019;98:2509–2521. doi: 10.3382/ps/pey596. [DOI] [PubMed] [Google Scholar]
- He L.Y., Xing Y., Ren X.X., Zheng M.J., Yu S.Q., Wang Y.B., Xiu Z.L., Dong Y.S. Mulberry leaf extract improves metabolic syndrome by alleviating lipid accumulation in vitro and In vivo. Molecules. 2022;27:5111. doi: 10.3390/molecules27165111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.W., Lv Z.P., Dai H.J., Li S.M., Jiang J.L., Ye N.W., Zhu S.L., Wei Q.W., Shi F.X. Dietary mulberry-leaf flavonoids supplementation improves liver lipid metabolism and ovarian function of aged breeder hens. J Anim Physiol Anim Nutr. 2021;106:1321–1332. doi: 10.1111/jpn.13658. [DOI] [PubMed] [Google Scholar]
- Ko H., Kim C., Lee M.S., Chang E., Kim C.T., Kim Y. High hydrostatic pressure extract of mulberry leaf attenuated obesity-induced inflammation in rats. J. Med. Food. 2022;25:251–260. doi: 10.1089/jmf.2021.K.0113. [DOI] [PubMed] [Google Scholar]
- Lee M.R., Kim J.E., Choi J.Y., Park J.J., Kim H.R., Song B.R., Choi Y.W., Kim K.M., Song H., Hwang D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019;17:2185–2193. doi: 10.3892/etm.2019.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Cai H., Liu G., Han Y., Qiu K., Liu W., Meng K., Yang P. Lactiplantibacillus plantarum FRT4 attenuates high-energy low-protein diet-induced fatty liver hemorrhage syndrome in laying hens through regulating gut-liver axis. J. Anim. Sci. Biotechnol. 2024;15:31. doi: 10.1186/s40104-023-00982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.L., Wang Y.L., Wang H.H., Yang Y., Ma H. Protective effects of genistein on the production performance and lipid metabolism disorders in laying hens with fatty liver hemorrhagic syndrome by activation of the GPER-AMPK signaling pathways. J. Anim. Sci. 2023;101:skad197. doi: 10.1093/jas/skad197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Hassan W.F., Peng L.J., Xie H., Liang X., Huang J.X., Huang F., Guo Y.X., Yang C.J. Mulberry flavonoids modulate rumen bacteria to alter fermentation kinetics in water buffalo. PeerJ. 2022;10:e14309. doi: 10.7717/peerj.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xiao Y., Xie J., Peng Y., Li F., Chen C., Li Y., Zhang X., He J., Xiao D., Yin Y. Dietary supplementation with flavonoids from mulberry leaves improves growth performance and meat quality and alters lipid metabolism of skeletal muscle in a Chinese hybrid Pig. Anim Feed Sci Technol. 2022;285 [Google Scholar]
- Liu Y.Y., Peng Y.L., Chen C., Ren H.B., Zhu J., Deng Y., Cui Q.M., Hu X.G., He J.H., Li H.L., Zhu X.H., Yin Y.L., He J., Xiao Y. Flavonoids from mulberry leaves inhibit fat production and improve fatty acid distribution in adipose tissue in finishing pigs. Anim Nutr. 2024;16:147–157. doi: 10.1016/j.aninu.2023.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.Z., Liu Q.H., Liu Z., Tang J.W., Chua E.G., Li F., Xiong X.S., Wang M.M., Wen P.B., Shi X.Y., Xi X.Y., Zhang X., Wang L. Ethanol extract of mulberry leaves partially restores the composition of intestinal microbiota and strengthens liver glycogen fragility in type 2 diabetic rats. BMC Complement Med Ther. 2021;21:172. doi: 10.1186/s12906-021-03342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Fan W., Qin J.P., Guo S.Y., Xiao H., Tang Z.H. Pharmacological and pathological effects of mulberry leaf extract on the treatment of type 1 diabetes mellitus mice. Curr Issues Mol Biol. 2023;45:5403–5421. doi: 10.3390/cimb45070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.Y., Ma H., Liu S.J., Wang J., Wang H.L., Zang J.J., Long S.F., Piao X.S. Effect of mulberry leaf powder of varying levels on growth performance, Immuno-Antioxidant status, meat quality and intestinal health in finishing pigs. Antioxidants. 2022;11:2243. doi: 10.3390/antiox11112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Qi X., Fu Y., Chen Q., Cheng P., Yu X., Sun X., Wu J., Li W., Zhang Q., Li Y., Wang A., Bian H. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J Ethnopharmacol. 2020;248 doi: 10.1016/j.jep.2019.112326. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th rev. ed. NAS-NRC; Washington, DC: 1994. [Google Scholar]
- Neopane K., Kozlov N., Negoita F., Murray-Segal L., Brink R., Hoque A., Ovens A.J., Tjin G., McAloon L.M., Yu D., Ling N., Sanders M.J., Oakhill J.S., Scott J.W., Steinberg G.R., Loh K., Kemp B.E., Sakamoto K., Galic S. Blocking AMPK β 1 myristoylation enhances AMPK activity and protects mice from high-fat diet-induced obesity and hepatic steatosis. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111862. [DOI] [PubMed] [Google Scholar]
- Peng G., Huang E.F., Ruan J.M., Huang L.M., Liang H.P., Wei Q., Xie X.H., Zeng Q.J., Huang J.Z. Effects of a high energy and low protein diet on hepatic and plasma characteristics and Cidea and Cidec mRNA expression in liver and adipose tissue of laying hens with fatty liver hemorrhagic syndrome. Anim. Sci. J. 2019;90:247–254. doi: 10.1111/asj.13140. [DOI] [PubMed] [Google Scholar]
- Qin L., Huang T., Jing R., Wen J., Cao M. Mulberry leaf extract reduces abdominal fat deposition via adenosine-activated protein kinase/sterol regulatory element binding protein-1c/acetyl-CoA carboxylase signaling pathway in female Arbor Acre broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So-In C., Sunthamala N. The effects of mulberry (Morus alba Linn.) leaf supplementation on growth performance, blood parameter, and antioxidant status of broiler chickens under high stocking density. Vet. World. 2022;15:2715–2724. doi: 10.14202/vetworld.2022.2715-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.X., Gao H.Q., Sun C., Huang L.X. Protective application of morus and its extracts in animal production. Animals. 2022;12 doi: 10.3390/ani12243541. 3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.F., Lian C.L., Pan L.X., Lai W.J., Zhang F., Peng L.M., Zhou S.J., Zhao G.H., Yang X.Z., Zhang G.H., Tan Z.F., Wang Y.K. N-acetyl-L-leucine protects MPTP-treated Parkinson’s disease mouse models by suppressing Desulfobacterota via the gut-brain axis. Brain Res. Bull. 2023;202:110729. doi: 10.1016/j.brainresbull.2023.110729. [DOI] [PubMed] [Google Scholar]
- Yang F., Ruan J.M., Wang T.C., Luo J.R., Cao H.B., Song Y.L., Huang J.Z., Hu G.L. Improving effect of dietary soybean phospholipids supplement on hepatic and serum indexes relevant to fatty liver hemorrhagic syndrome in laying hens. Anim. Sci. J. 2017;88:1860–1869. doi: 10.1111/asj.12832. [DOI] [PubMed] [Google Scholar]
- Yang S.J., Park N.Y., Lim Y. Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes. Nutr. Res. Pract. 2014;8:613–617. doi: 10.4162/nrp.2014.8.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.Y., Li D.P., Zhang M.H., Feng Y.Q., Jin X.L., Liu D., Guo Y.M., Hu Y.F. Ginkgo biloba extract alleviates fatty liver hemorrhagic syndrome in laying hens via reshaping gut microbiota. J. Anim. Sci. Biotechnol. 2023;14:97. doi: 10.1186/s40104-023-00900-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q., Li S., Cheng X., Zou Y., Shen Y., Zhang S. Yin Zhi Huang, a traditional Chinese herbal formula, ameliorates diet-induced obesity and hepatic steatosis by activating the AMPK/SREBP-1 and the AMPK/ACC/CPT1A pathways. Ann Transl Med. 2020;8:231. doi: 10.21037/atm.2020.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Wang H.H., Yang Y., Jiang Z.H., Ma H.T. Dehydroepiandrosterone activates the GPER-mediated AMPK signaling pathway to alleviate the oxidative stress and inflammatory response in laying hens fed with high-energy and low-protein diets. Life Sci. 2022;308 doi: 10.1016/j.lfs.2022.120926. 120926-120926. [DOI] [PubMed] [Google Scholar]
- You M.H., Zhang S.B., Shen Y.M., Zhao X.H., Chen L.G., Liu J.X., Ma N. Quantitative lipidomics reveals lipid perturbation in the liver of fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102352. 102352-102352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.F., Chen Y.H., Shi X.P., Ye C., Wang J.W., Huang J.Z., Zhang B., Deng Z.Y. Hepatoprotective effects of different mulberry leaf extracts against acute liver injury in rats by alleviating oxidative stress and inflammatory response. Food Funct. 2022;15:8593–8604. doi: 10.1039/d2fo00282e. 13. [DOI] [PubMed] [Google Scholar]
- Yu A.Y., Tang C.M., Wang S.T., Wang Y., Chen L., Li Z.Y., Luo G.Q., Zhong J.W., Fang Z.F., Wang Z.J., Lin S. Effects of dietary supplementation with mulberry leaf powder on the growth performance, lipid metabolism parameters, immunity indicators, and gut microbiota of dogs. Metabolites. 2023;13:918. doi: 10.3390/metabo13080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wang Z.B., Huang C.X., Wang D.H., Chang D.M., Shi X.W., Chen Y.F., Chen H. Positive effects of Mulberry leaf extract on egg quality, lipid metabolism, serum biochemistry, and antioxidant indices of laying hens. Front Vet Sci. 2022;9:1005643. doi: 10.3389/fvets.2022.1005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Shi Y., Huang C., Huang C., Xu P., Zhou C., Liu P., Hu R., Zhuang Y., Li G., Hu G., Guo X. Activation of AMP-activated protein kinase signaling pathway ameliorates steatosis in laying hen hepatocytes. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Chen Q., Chen Z., He T., Zhang L., Huang Q., Liu W., Zeng X., Zhang Y. Anti-obesity effects of mulberry leaf extracts on female high-fat diet-induced obesity: Modulation of white adipose tissue, gut microbiota, and metabolic markers. Food Res Int. 2024;177 doi: 10.1016/j.foodres.2023.113875. [DOI] [PubMed] [Google Scholar]
- Zhao X., Fu Z., Yao M., Cao Y., Zhu T., Mao R., Huang M., Pang Y., Meng X., Li L., Zhang B., Li Y., Zhang H. Mulberry (Morus alba L.) leaf polysaccharide ameliorates insulin resistance- and adipose deposition-associated gut microbiota and lipid metabolites in high-fat diet-induced obese mice. Food Sci. Nutr. 2021;10:617–630. doi: 10.1002/fsn3.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]