Abstract
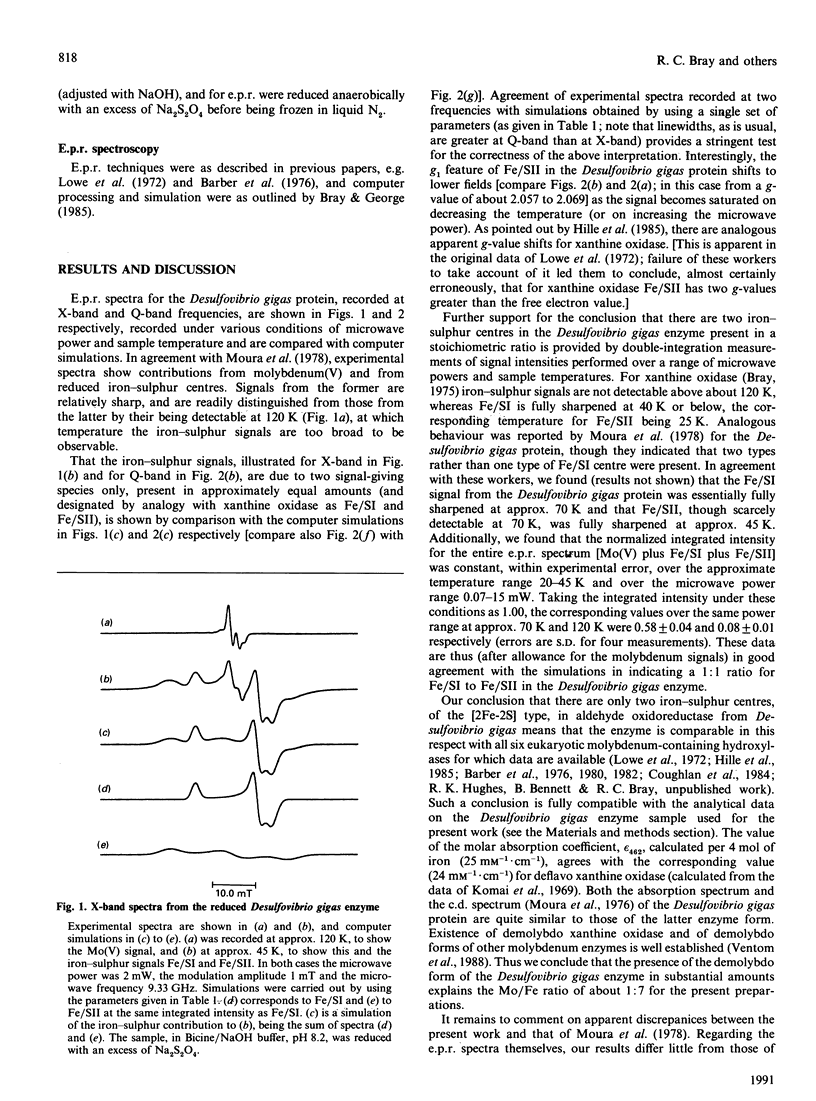
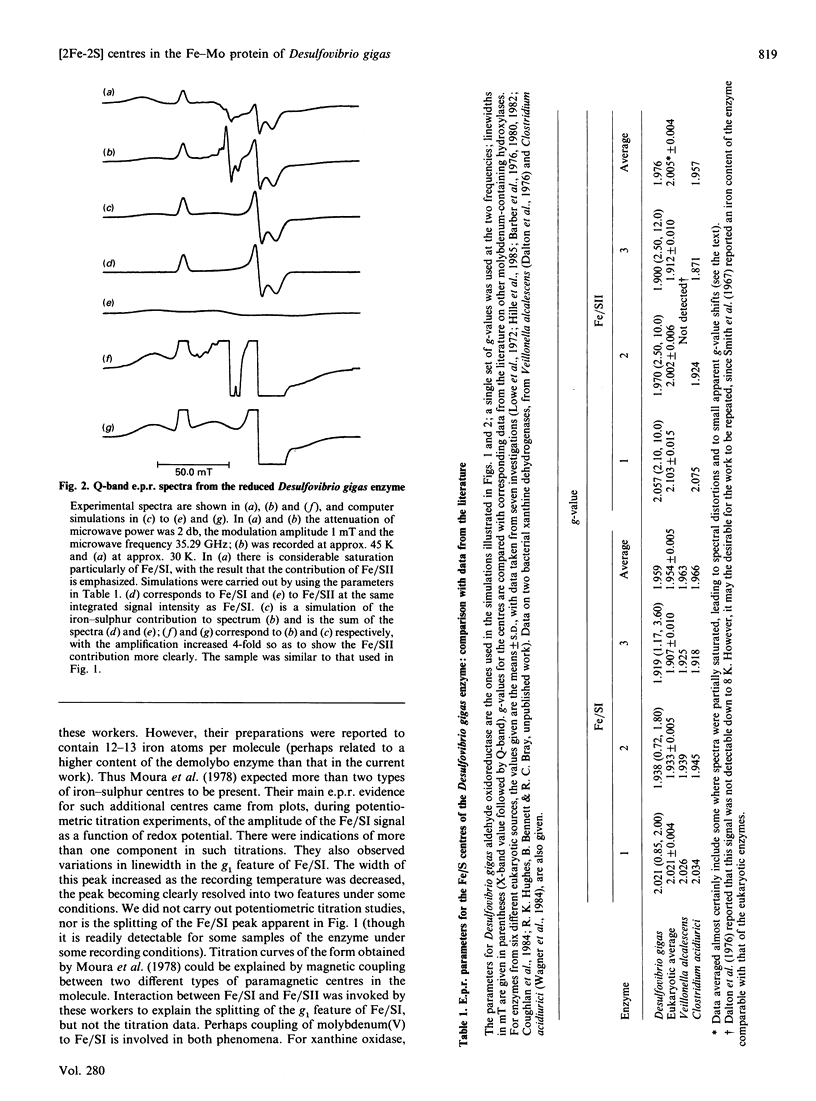
E.p.r. spectra of reduced iron-sulphur centres of the aldehyde oxidoreductase (iron-molybdenum protein) of Desulfovibrio gigas were recorded at X-band and Q-band frequencies and simulated. Results are consistent with the view that only two types of [2Fe-2S] clusters are present, as in eukaryotic molybdenum-containing hydroxylases. The data indicate the Fe/SI centre to be very similar, and the Fe/SII centre somewhat similar, to these centres in the eukaryotic enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya Y., Yamazaki K., Sato M., Noda K., Nishino T., Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem. 1990 Aug 25;265(24):14170–14175. [PubMed] [Google Scholar]
- Barber M. J., Bray R. C., Lowe D. J., Coughlan M. P. Studies by electron-paramagnetic-resonance spectroscopy and stopped-flow spectrophotometry on the mechanism of action of turkey liver xanthine dehydrogenase. Biochem J. 1976 Feb 1;153(2):297–307. doi: 10.1042/bj1530297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. J., Coughlan M. P., Kanda M., Rajagopalan K. V. Electron paramagnetic resonance properties and oxidation-reduction potentials of the molybdenum, flavin, and iron-sulfur centers of chicken liver xanthine dehydrogenase. Arch Biochem Biophys. 1980 May;201(2):468–475. doi: 10.1016/0003-9861(80)90535-4. [DOI] [PubMed] [Google Scholar]
- Barber M. J., Coughlan M. P., Rajagopalan K. V., Siegel L. M. Properties of the prosthetic groups of rabbit liver aldehyde oxidase: a comparison of molybdenum hydroxylase enzymes. Biochemistry. 1982 Jul 20;21(15):3561–3568. doi: 10.1021/bi00258a006. [DOI] [PubMed] [Google Scholar]
- Bray R. C., George G. N. Electron-paramagnetic-resonance studies using pre-steady-state kinetics and substitution with stable isotopes on the mechanism of action of molybdoenzymes. Biochem Soc Trans. 1985 Jun;13(3):560–567. doi: 10.1042/bst0130560. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The inorganic biochemistry of molybdoenzymes. Q Rev Biophys. 1988 Aug;21(3):299–329. doi: 10.1017/s0033583500004479. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P., Mehra R. K., Barber M. J., Siegel L. M. Optical and electron paramagnetic resonance spectroscopic studies on purine hydroxylase II from Aspergillus nidulans. Arch Biochem Biophys. 1984 Mar;229(2):596–603. doi: 10.1016/0003-9861(84)90192-9. [DOI] [PubMed] [Google Scholar]
- Dalton H., Lowe D. J., Pawlik T., Bray R. C. Studies by electron-paramagnetic-resonance spectroscopy on the mechanism of action of xanthine dehydrogenase from Veillonella alcalescens. Biochem J. 1976 Feb 1;153(2):287–295. doi: 10.1042/bj1530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R., Hagen W. R., Dunham W. R. Spectroscopic studies on the iron-sulfur centers of milk xanthine oxidase. J Biol Chem. 1985 Sep 5;260(19):10569–10575. [PubMed] [Google Scholar]
- Johnson J. L., Bastian N. R., Rajagopalan K. V. Molybdopterin guanine dinucleotide: a modified form of molybdopterin identified in the molybdenum cofactor of dimethyl sulfoxide reductase from Rhodobacter sphaeroides forma specialis denitrificans. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3190–3194. doi: 10.1073/pnas.87.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Chaudhury M., Rajagopalan K. V. Identification of a molybdopterin-containing molybdenum cofactor in xanthine dehydrogenase from Pseudomonas aeruginosa. Biofactors. 1991 Jun;3(2):103–107. [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V., Meyer O. Isolation and characterization of a second molybdopterin dinucleotide: molybdopterin cytosine dinucleotide. Arch Biochem Biophys. 1990 Dec;283(2):542–545. doi: 10.1016/0003-9861(90)90681-n. [DOI] [PubMed] [Google Scholar]
- Keith T. P., Riley M. A., Kreitman M., Lewontin R. C., Curtis D., Chambers G. Sequence of the structural gene for xanthine dehydrogenase (rosy locus) in Drosophila melanogaster. Genetics. 1987 May;116(1):67–73. doi: 10.1093/genetics/116.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K., Andreesen J. R. Xanthine dehydrogenase and 2-furoyl-coenzyme A dehydrogenase from Pseudomonas putida Fu1: two molybdenum-containing dehydrogenases of novel structural composition. J Bacteriol. 1990 Oct;172(10):5999–6009. doi: 10.1128/jb.172.10.5999-6009.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- Lee C. S., Curtis D., McCarron M., Love C., Gray M., Bender W., Chovnick A. Mutations affecting expression of the rosy locus in Drosophila melanogaster. Genetics. 1987 May;116(1):55–66. doi: 10.1093/genetics/116.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Bray R. C. Magnetic coupling of the molybdenum and iron-sulphur centres in xanthine oxidase and xanthine dehydrogenases. Biochem J. 1978 Mar 1;169(3):471–479. doi: 10.1042/bj1690471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura J. J., Xavier A. V., Bruschi M., Le Gall J., Hall D. O., Cammack R. A molybdenum-containing iron-sulphur protein from Desulphovibrio gigas. Biochem Biophys Res Commun. 1976 Oct 4;72(3):782–789. doi: 10.1016/s0006-291x(76)80201-x. [DOI] [PubMed] [Google Scholar]
- Moura J. J., Xavier A. V., Cammack R., Hall D. O., Bruschi M., Le Gall J. Oxidation-reduction studies of the Mo-(2Fe-2S) protein from Desulfovibrio gigas. Biochem J. 1978 Aug 1;173(2):419–425. doi: 10.1042/bj1730419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. K., Hederstedt L., Hasnain S., Rutberg L., Guest J. R. Nucleotide sequence encoding the flavoprotein and iron-sulfur protein subunits of the Bacillus subtilis PY79 succinate dehydrogenase complex. J Bacteriol. 1987 Feb;169(2):864–873. doi: 10.1128/jb.169.2.864-873.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan K. V. Novel aspects of the biochemistry of the molybdenum cofactor. Adv Enzymol Relat Areas Mol Biol. 1991;64:215–290. doi: 10.1002/9780470123102.ch5. [DOI] [PubMed] [Google Scholar]
- Rypniewski W. R., Breiter D. R., Benning M. M., Wesenberg G., Oh B. H., Markley J. L., Rayment I., Holden H. M. Crystallization and structure determination to 2.5-A resolution of the oxidized [2Fe-2S] ferredoxin isolated from Anabaena 7120. Biochemistry. 1991 Apr 30;30(17):4126–4131. doi: 10.1021/bi00231a003. [DOI] [PubMed] [Google Scholar]
- Smith S. T., Rajagopalan K. V., Handler P. Purification and properties of xanthine dehydroganase from Micrococcus lactilyticus. J Biol Chem. 1967 Sep 25;242(18):4108–4117. [PubMed] [Google Scholar]
- Turner N., Barata B., Bray R. C., Deistung J., Le Gall J., Moura J. J. The molybdenum iron-sulphur protein from Desulfovibrio gigas as a form of aldehyde oxidase. Biochem J. 1987 May 1;243(3):755–761. doi: 10.1042/bj2430755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventom A. M., Deistung J., Bray R. C. The isolation of demolybdo xanthine oxidase from bovine milk. Biochem J. 1988 Nov 1;255(3):949–956. doi: 10.1042/bj2550949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton J. C., Nicolson R. E., Cock J. M., Walters D. E., Burke J. F., Doyle W. A., Bray R. C. Enzymes depending on the pterin molybdenum cofactor: sequence families, spectroscopic properties of molybdenum and possible cofactor-binding domains. Biochim Biophys Acta. 1991 Mar 29;1057(2):157–185. doi: 10.1016/s0005-2728(05)80100-8. [DOI] [PubMed] [Google Scholar]


