Abstract
Objectives
The functions of eating, drinking, speaking, and breathing demand close coordination of the upper airway musculature which may be challenged by the long-term use of daytime non-invasive ventilation (NIV). This rapid review explores the challenges and practicalities of these interactions in people with neuromuscular disorders.
Methods
A search was performed on PubMed (period 2000-2023) using generic terms to refer to eating, drinking, and speaking related to people with neuromuscular disorders on NIV. A narrative approach was used to summarize the available literature.
Results
Our review shows only a small number of studies exist exploring the use of NIV on swallowing and speaking in people with neuromuscular disorders. We summarize study findings and provide practical advice on eating, drinking and speaking with NIV.
Conclusions
By understanding breathing, NIV mechanics and upper airway interactions, it is possible to optimize swallowing and speaking whilst using NIV. There is a lack of specific guidelines, and concerns regarding aspiration warrant further research and guideline development.
Key words: swallowing, speaking, non-invasive ventilation, neuromuscular disorders
Introduction
The increasing use of non-invasive mechanical ventilation (NIV) for respiratory support in individuals with neuromuscular disorders (NMD) has extended the use of NIV during the daytime 1. By contrast to nocturnal NIV, the use of diurnal NIV interacts with several vital physical and social functions of everyday life including eating, drinking and speaking. In health, these functions act in synchrony within the upper airway where the gastrointestinal and respiratory tracts cross. The upper airways, and especially the larynx – the gateway to the lungs – regulate these multiple functions and protect the lungs from aspiration 2 The use of NIV in various NMDs may disrupt the delicate coordination between breathing, swallowing and speaking, increasing the risk for aspiration to occur 3. Consequently, manufacturers may have advised against eating and swallowing during NIV use 4,5.
In addition to the interaction of NIV on the upper aerodigestive tract, individuals with NMD commonly experience bulbar dysfunction ranging from mild to severe 6-8. Dysarthria and dysphagia may be clinically apparent prior to initiating NIV or may be subclinical, leading to under-reporting by patients and under-detection by healthcare providers 9,10. As respiratory function declines, so too may swallowing and speaking 6,11. This decline, demanding greater dependency on daytime NIV use, may subsequently impact the individual’s participation and enjoyment in daily activities, social inclusion and quality of life 12,13.
Oral feeding may be supplemented or substituted by nutritional support delivered via an enteral feeding tube, such as a nasogastric tube or a gastrostomy tube 14. From the perspective of NIV use, nasogastric tubes can disrupt the patient-mask interface and cause an air leak. Moreover, the effectiveness of enteral tube feeding in terms of survival and quality of life, as well as the type and timing of tube placement in individuals with NMD is still not known 15. One advantage of tube feeding is that energy and hunger needs can be met, reducing the burden of eating and drinking by mouth and allowing oral intake for taste and pleasure. Larger quantities of liquids are almost always more easily and safely managed via feeding tubes than orally in the case of significant swallowing impairment 16. However, patients frequently wish to continue eating and drinking by mouth for as long as possible, and coordination of breathing and swallowing during NIV therefore remains a complex and long-term challenge.
This rapid review explores the challenges, practicalities, and technicalities of interactions between breathing, swallowing, and speaking during NIV focusing on individuals with NMD.
Methods
A search was performed in Medline database (accessed through PubMed, period 2000-2023) using generic terms to refer to eating, drinking, swallowing, and speaking specifically related to people with NMDs on NIV. Additional screening of the reference lists of key publications and searches using Google Scholar were performed to identify other relevant literature. Papers written in English were considered. A narrative approach was used to summarize the available literature as a rapid review.
Results
Practical challenges of swallowing and speaking while on NIV
In severe respiratory muscle weakness, individuals without bulbar dysfunction may be able to eat and drink safely and efficiently whilst using NIV, although more time is needed to coordinate and control bolus preparation and transfer with the addition of positive pressure ventilation 3,17. Further, patients have emphasized that eating can become harder during periods of respiratory illness or exacerbations, and upper airway secretions often become bothersome requiring more active management 18.
During disease progression, the work of chewing and especially swallowing may become too hard to perform with low respiratory capacity. The risk of aspiration may increase 19 and feeding while on NIV may be perceived as unsafe 20. Removal of the mask to allow for oral intake may not be tolerated in patients with respiratory distress 19. The priority is to establish control of respiration first, as a requirement for the subsequent act of swallowing during a meal. A certain degree of “ventilatory reserve” is indispensable to facilitate the swallowing process effectively 21.
For similar reasons, speaking while on NIV may become challenging. Speech intelligibility may be reduced by both the NIV delivery method and the type of mask used, resulting in altered oral-nasal resonance, fewer words per breath, longer pauses, lower audibility and a muffled speech quality 22,23. This can adversely impact communication effectiveness leading to anxiety, frustration and distress from frequent communication failure, affecting interpersonal relationships and quality of life. A range of alternative and augmentative communication approaches can improve communication effectiveness for individuals using NIV such as smartphone apps, writing and text-to-speech devices, alphabet and communication charts, signing and natural gestures 24.
Understanding respiratory-swallowing patterns
To be able to eat, drink and swallow safely and efficiently while on NIV, an understanding of the relationship between breathing and swallowing is first needed 4,25,26.
Moving food and liquid through the upper airway is a complex act requiring significant respiratory-swallow coordination in addition to orolingual and pharyngeal muscular effort 27. Respiratory-swallow coordination patterns in healthy adults occur predominantly via an «expiration-swallow-expiration» pattern 28,29, see Figure 1. This pattern is characterized by an initial inhalation and partial exhalation prior to swallowing such that swallowing occurs during the mid-expiratory phase and continues through the expiratory phase to the completion of the process. In other words, swallowing is immediately preceded and followed by expiration without an intervening inspiratory effort 28,29. This is believed to contribute to airway protection by: 1) generating positive subglottic pressure to resist the accidental penetration of food and liquid into the larynx, 2) allowing a pressurized expiratory airflow reflex to correct penetration of the larynx should it occur, and 3) preventing the inhalation of residual material in the pharynx after swallowing 26,28. Low subglottic pressure during swallowing has been shown to prolong bolus transit times, increase pharyngeal residue volume, and increase the risk of aspiration 30,31.
Figure 1.
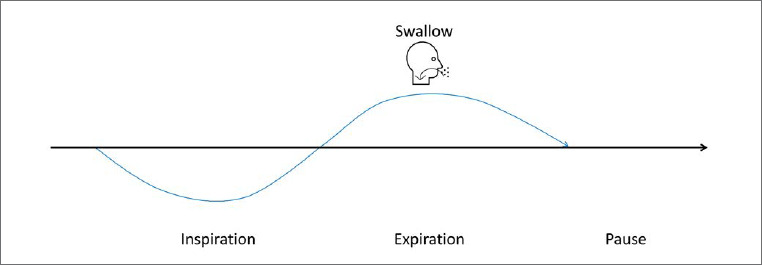
The normal pattern to synchronize the swallowing and breathing is ‘’expiration-swallow-expiration’’ pattern.
Other respiration-swallowing patterns become more prevalent with increasing age, in diseases that increase respiratory muscle elastic loading or respiratory center CO2 loading, or when eating and drinking with NIV. These patterns include “expiration-swallow-inspiration”, “inspiration-swallow-expiration’’, or “inspiration-swallow-inspiration” 26,32-34. Studies by Britton et al. 5,17,35 confirm that individuals using ventilatory support experience challenges and compensations related to coordinating breathing and swallowing. In people with NMD, exhalation times before and after swallowing can be reduced or even deleted 21, leading to inspiration efforts before or after swallowing. Indeed, inspiration rather than expiration was reported to follow swallowing in 40-50% of patients 21,26, leading to a higher risk for aspiration. In addition, individuals with NMD demonstrate longer times to swallow, increased number of chews and several swallows per bolus, requiring greater respiratory-swallow coordination 3,26.
Practical recommendations and strategies to improve swallowing and speaking on NIV
Challenges for swallowing and speaking on NIV relate to generating and maintaining subglottic pressure, suboptimal breathing-swallowing coordination, technical issues with NIV triggering, altered speech quality and risk of aspiration 3,5,17,19-21,25,26,35,36.
Practical recommendations for improving swallowing on NIV may include: 1) reducing the load on the swallowing system, 2) examining swallowing ability, 3) education around swallowing on NIV, 4) optimizing positioning and use of supportive devices, and 5) optimizing the NIV technique. The importance of assessment and guidance by a speech-language therapist is highlighted 5,37,38.
1) Reducing the load on the swallowing system
Chewing is complex and requires considerable respiratory-swallowing coordination and control. Patients using NIV should be encouraged to take smaller bites and to chew thoroughly before swallowing 3. In the case of tongue weakness or jaw muscle fatigue, one option is to modify the food consistency so that it is softer and easier to chew and move from the mouth to the pharynx. Occasionally, such as in the later stages of NMD, food that requires little to no chewing may be needed, e.g, pureed foods. Mealtime management in terms of consuming smaller portions at more regular intervals throughout the day, as well as food fortification and supplementation advice from a dietitian, may be beneficial in reducing the overall load on the swallowing system as dependency on NIV increases 39.
Additional compensatory strategies such as fluid washes may also be useful and are often spontaneously employed by individuals. In the presence of post-swallow residue of solids or puree, drinking liquids may help to clear residues from the pharynx 40. In children with NMD conditions, laryngeal penetration and aspiration (before, during, and after swallowing) with thin liquid were observed in only 6% of cases. Where signs or symptoms of dysphagia exist, or there are concerns of aspiration, clinical assessment of eating and drinking may be insufficient and swallow imaging via an instrumental examination is indicated 41.
2) Examining swallowing ability
Instrumental swallowing assessments, typically performed by a speech-language therapist, include the videofluoroscopic swallowing study (VFSS) and flexible endoscopic evaluation of swallowing (FEES). Both studies aim to examine swallowing capability and capacity including the types of food and liquids that can be managed safely and efficiently by the individual 42.
The VFSS constitutes a radiological evaluation of oral, pharyngeal, and esophageal swallowing of a range of food and liquids using a contrast agent and rapid sequence image acquisition. The assessment facilitates a comprehensive understanding of the anatomical and physiological factors contributing to inefficient swallowing, such as residue, and inadequate airway protection, encompassing both penetration and aspiration 43,44. FEES entails the transnasal passage of a flexible endoscope into the pharynx and larynx to visualize upper airway structure and signs of swallowing dysfunction 45-47. There is also evidence to support the utility of ultrasound as an adjunct clinical tool for the assessment of swallowing and laryngeal function 48.
3) Education around swallowing on NIV
Awareness of swallowing difficulties on NIV is needed among healthcare professionals providing care to people with NMD. In addition, educating individuals and their caregivers about the early signs and symptoms of swallowing impairment, such as food remaining in the mouth, coughing or a ‘wet-voice’ quality after swallowing, is essential in reducing the complications of dysphagia including malnutrition, dehydration, aspiration pneumonia, and other pulmonary sequelae 41.
Techniques for swallowing well during NIV address the critical interaction between breathing and swallowing. Some people naturally adapt their breathing-swallowing pattern to accommodate the delivery of positive pressure to the upper airway over time, for example by waiting to swallow until the expiratory phase of NIV is complete 3. Individuals with NMD report that swallowing during NIV requires conscious attention of the need to alternate eating and drinking with ‘sipping’ air for ventilation 17 Additionally, volitional clearing swallows may help clear the mouth and pharynx of residue and secretions, which can reduce the risk of subsequent post-swallow aspiration 49.
Kinnear and colleagues found non-bulbar patients were able to block several NIV breaths whilst eating and drinking. The mechanism for this was not studied but is likely mediated by volitional upper airway closure. They highlight a learning process primarily driven by trial and error to effectively manage the combination of NIV use with eating, drinking, and speaking. Building on the insights gained in their study, they formulated a hierarchy for teaching these skills providing valuable guidance for those facing this challenge for the first time 3. See an adapted version in Table I.
Table I.
The four E’s protocol to teach patients how to eat whilst on NIV, adapted from Kinnear et al.
| Explain | The role of the pharynx and larynx as pathways for both breathing and swallowing. |
| Explore | Practice upper airway closure to block the ventilator.
Discuss optimal food consistency. Use of smaller food boluses. |
| Experiment | Extend chewing over several breaths.
Swallow food between NIV breaths. Block ventilator after swallowing. Divide larger bolus into several swallows. |
| Expel | Post-meal mechanically assisted cough practice. |
Strategies to facilitate drinking on NIV may include use of a straw or specialized drinking cup to enable delivery to the mouth, and/or adjusting the position of the NIV mask during drinking 3. Thickened liquids may be associated with adverse health outcomes including dehydration, aspiration, and pneumonia, and are not routinely recommended unless demonstrated to improve swallowing physiology and safety on instrumental assessment 39,50,51.
4) Optimizing positioning and use of supportive devices
Ideally, the individual should be seated in an upright position in a chair or wheelchair during eating and drinking. This is because well-supported vertical positioning positively impacts breathing function, neck alignment and forward head position resulting in improved respiratory, laryngeal and pharyngeal biomechanics during swallowing 52. In particular, there is a need to avoid both excessive neck extension and head drop, which may challenge airway protection and swallow efficiency respectively 53. A chin support or neck brace that allows for head stability and a ‘chin-tuck’ position during eating and drinking may facilitate swallowing for some 3,54.
5) Optimizing the NIV technique
The use of NIV may both help and hinder swallowing in individuals with NMD 17 Successful use of NIV includes increased subglottic pressure or reduced swallowing duration 25 which may contribute to the overall timing and efficiency of the swallowing process 34. The challenges are associated with ventilator triggering and autotriggering induced by swallowing 3,4,17, as well as the time needed for chewing interacting with ventilatory requirements 3. While patient-triggered pressure support may offer increased comfort during ventilation, the delivery of breaths at a fixed rate leads to the unpredictability of back-up breaths posed challenges during activities such as chewing and swallowing 3.
Effective coping strategies are associated with awareness of these issues and the practical advice described earlier. To date, there are no dedicated ventilation modes for use when eating. However, supportive strategies for nasal NIV settings have been suggested 3,17,35, the use of mouthpiece ventilation (MPV) has been explored 3,17, and innovative technological developments have also been investigated 21. Using a tailored NIV-program while eating and drinking may facilitate a respiratory-swallow pattern that allows individuals to time swallowing immediately after the ventilator-delivered inspiration, followed by post-swallow exhalation. Such a strategy needs to be explored and investigated further but could represent a beneficial and pragmatic NIV optimization strategy that is likely underutilized.
A. Supportive strategies for nasal NIV settings
An allocated program for nasal NIV use during eating outlining adjustments may be beneficial for some individuals 5. Although the frequency and timing of settings will likely differ between NIV devices and for speaking or swallowing activities 5.
In practice, a ‘swallow-optimized NIV mode’ should include: a fairly slow flow rate, a non-triggered or less sensitive trigger to avoid inspiratory triggering during swallowing, and the use of a volume cycle (preferably) or pressure adjustment mode by the delivery of breaths at a fixed rate 3.
Firstly, ensure that NIV maintains the ventilation needs with adequate inspiratory rate and properly timed inspirations. Thereafter, try to adjust the inspiratory time and -flow, rate, timing of inspirations to improve speaking and swallowing 5.
B. Use of mouthpiece ventilation (MPV)
Within the technology available to date, volume-controlled ventilation via a mouthpiece is suggested to be the best method for speaking and swallowing 5,17,35,55,56. It may positively affect breathing-swallowing coordination, improve eating/drinking experience 17, speech and communication 57, and additionally, it may be safer for swallowing than nasal NIV considering aspirations 17. Moreover, the use of MPV over nasal NIV appears to be preferred by patients during eating, drinking and speaking 17
With MPV, the user can switch between taking sips of air (inspiration) and moving the mouth away for swallowing or coughing 5. In dyspneic NMD patients, the addition of daytime MPV to effective nocturnal NIV was reported to restore some respiratory muscle endurance 36, leading to possible improvements in swallowing function from increased respiratory recoil on subglottic pressure generation 8,17,55.
Allowing for the long inspiratory pauses interrupting the flow of speech, MPV enabled longer breaths with more syllables 55.
An optimal placement of the mouthpiece may include close approximation to the mouth to minimize the need to turn the head repeatedly and reduce pause time during speaking, with the ability to adjust the mouthpiece to an alternative position to facilitate coordination with eating efforts 5.
C. Innovative technological developments
In an experimental study of ten patients with severe NMD requiring daytime NIV, a novel patient-controlled off-switch to pause inspirations during swallowing was investigated 21. Use of the device improved respiratory-swallowing control and coordination, measured by fewer ventilator interruptions during swallowing, increased post-swallow expiration airflow, and decreased perception of dyspnoea during swallowing, without impairing swallowing comfort 21. Unfortunately, such off-switches are not yet commercially available, limiting the ability to use this ventilatory-pause technique in clinical practice.
6) Teaching how to speak whilst on NIV
A protocol for teaching effective speech on NIV has been suggested by Kinnear et al. 3, see Table II for adapted version. The protocol helps patients understand the role of phonation, practice respiratory-phonatory coordination, and increase voice volume with ventilator support 3.
Table II.
The four E’s protocol to teach patients how to speak whilst on NIV, adapted from Kinnear et al.
| Explain | Phonation occurs through the vocal cords during exhalation |
| Explore | Repetitive phonation on single syllables throughout the breathing cycle to demonstrate the loss of volume during inspiration.
Repeated phonation with single syllable during expiration only, counting the syllables achieved on each breath. |
| Experiment | Practice short phrases with the mastered number of syllables per breath. |
| Extend | Try to increase voice volume by breath stacking using the ventilator. |
Discussion
A variety of mechanisms can affect swallowing and speaking during NIV, with the potential for upper aerodigestive functions to be both improved and disrupted by physiological changes imposed by ventilatory support. Awareness of the interaction between breathing, speaking, and swallowing is essential for patients, caregivers, and healthcare professionals, as is an understanding of the early signs and symptoms of difficulty swallowing. Practical strategies for enhancing eating and drinking while on NIV include reducing the eating load, optimizing body and neck positioning, assessing the safety and efficiency of swallowing, instruction on eating techniques, and consideration of ventilatory approaches beyond nasal/face mask NIV. The significance of evaluation and guidance from a speech-language therapist is emphasised.
Swallowing is a coordinated action that usually occurs smoothly alongside breathing in healthy individuals 29. However, breathing-swallowing coordination can be challenged in different ways for individuals with NMD using NIV 17. In people with severe respiratory failure, the typical respiratory pattern without NIV is characterized by rapid shallow breathing and low tidal volumes 58 NIV enhances vital capacity and tidal volumes which are likely to improve swallowing through the transfer of greater respiratory recoil on raised subglottic pressure 4,21,59,60. Maintaining adequate expiratory subglottic pressure is a critical component of swallowing performance. Low subglottic pressure has been found to prolong bolus transit times and increase the volume of post-swallow pharyngeal residue, increasing the risk of aspiration 4,21.
Despite the incidence of dysphagia in adults with NMD, many individuals are able to eat, drink, and speak effectively while using NIV 3,61 Our clinical experience suggests that individuals growing up using NIV due to chronic NMD adapt better to altered breathe-swallow interactions than older adults initiating NIV due to acquired progressive conditions or acute post-intubation situations. Simple adaptations to the texture and delivery of food and liquids may be helpful, e.g. softer foods, smaller bites and mouthfuls, single sequential sips of liquids and use of a straw. Swallowing assessments including VFSS and FEES are important aspects of care 39,47.
In muscular dystrophy, the use of NIV has been shown to reduce dyspnoea and fatigue associated with eating and drinking compared to no ventilatory support 17. The results indicate that NIV may improve performance by establishing better control of breathing and ‘ventilatory reserve’. Unloading the respiratory muscles with mechanical ventilation is liable to improve breathing-swallowing patterns 21. Nasal NIV allows patients to eat while receiving ventilation and provides the flexibility to interrupt ventilation at their discretion for swallowing, which may promote swallow synchronization with the ventilatory cycle 17. The administration of daytime NIV through a mouthpiece has become more common in ventilator-dependent patients, and MPV is indicated to be more convenient in coordinating breathing, eating and drinking 17. However, two important practical issues have been identified: ventilator inspirations triggered by swallowing, and chewing effort interfering with ventilatory requirements 4,17,19.
Logically, the issue of NIV triggering of inspirations coinciding with swallowing is greater with the nasal mask NIV than with mouthpiece NIV. Indeed, trigger via nasal mask can be used unintentionally, while MPV trigger is always voluntarily controlled by the patient. Nasal mask trigger can therefore lead to concerns about the potential for food or liquid to be blown into the airway while eating and drinking. This occurs because pressure-targeted NIV systems automatically trigger inspiration when detecting a pressure drop, such as when the mouth opens for eating or drinking, when the upper airway re-opens after the pharyngeal swallow 17,19. Recognizing this problem, studies have explored modifications to NIV systems permitting users to temporarily pause the inspiratory flow during each sip of liquid and bite of food 4,21 A study by Terzi et al. used the same off-switch strategy (like the study performed in individuals with NMD) 21 in individuals with hypercapnic chronic obstructive pulmonary disease. They showed that stopping positive pressure inspirations during swallowing eliminated ventilator triggering and autotriggering induced by swallowing. Further, the use of NIV for acute respiratory failure was associated with improvements in swallowing efficiency and more favourable breathing-swallowing interactions than spontaneous breathing 4. In clinical settings, there’s a critical need for such innovations, with hopes for future commercial accessibility.
The time required for chewing food may interfere with ventilation. Typically, the nasopharynx remains open with the soft palate lowered during chewing, allowing tidal breathing to continue prior to initiation of the pharyngeal phase of swallowing 17. In contrast to nasal NIV, inspiration via MPV takes place orally 56, which could be challenging and even risky when chewing. The longer the chewing process persists, the more ventilation may be delayed, potentially intensifying the experience of dyspnoea – a phenomenon commonly described by individuals particularly when consuming solid foods 17. In the muscular dystrophy population, the prolonged chewing time attributed to masticatory muscle weakness and dental malocclusion may worsen this effect 62,63. However, MPV is potentially less favorable in chewing than nasal NIV, but this is not evident. For instance, it seems that individuals with DMD using daytime MPV have little or no problem to manage chewing and breathing when using MPV.
Factors such as acute respiratory exacerbation can also complicate eating and upper airway function 18. The use of manually or mechanically assisted cough techniques may be beneficial in supporting continued oral intake in individuals with NMD on full-time NIV, providing prophylactic airway clearance in the event of silent aspiration 3,64. Special attention should be given to the use of mechanical cough devices in the presence of concomitant bulbar dysfunction, where adverse outcomes associated with laryngeal obstruction or induced aspiration of secretions and residues may occur 65,66. Understanding how NIV can support or hinder swallowing, coupled with a basic grasp of the mechanics of NIV systems, can be beneficial for the clinical assessment and treatment of dysphagia in NIV users 17.
A qualitative study showed that individuals with NMD preferred MPV compared to nasal NIV during speaking 5,35. Three major themes emerged from the interview data of Britton et al. Firstly, MPV aids speech by increasing voice loudness, utterance duration, clarity, and speaking endurance. Secondly, MPV interferes with the flow of speaking due to the need to pause to take a breath, problems with mouthpiece placement, and difficulty in using speech recognition software. Thirdly, nasal NIV interferes with speaking by causing abnormal nasal resonance, muffled speech, mask discomfort, and difficulty in coordinating speaking with ventilator-delivered inspirations 35. NIV interfaces and modes/settings provide an external challenge to the usual aspects of speech production, leading to reduced intelligibility and listener comprehensibility. Communication vulnerability, defined as a struggle to share information in a particular environment 67, may be experienced by individuals and is likely to be exacerbated in those with bulbar-related speech impairment. Future research is needed to determine the most effective way to use MPV for speaking and whether training participants in its use can bring even greater speech benefits 55.
Future aspects
Future research should explore the breathing and swallowing interactions in neuromuscular NIV users, specifically the respiratory phase, timing, and lung volumes. A particularly useful avenue would be the optimization of NIV parameters, such as flow rate and inspiratory pressure, and behavioural training, which may help patients improve chewing and promote post-swallow expiration. Additionally, to examine swallowing dynamics in individuals with NMD who have recently initiated NIV therapy 3.
The paucity of guidelines for the management of swallowing and speaking during NIV is apparent in the current literature. The consequences for disrupted swallowing can be serious and life-threatening, particularly aspiration. Notably, many studies have not included visualization of swallowing using flexible endoscopy or videofluoroscopy, and thus microaspiration and silent aspiration while eating and drinking on NIV cannot be ruled out during reported practicalities 3,5,17,21,35. The technique of transnasal flexible laryngoscopy during mechanically assisted cough has been shown to be feasible to assess laryngeal function during ongoing NIV treatment 65,68-71, and a similar method could be applied to assess the impact and outcomes of NIV on swallowing in NMD patients with and without bulbar dysfunction. NIV services should adopt a coordinated, multidisciplinary care approach that includes early and regular review by speech-language therapists and respiratory physiotherapists to optimise breathing with the upper airway functions of speaking, swallowing and coughing 72. The main goal of long-term NIV is to maintain or improve good quality of life 73. Swallowing and speaking are critical to this, and future studies investigating the effectiveness of NIV therapy for respiratory failure should routinely incorporate a range of standardized outcome measures to evaluate the impact on swallowing and speaking from the perspectives of patient, caregiver and clinician. Further research is needed to improve effective speech with different communication partners in a variety of settings, either through modifications of NIV delivery methods and interfaces or the addition of external devices such as microphones, in order to reduce the psychosocial burden of NIV dependency on day to day life 22,6.
Further, it remains to be investigated if Intermittent abdominal pressure ventilation (IAPV) can be used to assist eating, drinking, and speaking during ventilation. One significant advantage of IAPV is that it doesn’t require a traditional interface, such as a mask or mouthpiece. This can be particularly beneficial for patients who have difficulties with these parallel functional tasks with traditional interfaces.
Conclusions
This review highlights the intricate interactions between breathing, swallowing, and speaking, particularly in the context of NIV. Understanding these interactions and implementing strategies to overcome challenges is essential for improving the physical, functional, and psychosocial well-being of individuals with respiratory and neuromuscular disorders who rely on NIV. By understanding the breathing-swallowing interaction, it is possible to introduce practical strategies to optimize swallowing and speaking while using NIV. The impact of NIV on speech and swallowing is a relatively underexplored area of research compared to tracheostomy-related studies. The limited evidence to support best practice in managing swallowing and speaking in people with NIV dependency, and concerns regarding aspiration during NIV, warrant further research and guideline development.
Acknowledgements
The authors extend many thanks to William Kinnear for providing additional practical details concerning the use of NIV during eating, drinking and speaking.
Conflicts of interest statement
The authors declare no conflict of interest
Funding
None.
Authors’ contributions
All authors made a significant contribution to the conception and the design of the article and of the collection, analysis, and interpretation of the data, drafting of the article and revising it critically for content and final approval of the version to be published.
History
Received: December 19, 2023
Accepted: February 7, 2024
Figures and tables
References
- 1.Toussaint M, Steens M, Wasteels G, et al. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J. 2006;28(3):549-555. https://doi.org/10.1183/09031936.06.00004906. 10.1183/09031936.06.00004906 [DOI] [PubMed] [Google Scholar]
- 2.Stein JF. Why did language develop? Int J Pediatr Otorhinolaryngol. 2003;67 Suppl 1:S131-5. https://doi.org/10.1016/j.ijporl.2003.08.011. 10.1016/j.ijporl.2003.08.011 [DOI] [PubMed] [Google Scholar]
- 3.Kinnear W, Dring K, Kinnear K, et al. How to eat, drink and speak on non-invasive ventilation. Chron Respir Dis. 2021;18:14799731211061156.. https://doi.org/10.1177/14799731211061156. 10.1177/14799731211061156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terzi N, Normand H, Dumanowski E, et al. Noninvasive ventilation and breathing-swallowing interplay in chronic obstructive pulmonary disease*. Crit Care Med. 2014:42(3):565-573. https://doi.org/10.1097/CCM.0b013e3182a66b4a. 10.1097/CCM.0b013e3182a66b4a [DOI] [PubMed] [Google Scholar]
- 5.Britton D, Benditt JO, Hoit JD. Beyond Tracheostomy: Noninvasive Ventilation and Potential Positive Implications for Speaking and Swallowing. Semin Speech Lang. 2016;37(3):173-84. https://doi.org/10.1055/s-0036-1583545. 10.1055/s-0036-1583545 [DOI] [PubMed] [Google Scholar]
- 6.Sarmet M, Mangilli LD, Costa GP, et al. The relationship between pulmonary and swallowing functions in patients with neuromuscular diseases followed up by a tertiary referral center: a cross-sectional study. Logoped Phoniatr Vocol. 2022;47(2):117-124. https://doi.org/10.1080/14015439.2021.1879254. 10.1080/14015439.2021.1879254 [DOI] [PubMed] [Google Scholar]
- 7.Perry BJ, Nelson J, Wong JB, et al. Predicting dysphagia onset in patients with ALS: the ALS dysphagia risk score. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23(3-4):271-278. https://doi.org/10.1080/21678421.2021.1961805. 10.1080/21678421.2021.1961805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toussaint M, Davidson Z, Bouvoie V, et al. Dysphagia in Duchenne muscular dystrophy: practical recommendations to guide management. Disabil Rehabil 2016;38(20):2052-62. https://doi.org/10.3109/09638288.2015.1111434. 10.3109/09638288.2015.1111434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9(2):177-89. https://doi.org/10.1016/s1474-4422(09)70272-8. 10.1016/s1474-4422(09)70272-8 [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Madhavan A, Krajewski E, et al. Assessment of dysarthria and dysphagia in patients with amyotrophic lateral sclerosis: Review of the current evidence. Muscle Nerve. 2021;64(5):520-531. https://doi.org/https://doi.org/10.1002/mus.27361. 10.1002/mus.27361 [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Oh HJ, Choi WA, et al. Relationship between Eating and Digestive Symptoms and Respiratory Function in Advanced Duchenne Muscular Dystrophy Patients. J Neuromuscul Dis. 2020;7(2):101-107. https://doi.org/10.3233/jnd-190435. 10.3233/jnd-190435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith R, Bryant L, Hemsley B. The true cost of dysphagia on quality of life: The views of adults with swallowing disability. Int J Lang Commun Disord. 2023;58(2):451-466. https://doi.org/https://doi.org/10.1111/1460-6984.12804. 10.1111/1460-6984.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walshe M, Miller N. Living with acquired dysarthria: the speaker’s perspective. Disabil Rehabil. 2011;33(3):195-203. https://doi.org/10.3109/09638288.2010.511685. 10.3109/09638288.2010.511685 [DOI] [PubMed] [Google Scholar]
- 14.Ambrosino N, Clini E. Long-term mechanical ventilation and nutrition. Respir Med. 2004;98(5):413-20. https://doi.org/10.1016/j.rmed.2003.11.008. 10.1016/j.rmed.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 15.Sulistyo A, Abrahao A., Freitas M.E., et al. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev, 2023. 8(8): p. CD004030. https://doi.org/10.1002/14651858.CD004030.pub4. 10.1002/14651858.CD004030.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kringelbach ML. The pleasure of food: underlying brain mechanisms of eating and other pleasures. Flavour. 2015;4(1):20. https://doi.org/10.1186/s13411-014-0029-2. 10.1186/s13411-014-0029-2 [DOI] [Google Scholar]
- 17.Britton D, Hoit JD, Benditt JO, et al. Swallowing with Noninvasive Positive-Pressure Ventilation (NPPV) in Individuals with Muscular Dystrophy: A Qualitative Dysphagia. 2020;35(1):32-41. https://doi.org/10.1007/s00455-019-09997-6. 10.1007/s00455-019-09997-6 [DOI] [PubMed] [Google Scholar]
- 18.Toussaint M, Chatwin M, Gonzales J, et al. 228th ENMC International Workshop:: Airway clearance techniques in neuromuscular disorders Naarden, The Netherlands, 3-5 March, 2017. Neuromuscul Disord, 2018. 28(3): p. 289-298. https://doi.org/10.1016/j.nmd.2017.10.008. 10.1016/j.nmd.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 19.Hori R, Isaka M, Oonishi K, et al. Coordination between respiration and swallowing during non-invasive positive pressure ventilation. Respirology. 2016;21(6):1062-7. https://doi.org/10.1111/resp.12790. 10.1111/resp.12790 [DOI] [PubMed] [Google Scholar]
- 20.Singer P, Rattanachaiwong S. To eat or to breathe? The answer is both! Nutritional management during noninvasive ventilation. Crit Care 2018;22(1):27. https://doi.org/10.1186/s13054-018-1947-7. 10.1186/s13054-018-1947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garguilo M, Lejaille M, Vaugier I, et al. Noninvasive Mechanical Ventilation Improves Breathing-Swallowing Interaction of Ventilator Dependent Neuromuscular Patients: A Prospective Crossover Study. PLoS One. 2016;11(3):e0148673. https://doi.org/10.1371/journal.pone.0148673. 10.1371/journal.pone.0148673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young ED, Ferguson SH, Brewer LM, et al. Using a novel in-mask non-invasive ventilator microphone to improve talker intelligibility in healthy and hospitalised adults. Int J Speech Lang Pathol. 2023. Oct 13:1-16. https://doi.org/10.1080/17549507.2023.2251726. 10.1080/17549507.2023.2251726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong A-KI, Cheung PC, Happ MB, et al. Consequences and Solutions for the Impact of Communication Impairment on Noninvasive Ventilation Therapy for Acute Respiratory Failure: A Focused Review. Crit Care Explor. 2020;2(6):e0121. https://doi.org/10.1097/cce.0000000000000121. 10.1097/cce.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vansteensel MJ, Jarosiewicz B. Brain-computer interfaces for communication. Handb Clin Neurol. 2020;168:67-85. https://doi.org/10.1016/B978-0-444-63934-9.00007-X. 10.1016/B978-0-444-63934-9.00007-X [DOI] [PubMed] [Google Scholar]
- 25.Shaikh H, Laghi F. Eating on noninvasive ventilation*. Crit Care Med 2014;42(3):737-8. https://doi.org/10.1097/CCM.0000000000000002. 10.1097/CCM.0000000000000002 [DOI] [PubMed] [Google Scholar]
- 26.Terzi N, Orlikowski D, Aegerter P, et al. Breathing-swallowing interaction in neuromuscular patients: a physiological evaluation. Am J Respir Crit Care Med. 2007;175(3):269-76. https://doi.org/10.1164/rccm.200608-1067OC. 10.1164/rccm.200608-1067OC [DOI] [PubMed] [Google Scholar]
- 27.Martin-Harris B, Brodsky MB, Michel Y, et al. Breathing and Swallowing Dynamics Across the Adult Lifespan. Arch Otolaryngol Head Neck Surg. 2005;131(9):762-70. https://doi.org/10.1001/archotol.131.9.762. 10.1001/archotol.131.9.762 [DOI] [PubMed] [Google Scholar]
- 28.Preiksaitis HG, Mayrand S, Robins K, et al. Diamant, Coordination of respiration and swallowing: effect of bolus volume in normal adults. Am J Physiol.1992;263(3 Pt 2):R624-30. https://doi.org/10.1152/ajpregu.1992.263.3.R624. 10.1152/ajpregu.1992.263.3.R624 [DOI] [PubMed] [Google Scholar]
- 29.Martin-Harris B, Brodsky MB, Michel Y, et al. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131(9):762-70. https://doi.org/10.1001/archotol.131.9.762. 10.1001/archotol.131.9.762 [DOI] [PubMed] [Google Scholar]
- 30.Stachler RJ, Hamlet SL, Choi J, et al. Scintigraphic quantification of aspiration reduction with the Passy-Muir valve. Laryngoscope. 1996;106(2 Pt 1):231-4. https://doi.org/10.1097/00005537-199602000-00024. 10.1097/00005537-199602000-00024 [DOI] [PubMed] [Google Scholar]
- 31.Logemann JA, Pauloski BR, Colangelo L. Light digital occlusion of the tracheostomy tube: a pilot study of effects on aspiration and biomechanics of the swallow. Head Neck. 1998;20(1):52-7. https://doi.org/10.1002/(sici)1097-0347(199801)20:1<52::aid-hed8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Terzi N, Prigent H, Lejaille M, et al. Impact of tracheostomy on swallowing performance in Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20(8):493-8. https://doi.org/10.1016/j.nmd.2010.05.009. 10.1016/j.nmd.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Shaker R, Li Q, Ren J, et al. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol. 1992;263(5 Pt 1):G750-5. https://doi.org/10.1152/ajpgi.1992.263.5.G750. 10.1152/ajpgi.1992.263.5.G750 [DOI] [PubMed] [Google Scholar]
- 34.Gross RD, Atwood CW, Jr, Ross SB, et al. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(7):559-65. https://doi.org/10.1164/rccm.200807-1139OC. 10.1164/rccm.200807-1139OC [DOI] [PubMed] [Google Scholar]
- 35.Britton D, Hoit JD, Pullen E, et al. Experiences of Speaking With Noninvasive Positive Pressure Ventilation: A Qualitative Investigation. Am J Speech Lang Pathol. 2019;28(2S):784-792. https://doi.org/10.1044/2019_AJSLP-MSC18-18-0101. 10.1044/2019_AJSLP-MSC18-18-0101 [DOI] [PubMed] [Google Scholar]
- 36.Toussaint M, Soudon P, Kinnear W. Effect of non-invasive ventilation on respiratory muscle loading and endurance in patients with Duchenne muscular dystrophy. Thorax. 2008;63(5):430-4. https://doi.org/10.1136/thx.2007.084574. 10.1136/thx.2007.084574 [DOI] [PubMed] [Google Scholar]
- 37.Kooi-van Es M, Erasmus CE, Voet NBM, et al. Dysphagia and Dysarthria in Children with Neuromuscular Diseases, a Prevalence Study. J Neuromuscul Dis. 2020;7(3):287-295. https://doi.org/10.3233/JND-190436. 10.3233/JND-190436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KKooi-van Es M, Erasmus CE, Voet NBM, et al. Best practice recommendations for speech-language pathology in children with neuromuscular disorders: A Delphi-based consensus study. Int J Speech Lang Pathol. 2023;10.1080/17549507.2023.2181224:1-14. https://doi.org/10.1080/17549507.2023.2181224. 10.1080/17549507.2023.2181224 [DOI] [PubMed] [Google Scholar]
- 39.Narayan S, Pietrusz A, Allen J, et al. Adult North Star Network (ANSN): Consensus Document for Therapists Working with Adults with Duchenne Muscular Dystrophy (DMD) - Therapy Guidelines. J Neuromuscul Dis. 2022;9(3):365-381. https://doi.org/10.3233/jnd-210707. 10.3233/jnd-210707 [DOI] [PubMed] [Google Scholar]
- 40.van den Engel-Hoek L, Erasmus CE, van Hulst KC, et al. Children with central and peripheral neurologic disorders have distinguishable patterns of dysphagia on videofluoroscopic swallow study. J Child Neurol. 2014;29(5):646-53. https://doi.org/10.1177/0883073813501871. 10.1177/0883073813501871 [DOI] [PubMed] [Google Scholar]
- 41.Audag N, Goubau C, Toussaint M, et al. Screening and evaluation tools of dysphagia in adults with neuromuscular diseases: a systematic review. Therapeutic Advances in Chronic Disease. 2019;10:2040622318821622. https://doi.org/10.1177/2040622318821622. 10.1177/2040622318821622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady S, Donzelli J. The modified barium swallow and the functional endoscopic evaluation of swallowing. Otolaryngol Clin North Am. 2013;46(6):1009-22. https://doi.org/10.1016/j.otc.2013.08.001. 10.1016/j.otc.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 43.Hanayama K, Liu M, Higuchi Y, et al. Dysphagia in patients with Duchenne muscular dystrophy evaluated with a questionnaire and videofluorography. Disabil Rehabil. 2008;30(7):517-522. https://doi.org/10.1080/09638280701355595. 10.1080/09638280701355595 [DOI] [PubMed] [Google Scholar]
- 44.Waito AA, Plowman EK, Barbon CEA, et al. A Cross-Sectional, Quantitative Videofluoroscopic Analysis of Swallowing Physiology and Function in Individuals With Amyotrophic Lateral Sclerosis. J Speech Lang Hear Res. 2020;63(4):948-962. https://doi.org/doi:10.1044/2020_JSLHR-19-00051. 10.1044/2020_JSLHR-19-00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leder SB, Novella S, Patwa H. Use of Fiberoptic Endoscopic Evaluation of Swallowing (FEES) in Patients with Amyotrophic Lateral Sclerosis. Dysphagia. 2004;19(3):177-181. https://doi.org/10.1007/s00455-004-0009-2. 10.1007/s00455-004-0009-2 [DOI] [PubMed] [Google Scholar]
- 46.Pilz W, Baijens LWJ, Passos VL, et al. Swallowing assessment in myotonic dystrophy type 1 using fiberoptic endoscopic evaluation of swallowing (FEES). Neuromuscular Disorders. 2014;24(12):1054-1062. https://doi.org/https://doi.org/10.1016/j.nmd.2014.06.002. 10.1016/j.nmd.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 47.Kawamoto-Hirano A, Ikeda R, Takahashi T, et al. Bedside evaluation of swallowing function to predict aspiration pneumonia in Duchenne muscular dystrophy. Auris Nasus Larynx. 2023;50(2):247-253. https://doi.org/https://doi.org/10.1016/j.anl.2022.07.006. 10.1016/j.anl.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 48.Allen JE, Cleland J, Smith M. An initial framework for use of ultrasound by speech and language therapists in the UK: Scope of practice, education and governance. Ultrasound. 2023;31(2):92-103. https://doi.org/10.1177/1742271X221122562. 10.1177/1742271X221122562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank K, Frank U. [Respiratory therapy (bagging, air stacking) for patients in early neurorehabilitation]. Pneumologie. 2011;65(5):314-9. https://doi.org/10.1055/s-0030-1256181. 10.1055/s-0030-1256181 [DOI] [PubMed] [Google Scholar]
- 50.van den Engel-Hoek L, de Groot IJM, de Swart BJM, et al. Feeding and Swallowing Disorders in Pediatric Neuromuscular Diseases: An Overview. J Neuromuscul Dis. 2015;2:357-369. https://doi.org/10.3233/JND-150122. 10.3233/JND-150122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werden Abrams S, Gandhi P, Namasivayam-MacDonald A. The Adverse Effects and Events of Thickened Liquid Use in Adults: A Systematic Review. Am J Speech Lang Pathol. 2023;32(5):2331-2350. https://doi.org/10.1044/2023_AJSLP-22-00380. 10.1044/2023_AJSLP-22-00380 [DOI] [PubMed] [Google Scholar]
- 52.Rosen SP, Abdelhalim SM, Jones CA, et al. Effect of Body Position on Pharyngeal Swallowing Pressures Using High-Resolution Manometry. Dysphagia. 2018;33(3):389-398. https://doi.org/10.1007/s00455-017-9866-3. 10.1007/s00455-017-9866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ertekin C, Keskin A, Kiylioglu N, et al. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Arch Phys Med Rehabil. 2001;82(9):1255-60. https://doi.org/10.1053/apmr.2001.25156. 10.1053/apmr.2001.25156 [DOI] [PubMed] [Google Scholar]
- 54.Leigh J-H, Oh B-M, Seo HG, et al. Influence of the Chin-Down and Chin-Tuck Maneuver on the Swallowing Kinematics of Healthy Adults. Dysphagia. 2015;30(1):89-98. https://doi.org/10.1007/s00455-014-9580-3. 10.1007/s00455-014-9580-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britton Pullen DE, Hoit JD, Benditt JO. Effects of Mouthpiece Noninvasive Ventilation on Speech in Men With Muscular Dystrophy: A Pilot Study. Am J Speech Lang Pathol. 2021;30(3S):1373-1381. https://doi.org/doi:10.1044/2020_AJSLP-20-00146. 10.1044/2020_AJSLP-20-00146 [DOI] [PubMed] [Google Scholar]
- 56.Pierucci P, Portacci A, Carpagnano GE, et al. The right interface for the right patient in noninvasive ventilation: a systematic review. Expert Rev Respir Med. 2022;16(8):931-944. https://doi.org/10.1080/17476348.2022.2121706. 10.1080/17476348.2022.2121706 [DOI] [PubMed] [Google Scholar]
- 57.Chatwin M, Goncalves M, Gonzalez-Bermejo J, et al. 252nd ENMC international workshop: Developing best practice guidelines for management of mouthpiece ventilation in neuromuscular disorders. March 6th to 8th 2020, Amsterdam, the Netherlands. Neuromuscul Disord. 2020;30(9):772-781. https://doi.org/10.1016/j.nmd.2020.07.008. 10.1016/j.nmd.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanini B, Misuri G, Gigliotti F, et al. Perception of dyspnea in patients with neuromuscular disease. Chest. 2001;120(2):402-8. https://doi.org/10.1378/chest.120.2.402. 10.1378/chest.120.2.402 [DOI] [PubMed] [Google Scholar]
- 59.Gross RD, Atwood CW, Jr, Grayhack JP, et al. Lung volume effects on pharyngeal swallowing physiology. J Appl Physiol (1985). 2003;95(6):2211-7. https://doi.org/10.1152/japplphysiol.00316.2003. 10.1152/japplphysiol.00316.2003 [DOI] [PubMed] [Google Scholar]
- 60.Gross RD, Carrau RL, Slivka WA, et al. Deglutitive subglottic air pressure and respiratory system recoil. Dysphagia. 2012;27(4):452-9. https://doi.org/10.1007/s00455-011-9389-2. 10.1007/s00455-011-9389-2 [DOI] [PubMed] [Google Scholar]
- 61.Wollinsky KH, Kutter B, Geiger PM. Long-term ventilation of patients with Duchenne muscular dystrophy: experiences at the Neuromuscular Centre Ulm. Acta Myol. 2012;31(3):170-8. [PMC free article] [PubMed] [Google Scholar]
- 62.van den Engel-Hoek L, de Groot IJ, Sie LT, et al. Dystrophic changes in masticatory muscles related chewing problems and malocclusions in Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26(6):354-60. https://doi.org/10.1016/j.nmd.2016.03.008. 10.1016/j.nmd.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 63.Kroon RHMJM, Horlings CGC, de Swart BJM, et al. Swallowing, Chewing and Speaking: Frequently Impaired in Oculopharyngeal Muscular Dystrophy. JJ Neuromuscul Dis. 2020;7:483-494. https://doi.org/10.3233/JND-200511. 10.3233/JND-200511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miura T, Takami A, Makino M, et al. Rate of oral intake and effects of mechanical insufflation-exsufflation on pulmonary complications in patients with duchenne muscular dystrophy. J Phys Ther Sci. 2017;29(3):487-490. https://doi.org/10.1589/jpts.29.487. 10.1589/jpts.29.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vollsaeter M, Skjoldmo A, Roksund O, et al. Tailoring NIV by dynamic laryngoscopy in a child with spinal muscular atrophy type I. Clin Case Rep. 2021;9(4):1925-1928. https://doi.org/10.1002/ccr3.3905. 10.1002/ccr3.3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen JE, O’Leary EL. Considerations for chest clearance and cough augmentation in severe bulbar dysfunction: a case study. Can J Respir Ther. 2018;54(3):66-70. https://doi.org/10.29390/cjrt-2018-014. 10.29390/cjrt-2018-014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dubin R, Ackrivo J. Giving Patients a Voice Among the Inpatient Orchestra: Communication Vulnerability in Noninvasive Ventilation Therapy. CHEST. 2021;159(4):1324-1325. https://doi.org/10.1016/j.chest.2020.10.068. 10.1016/j.chest.2020.10.068 [DOI] [PubMed] [Google Scholar]
- 68.Andersen T, Sandnes A, Brekka AK, et al. Laryngeal response patterns influence the efficacy of mechanical assisted cough in amyotrophic lateral sclerosis. Thorax. 2017;72(3):221-229. https://doi.org/10.1136/thoraxjnl-2015-207555. 10.1136/thoraxjnl-2015-207555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen T, Sandnes A, Hilland M, et al. Laryngeal response patterns to mechanical insufflation-exsufflation in healthy subjects. Am J Phys Med Rehabil. 2013;92(10):920-9. https://doi.org/10.1097/PHM.0b013e3182a4708f. 10.1097/PHM.0b013e3182a4708f [DOI] [PubMed] [Google Scholar]
- 70.Andersen TM, Sandnes A, Fondenes O, et al. Laryngeal Responses to Mechanically Assisted Cough in Progressing Amyotrophic Lateral Sclerosis. Respir Care. 2018;63(5):538-549. https://doi.org/10.4187/respcare.05924. 10.4187/respcare.05924 [DOI] [PubMed] [Google Scholar]
- 71.Andersen T, Fondenes O, Roksund OD, et al. From bedside to bench - In vivo and in vitro evaluation of mechanically assisted cough treatment in a patient with bulbar Amyotrophic Lateral Sclerosis. Respir Med Case Rep. 2022;37:101649. https://doi.org/10.1016/j.rmcr.2022.101649. 10.1016/j.rmcr.2022.101649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Brien D, Stavroulakis T, Baxter S, et al. The optimisation of noninvasive ventilation in amyotrophic lateral sclerosis: a systematic review. Eur Respir J. 2019;54(3):1900261. https://doi.org/10.1183/13993003.00261-2019. 10.1183/13993003.00261-2019 [DOI] [PubMed] [Google Scholar]
- 73.Kohler M, Clarenbach CF, Böni L, et al. Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2005;172(8):1032-6. https://doi.org/10.1164/rccm.200503-322OC. 10.1164/rccm.200503-322OC [DOI] [PubMed] [Google Scholar]


