Abstract
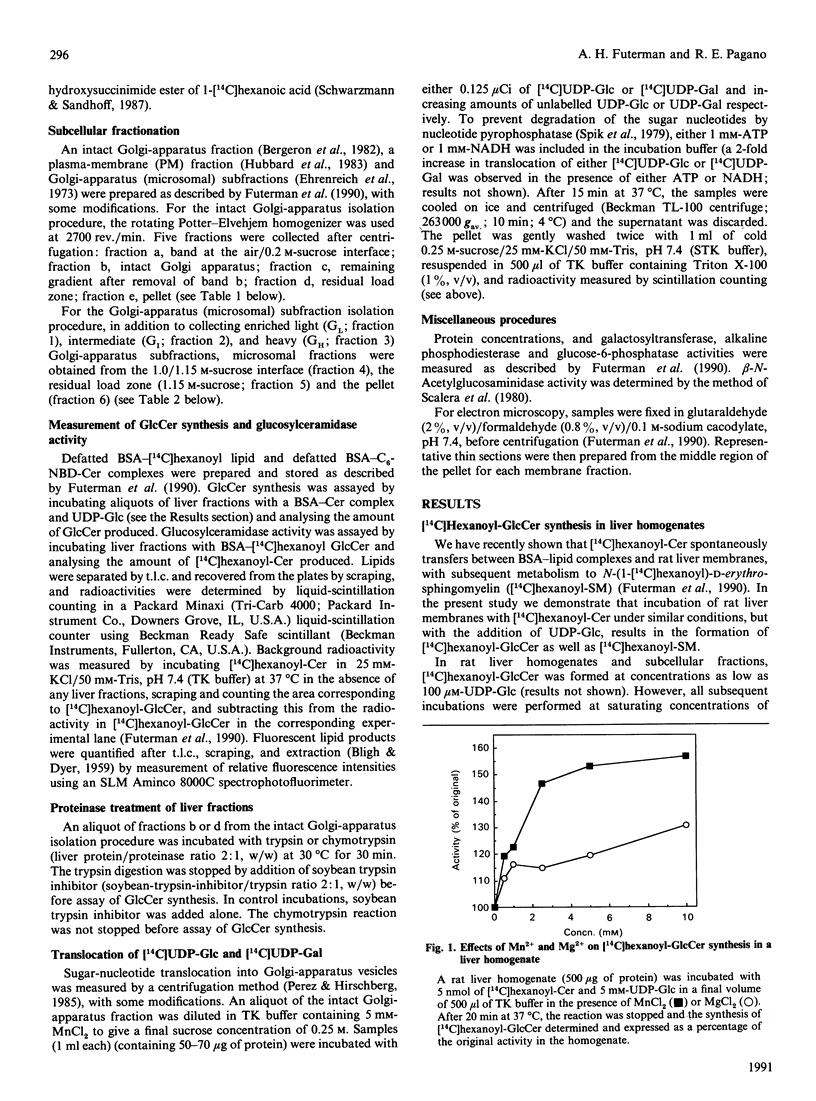
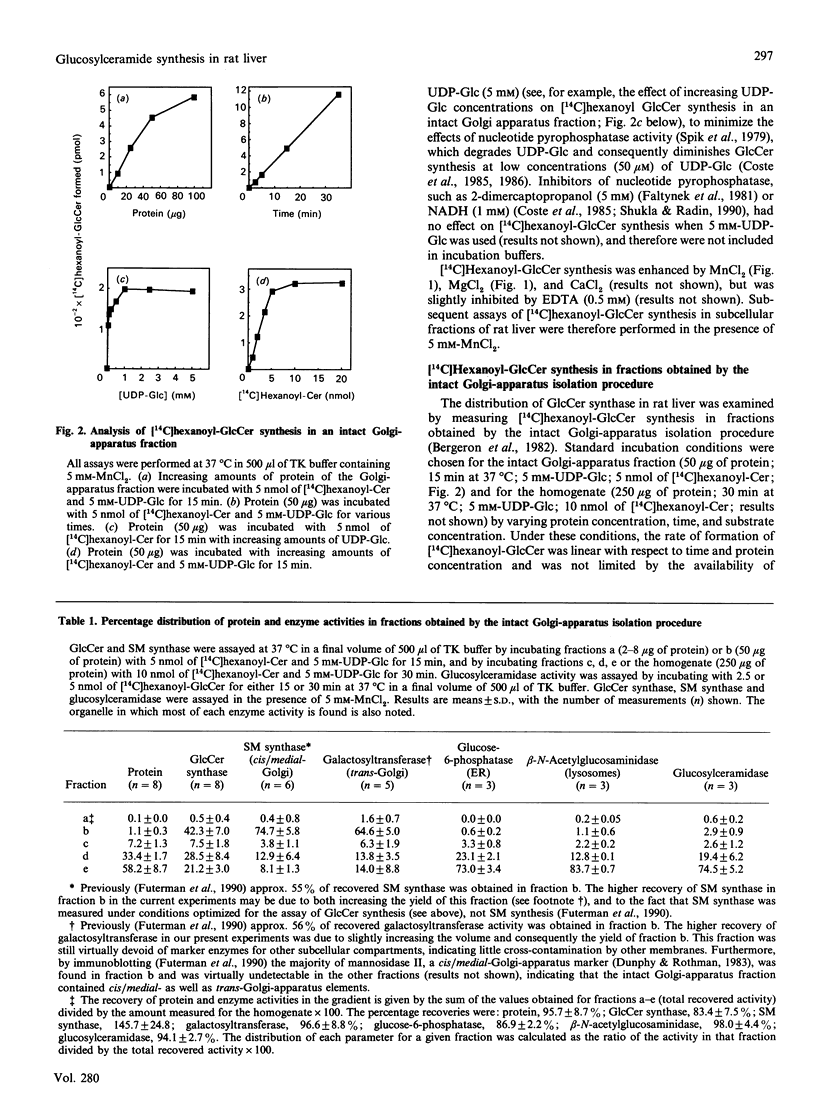
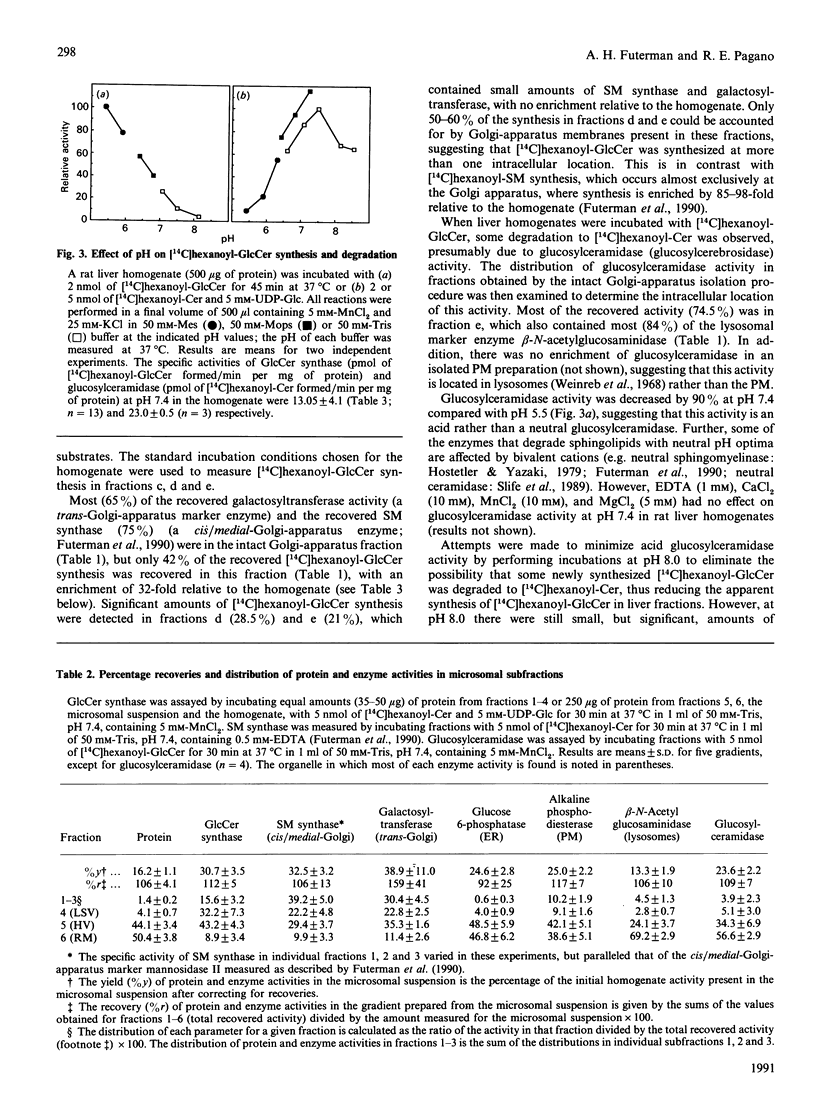
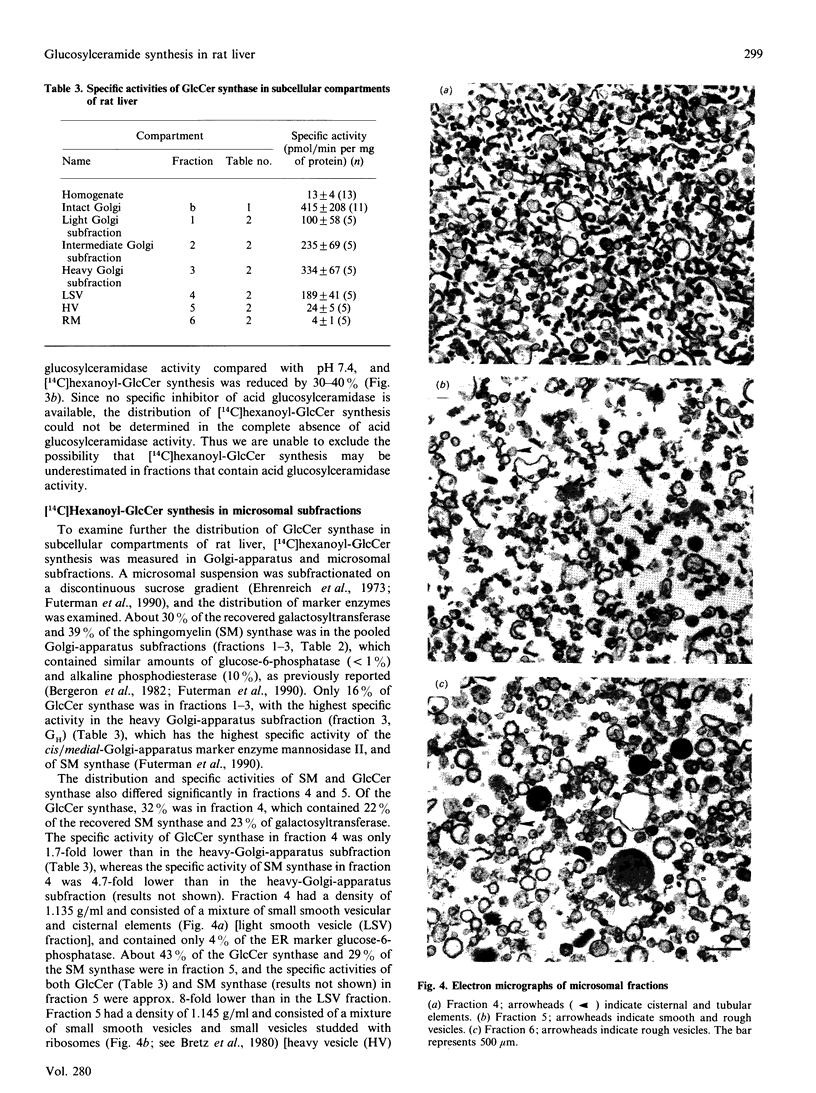
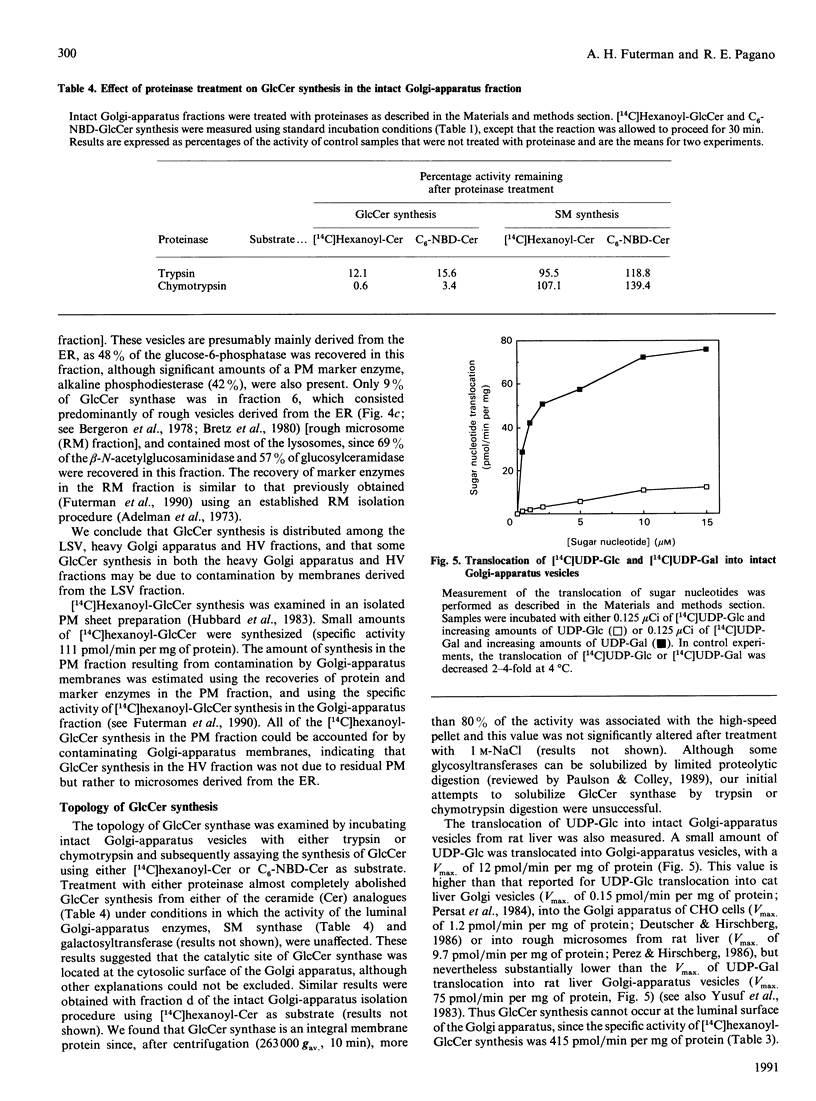
We examined the intracellular site(s) and topology of glucosylceramide (GlcCer) synthesis in subcellular fractions from rat liver, using radioactive and fluorescent ceramide analogues as precursors, and compared these results with those obtained in our recent study of sphingomyelin (SM) synthesis in rat liver [Futerman, Stieger, Hubbard & Pagano (1990) J. Biol. Chem. 265, 8650-8657]. In contrast with SM synthesis, which occurs principally at the cis/medial Golgi apparatus, GlcCer synthesis was more widely distributed, with substantial amounts of synthesis detected in a heavy (cis/medial) Golgi-apparatus subfraction, a light smooth-vesicle fraction that is almost devoid of an endoplasmic-reticulum marker enzyme (glucose-6-phosphatase), and a heavy vesicle fraction. Furthermore, no GlcCer synthesis was detected in an enriched plasma-membrane fraction after accounting for contamination by Golgi-apparatus membranes. These results suggest that a significant amount of GlcCer may be synthesized in a pre- or early Golgi-apparatus compartment. Unlike SM synthesis, which occurs at the luminal surface of the Golgi apparatus, GlcCer synthesis appeared to occur at the cytosolic surface of intracellular membranes, since (i) limited proteolytic digestion of intact Golgi-apparatus vesicles almost completely inhibited GlcCer synthesis, and (ii) the extent of UDP-glucose translocation into the Golgi apparatus was insufficient to account for the amount of GlcCer synthesis measured. These findings imply that, after its synthesis, GlcCer must undergo transbilayer movement to the luminal surface to account for the known topology of higher-order glycosphingolipids within the Golgi apparatus and plasma membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Blobel G., Sabatini D. D. An improved cell fractionation procedure for the preparation of rat liver membrane-bound ribosomes. J Cell Biol. 1973 Jan;56(1):191–205. doi: 10.1083/jcb.56.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Borts D., Cruz J. Passage of serum-destined proteins through the Golgi apparatus of rat liver. An examination of heavy and light Golgi fractions. J Cell Biol. 1978 Jan;76(1):87–97. doi: 10.1083/jcb.76.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Rachubinski R. A., Sikstrom R. A., Posner B. I., Paiement J. Galactose transfer to endogenous acceptors within Golgi fractions of rat liver. J Cell Biol. 1982 Jan;92(1):139–146. doi: 10.1083/jcb.92.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste H., Martel M. B., Azzar G., Got R. UDPglucose-ceramide glucosyltransferase from porcine submaxillary glands is associated with the Golgi apparatus. Biochim Biophys Acta. 1985 Mar 28;814(1):1–7. doi: 10.1016/0005-2736(85)90412-2. [DOI] [PubMed] [Google Scholar]
- Coste H., Martel M. B., Got R. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta. 1986 Jun 13;858(1):6–12. doi: 10.1016/0005-2736(86)90285-3. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmentation of asparagine-linked oligosaccharide processing in the Golgi apparatus. J Cell Biol. 1983 Jul;97(1):270–275. doi: 10.1083/jcb.97.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux I., Martel M. B., Got R. Solubilization of UDPglucose-ceramide glucosyltransferase from the Golgi apparatus. Biochim Biophys Acta. 1990 May 24;1024(2):263–266. doi: 10.1016/0005-2736(90)90352-o. [DOI] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltynek C. R., Silbert J. E., Hof L. Inhibition of the action of pyrophosphatase and phosphatase on sugar nucleotides. J Biol Chem. 1981 Jul 25;256(14):7139–7141. [PubMed] [Google Scholar]
- Futerman A. H., Stieger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990 May 25;265(15):8650–8657. [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groesch M. E., Ruohola H., Bacon R., Rossi G., Ferro-Novick S. Isolation of a functional vesicular intermediate that mediates ER to Golgi transport in yeast. J Cell Biol. 1990 Jul;111(1):45–53. doi: 10.1083/jcb.111.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Yazaki P. J. The subcellular localization of neutral sphingomyelinase in rat liver. J Lipid Res. 1979 May;20(4):456–463. [PubMed] [Google Scholar]
- Hubbard A. L., Wall D. A., Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983 Jan;96(1):217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. Intracellular translocation of fluorescent sphingolipids in cultured fibroblasts: endogenously synthesized sphingomyelin and glucocerebroside analogues pass through the Golgi apparatus en route to the plasma membrane. J Cell Biol. 1985 Jan;100(1):27–34. doi: 10.1083/jcb.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K. W., Touster O. Biosynthesis and modification of Golgi mannosidase II in HeLa and 3T3 cells. J Biol Chem. 1985 Jun 10;260(11):6654–6662. [PubMed] [Google Scholar]
- Pagano R. E. Lipid traffic in eukaryotic cells: mechanisms for intracellular transport and organelle-specific enrichment of lipids. Curr Opin Cell Biol. 1990 Aug;2(4):652–663. doi: 10.1016/0955-0674(90)90107-p. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J Biol Chem. 1986 May 25;261(15):6822–6830. [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Translocation of UDP-N-acetylglucosamine into vesicles derived from rat liver rough endoplasmic reticulum and Golgi apparatus. J Biol Chem. 1985 Apr 25;260(8):4671–4678. [PubMed] [Google Scholar]
- Persat F., Azzar G., Martel M. B., Got R. Evidence for coupling between transport of UDP-glucose and its synthesis by membrane-bound pyrophosphorylase in Golgi apparatus of cat liver. Biochim Biophys Acta. 1984 Jan 25;769(2):377–380. doi: 10.1016/0005-2736(84)90320-1. [DOI] [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Dissection of the Golgi complex. II. Density separation of specific Golgi functions in virally infected cells treated with monensin. J Cell Biol. 1983 Mar;96(3):851–856. doi: 10.1083/jcb.96.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Saito M., Rosenberg A. Action of monensin, a monovalent cationophore, on cultured human fibroblasts: evidence that it induces high cellular accumulation of glucosyl- and lactosylceramide (gluco- and lactocerebroside). Biochemistry. 1984 Mar 13;23(6):1043–1046. doi: 10.1021/bi00301a001. [DOI] [PubMed] [Google Scholar]
- Sasaki T. Glycolipid transfer protein and intracellular traffic of glucosylceramide. Experientia. 1990 Jun 15;46(6):611–616. doi: 10.1007/BF01939700. [DOI] [PubMed] [Google Scholar]
- Scalera V., Storelli C., Storelli-Joss C., Haase W., Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J. 1980 Jan 15;186(1):177–181. doi: 10.1042/bj1860177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann G., Sandhoff K. Lysogangliosides: synthesis and use in preparing labeled gangliosides. Methods Enzymol. 1987;138:319–341. doi: 10.1016/0076-6879(87)38028-0. [DOI] [PubMed] [Google Scholar]
- Schwarzmann G., Sandhoff K. Metabolism and intracellular transport of glycosphingolipids. Biochemistry. 1990 Dec 11;29(49):10865–10871. doi: 10.1021/bi00501a001. [DOI] [PubMed] [Google Scholar]
- Shukla G. S., Radin N. S. Glucosyceramide synthase of mouse kidney: further characterization with an improved assay method. Arch Biochem Biophys. 1990 Dec;283(2):372–378. doi: 10.1016/0003-9861(90)90657-k. [DOI] [PubMed] [Google Scholar]
- Slife C. W., Wang E., Hunter R., Wang S., Burgess C., Liotta D. C., Merrill A. H., Jr Free sphingosine formation from endogenous substrates by a liver plasma membrane system with a divalent cation dependence and a neutral pH optimum. J Biol Chem. 1989 Jun 25;264(18):10371–10377. [PubMed] [Google Scholar]
- Spik G., Six P., Montreuil J. Chemical and enzymic degradations of nucleoside mono- and diphosphate sugars. I. Determination of the degradation rate during the glycosyltransferase assays. Biochim Biophys Acta. 1979 May 1;584(2):203–215. doi: 10.1016/0304-4165(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Ecker C. P., Blough H. A. Enzymatic glucosylation of dolichol monophosphate and transfer of glucose from isolated dolichyl-D-glucosyl phosphate to ceramides by BHK-21 cell microsomes. Eur J Biochem. 1984 Sep 3;143(2):447–453. doi: 10.1111/j.1432-1033.1984.tb08392.x. [DOI] [PubMed] [Google Scholar]
- Trinchera M., Ghidoni R. Subcellular biosynthesis and transport of gangliosides formed from exogenous lactosylceramide in rat liver. Biochem J. 1990 Mar 1;266(2):363–369. doi: 10.1042/bj2660363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchera M., Ghidoni R. Two glycosphingolipid sialyltransferases are localized in different sub-Golgi compartments in rat liver. J Biol Chem. 1989 Sep 25;264(27):15766–15769. [PubMed] [Google Scholar]
- Trinchera M., Pirovano B., Ghidoni R. Sub-Golgi distribution in rat liver of CMP-NeuAc GM3- and CMP-NeuAc:GT1b alpha 2----8sialyltransferases and comparison with the distribution of the other glycosyltransferase activities involved in ganglioside biosynthesis. J Biol Chem. 1990 Oct 25;265(30):18242–18247. [PubMed] [Google Scholar]
- Wattenberg B. W. Glycolipid and glycoprotein transport through the Golgi complex are similar biochemically and kinetically. Reconstitution of glycolipid transport in a cell free system. J Cell Biol. 1990 Aug;111(2):421–428. doi: 10.1083/jcb.111.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb N. J., Brady R. O., Tappel A. L. The lysosomal localization of sphingolipid hydrolases. Biochim Biophys Acta. 1968 Apr 24;159(1):141–146. doi: 10.1016/0005-2744(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Yusuf H. K., Pohlentz G., Sandhoff K. Tunicamycin inhibits ganglioside biosynthesis in rat liver Golgi apparatus by blocking sugar nucleotide transport across the membrane vesicles. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7075–7079. doi: 10.1073/pnas.80.23.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski A., Devaux P. F. Transmembrane movements of lipids. Experientia. 1990 Jun 15;46(6):644–656. doi: 10.1007/BF01939703. [DOI] [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Modulation of ganglioside biosynthesis in primary cultured neurons. J Neurochem. 1989 Jan;52(1):207–214. doi: 10.1111/j.1471-4159.1989.tb10918.x. [DOI] [PubMed] [Google Scholar]



