Abstract
Rash and fever are some of the most common chief complaints present in paediatric dermatology emergencies. The spectrum of differential diagnosis is broad, including many different infectious and some non-infectious agents. A systematic approach involving detailed history taking, careful clinical examination along with particular attention to epidemiological features are the most important factors to make a diagnosis. This article reviews the morphological patterns of various causes of fever with rash in children, including infectious as well as non-infectious causes, with special emphasis on the Indian scenario. We intend to highlight the clinical characteristics of each cause, which will not only help make a clinical diagnosis but also distinguish benign versus life-threatening causes of skin rash in febrile paediatric patients and provide early medical intervention.
Keywords: Child, fever, India, rash
Introduction
Fever with rash is perhaps one of the commonest presenting complaints in routine and emergency paediatric dermatology setup. A wide range of both infectious and non-infectious aetiologies may be implicated [Flowchart 1]. Aetiological agents of fever with rash can also be grouped into agents causing scarlatiniform rash and morbiliform rash [Flowcharts 2 and 3]. While some conditions are benign and self-limiting, others may progress to systemic involvement and thus warrant hospital admission [Flowchart 4][1]. Morphology of rash is important, as it will help us narrow down the list of our differentials and aid in making the correct clinical diagnosis. Table 1 classifies the aetiologies of fever with rash based on the morphology of rash[1,2]. Thus, it is extremely important for a dermatologist to be able to diagnose the underlying cause for these febrile eruptions with confidence, as is it will not only allay parental anxiety but also decrease the associated morbidity. This review will delineate the key features of the most commonly encountered causes of fever with a rash on the basis of the morphology of the rash.
Flowchart 1.
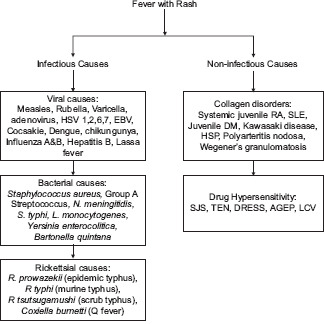
Infectious and non-infectious causes of fever and rash in children. HSV- Herpes simplex virus; EBV- Epstein Barr virus; RA - Rheumatoid arthritis; SLE - Systemic lupus erythematosus; DM - Dermatomyositis; HSP - Henoch-Schonlein purpura; SJS - Steven Johnson syndrome; TEN - Toxic epidermal necrolysis; DRESS - Drug reaction with Eosinophilia and systemic symptoms; AGEP - Acute generalised exanthematous pustulosis LCV
Flowchart 2.
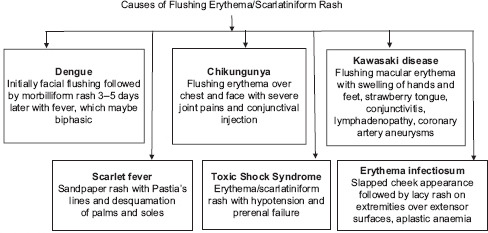
Causes of scarlatiniform rash
Flowchart 3.
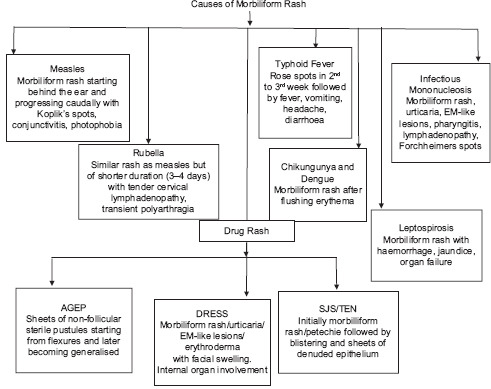
Causes of Morbiliform Rash
Flowchart 4.
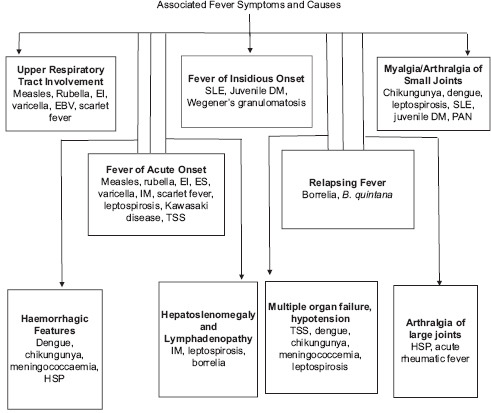
Symptoms associated with fever and their causes
Table 1.
Classification of Aetiological Agents on the Basis of Morphology of Rash
| Morphology | Aetiological Agent |
|---|---|
| Maculopapular rash | Centrally distributed |
| • Viral: Measles, rubella, dengue, erythema infectiosum, exanthem subitum, infectious mononucleosis | |
| • Drug induced: Antibiotics, anti-convulsants | |
| • Bacterial: Epidemic typhus, endemic typhus, scrub typhus, rheumatic fever, leptospirosis, relapsing fever, typhoid fever | |
| • Autoimmune: SLE, Still’s disease | |
| Peripherally distributed | |
| • Bacterial: Rocky mountain spotted fever, secondary syphilis, bacterial endocarditis | |
| • Viral: Chikungunya, hand and foot mouth disease | |
| • Erythema multiforme | |
| Confluent desquamative erythemas | Scarlet fever, toxic shock syndrome, staphylococcal scalded skin syndrome |
| Vesiculobullous or pustular rash | Varicella, variola, primary herpes infection, disseminated herpes infection, rickettsial pox |
| Nodular rash | Erythema nodosum, disseminated fungal infection, Sweet syndrome |
| Purpuric rash | • Bacterial: Meningococcaemia, disseminated gonococcal infection, purpura fulminans |
| • Viral: Viral haemorrhagic fever, Coxsackie A9, Echovirus 9, EBV, CMV | |
| • Vasculitis | |
| Rash with ulcers or eschars | Rickettsial infections, tularaemia, anthrax |
Maculopapular Rash
Centrally distributed
Viral causes
Measles
Measles has proven to be a disease of public health concern. The incubation period from exposure to prodrome averages 11–12 days. The time from exposure to onset of rash averages about 14 days.
It starts with a prodrome of cough, conjunctivitis, coryza, fever and Koplik spots. About 2–4 days later, there is an appearance of pink macules and papules around the hairline and behind the ears. This eruption spreads in a cephalocaudal direction and resolves in the same order [Figure 1].
Figure 1.
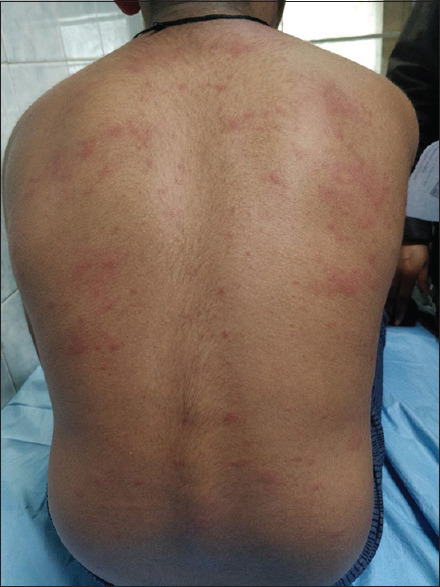
Maculopapular rash present over back in a case of measles infection
Complications include pneumonia, otitis, gastroenteritis, myocarditis, encephalitis and subacute sclerosing panencephalitis, which can occur years later[2].
Rubella
Rubella is usually a mild viral illness most commonly affecting the skin and lymph nodes. It mainly affects young children, but adolescents and adults can also be affected.
The average incubation period is 17 days. It often begins with a prodrome of low-grade fever, headache and upper respiratory tract symptoms. Occipital, posterior cervical and posterior auricular nodes may be enlarged. After about 5 days, a rash of rose-coloured macules and papules begins on the face, spreads in a cephalocaudal direction and resolves over the next 2–3 days. Forchheimer spots may be encountered.
Complications include arthralgias (more common in females), anaemia, neutropenia, thrombocytopenia, encephalitis, myocarditis, pericarditis and hepatitis[3].
Parvovirus B19
Parvovirus B19 is a small, single-stranded DNA virus that infects only humans. Almost 20% of the cases are asymptomatic. Erythema intectiosum, also known as the fifth disease or ‘slapped cheek disease’, is most common in school-aged children between 4 and 10 years.
It begins with a prodrome of fever, headache and respiratory symptoms, followed 2–3 days later by bright red macular erythema on the cheeks with circumoral pallor. About 4 days later, pink macules and papules appear on the extremities and, to a lesser extent, the trunk. Central clearing results in a characteristic lacy or reticular pattern. This cutaneous eruption lasts 1–3 weeks and may worsen with sun or heat exposure[4].
Rare complications of parvovirus B19 include cardiac involvement, neurologic problems, hepatitis and haemophagocytic syndrome.
• Papular-purpuric gloves and socks syndrome (PPGSS) is a rare exanthematous condition that affects mostly young adults and rarely children, predominantly during the spring and summer. It is characterized by acute onset of fever with intense erythema and oedema on the hands and feet, with a sharp demarcation at the wrists and ankles, followed by the gradual appearance of erythematous papules, petechiae or purpura and, rarely, blisters on the same sites. Oral erosions, petechiae and oedema involving the lips, buccal mucosa and palate are often present. Genital mucosa may also be affected.
While Parvovirus B19 is the most common causative organism, cytomegalovirus (CMV), EBV, measles, rubella and drugs such as trimethoprim/sulfa-methoxazole have also been implicated as causative agents for PPGSS.
It is self-limiting with resolution occurring spontaneously in 7–14 days, with slight peeling on palms and soles[5,6,7,8,9].
• Unilateral Laterothoracic Exanthem (ULE)/Asymmetric Periflexural Exanthem of Childhood.
Children aged 1–5 years are typically affected during the winter or spring. Symptoms of fever, upper respiratory symptoms, vomiting, diarrhoea or lymphadenopathy are often present. Viral infections including adenovirus, parainfluenza 2 and 3, parvovirus B19, human herpes Virus 6 and 7, cytomegalovirus (CMV) and Epstein–Barr Virus (EBV) have been associated with ULE.
It is a mildly pruritic eruption that begins unilaterally near the axilla or inguinal crease, and spreads centrifugally but maintains its asymmetric nature. Early lesions are erythematous coalescing papules that are 1–2 mm in size with a surrounding pale halo. Over time, papules become scalier and more eczematous with a central dusky colour. The lesions resolve with fine desquamation and post-inflammatory pigmentation in 5 weeks[10,11].
Dengue
Dengue viral infection is a cause of considerable morbidity and mortality, and thus early diagnosis by a dermatologist is of the utmost importance.
Classical dengue fever is characterized by sudden onset of very severe headache, retro-orbital pain, fatigue, severe arthralgia and myalgia. The fever usually lasts for 5–7 days after which a rash appears, which is maculopapular, confluent with sparing of normal islands of circular coin shaped skin (very characteristic). The rash subsides by 3–7 days with scaling and mild pruritus. The patients may have additional haemorrhagic manifestation, e.g., petechiae, purpura and a positive capillary fragility test[12].
Gum bleeding, menorrhagia, epistaxis and bleeding per rectum is occasionally seen secondary to trauma. Flagellate purpura can be rarely associated with dengue fever[13].
Bacterial causes
Rickettsial diseases
Rickettsial illnesses, caused by organisms within the genus of Rickettsiae, can be divided into the following three biogroups:
Spotted fever biogroup (15 rickettsioses)
Typhus group
Scrub typhus biogroup (Tsutsugamushi disease)[14].
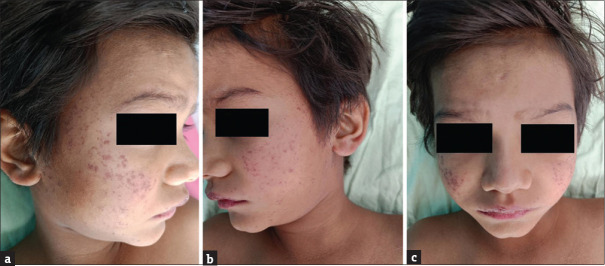
It typically begins with a prodrome of fever, headache, nausea, myalgia, vomiting and abdominal pain. The typical cutaneous feature is the presence of an eschar consisting of a central area of dermal and epidermal necrosis (0.5–2 cm) surrounded by a zone of erythema that appears during the initial 4–10 days. The rash begins at the wrists and ankles in case of spotted fever and axillae in typhus fever. The exanthem involves the entire body with relative sparing of the face. Palms and soles are generally involved with a maculopapular rash. Petechiae and purpura may develop in cases of severe endothelial injury, and cutaneous necrosis can lead to the necrosis of digits, ear lobes and tip of the nose [Figure 2a-c]. Lethargy and confusion may be early features of central nervous involvement. As the clinical course progresses, hypotension, acute renal failure, respiratory failure and coma may develop[15,16].
Figure 2.
(a-c) Numerous petechiae present over bilateral cheeks in a case of rickettsial infection
Connective tissue diseases
Systemic lupus erythematosus (SLE)
Lupus erythematosus is an autoimmune condition that has a wide range of manifestations, including the mild form involving the skin to the devastating multisystem involvement. Pediatric SLE is more acute and severe than adult SLE. There is a higher frequency of renal, neurologic, haematologic involvement with fever and lymphadenopathy in paediatric SLE[17] [Figures 3 and 4].
Figure 3.
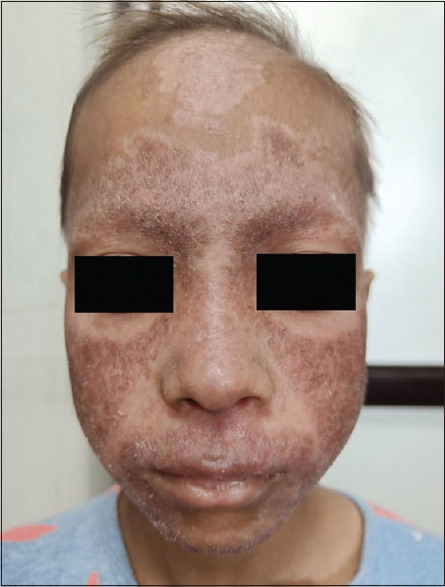
Characteristic malar rash with non-scarring alopecia in a patient of systemic lupus erythematosus
Figure 4.
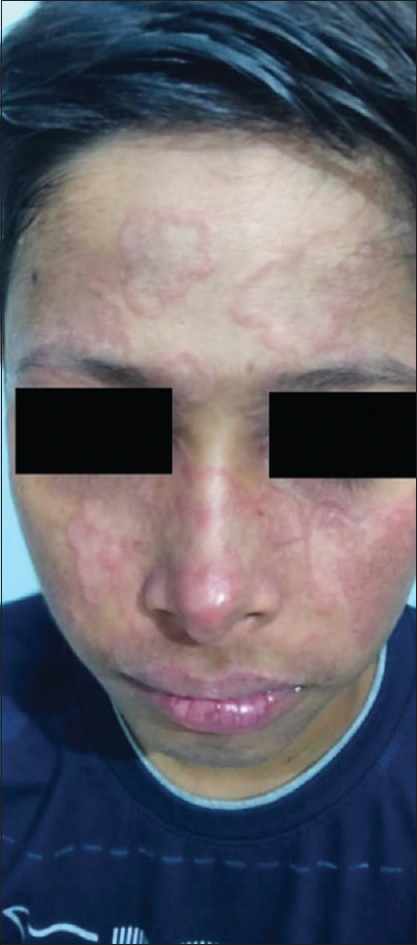
Annular lesions over face in a child with subacute cutaneous lupus erythematosus
Systemic juvenile idiopathic arthritis (JIA)
Systemic juvenile idiopathic arthritis (JIA) is characterized by the classic triad of fever, rash and arthritis. The fever needs to be present for at least 2 weeks and be quotidian. The classic rash is a salmon-coloured evanescent rash that occurs most often on the trunk and proximal extremities. The arthritis is typically polyarticular and needs to be present for 6 weeks to meet diagnostic criteria. Other features from diagnostic criteria include hepatosplenomegaly, lymphadenopathy and serositis[18].
Drug-related causes
Drug reaction with eosinophilia and systemic symptoms (DRESS)
The triad of fever, skin rash and symptomatic or asymptomatic internal organ involvement characterizes this specific entity. The culprit drugs are phenytoin, carbamazepine, phenobarbital, sulphonamides, dapsone, minocycline, allopurinol, non-steroidal anti-inflammatory drugs, etc.
It affects various organs, skin, liver and haematological system being most commonly targeted. It presents with high, spiking fever followed in 1–2 days by other manifestations. Skin lesions are most commonly exanthematic and rarely more severe skin reactions, such as erythroderma, erythema multiforme, may occur. Facial and periorbital oedema is a characteristic sign of potentially serious reaction[19,20].
Peripherally distributed
Causes of peripherally distributed type of maculopapular rash are mostly viral.
Chikungunya fever
Chikungunya fever is seen in all age groups from newborn to adults. The incubation period is typically 3–7 days. There is high-grade fever associated with arthralgia, myalgia, lymphadenopathy, skin eruptions, conjunctivitis, photophobia, meningeal syndrome and acute encephalopathy.
Clinical presentation is varied, including generalized maculopapular rash, hyperpigmentation, xerosis, desquamation of palms, generalized urticarial lesions, transient nasal erythema, vesiculobullous lesions, vasculitis, erythema multiforme-like lesions, aphthous ulcers and oral mucosal pigmentation[21].
Nasal hyperpigmentation (chik sign) is a clue to diagnose chikungunya in neonates, which is not seen in any other viral infections. This pigmentation persists for months even after remission[22].
Hand-foot-and-mouth disease (HFMD)
It frequently affects children younger than 5 years. Hand-foot-and-mouth disease (HFMD) is not only most commonly associated with coxsackievirus A16 but has also been caused by coxsackie viruses A5, A7, A9, A10, B1, B2, B3, B5 and enterovirus 71[23].
Clinical features may include fever, malaise, upper respiratory symptoms and odynophagia. The characteristic exanthem consists of triangular or elliptical-shaped vesicles surrounded by erythema on the hands and feet [Figure 5a and b]. The associated enanthem involves painful vesicles and erosions on the buccal surfaces, tongue, uvula, tonsillar pillars, and hard and soft wing palates. Onychomadesis is common following HFMD.
Figure 5.
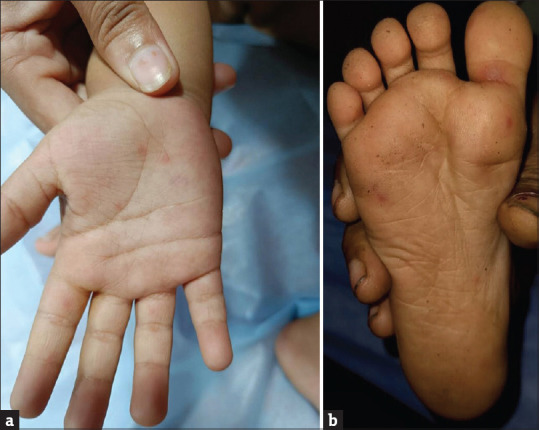
(a and b) Discrete erythematous macules present over palm and sole of hand-foot-and-mouth disease
Vesicles, bullae, haemorrhagic bullae, erosions, papules and petechiae tend to have a predilection for the peri-oral region and sites of atopic dermatitis similar to eczema herpeticum. It has therefore been labelled as ‘eczema coxsackium’[24].
Echoviruses cause an array of exanthems with morbilliform, vesicular, urticarial, erythema multiforme-like and petechial morphologies. There are often concomitant systemic symptoms of fever, upper respiratory symptoms and gastrointestinal symptoms[25,26,27,28].
Gianotti–Crosti syndrome (GCS)
It is also known as papular acrodermatitis of childhood. Gianotti–Crosti syndrome (GCS) predominantly affects children of 1–6 years of age, and there is an increased incidence in children with a history of atopy.
After a prodrome of low-grade fever, upper respiratory symptoms, diarrhoea, and axillary or inguinal lymphadenopathy, asymptomatic, or mildly pruritic erythematous monomorphic papules and papulovesicles appear symmetrically over the face, buttocks, and extensor upper and lower extremities, and resolves without scarring in 3–4 weeks. Mucous membranes are not involved[29,30].
Confluent desquamative erythemas
Causes of confluent desquamative erythemas are mostly bacterial.
Staphylococcal scalded skin syndrome
This syndrome is mostly seen in children less than two years of age.
It is caused by epidermolytic toxin producing strains of Staphylococci belonging to phage group 2. The disorder usually affects children younger than 5 years of age and begins as malaise, fever, irritability[31].
A generalized macular erythema and a fine, stippled, sandpaper or nutmeg-like appearance that progresses to a tender scarlatiniform phase over 1–2 days. The erythema and tenderness spread from the intertriginous, periorificial areas and trunk to the entire body. The lesions then exfoliate with exudation and crusting around the mouth, and sometimes the periorbital area. Large fragments of crusts often become separated leaving radial fissures that give the disorder its characteristic and diagnostic appearance. Within 2–3 days the upper layer of the epidermis becomes wrinkled and can be easily peeled off like wet tissue paper. Nikolsky’s sign is positive. Shortly, thereafter the patient develops flaccid bullae and eventual exfoliation of the skin (the desquamative phase). If there is no secondary infection, the entire skin heals without scarring within 14 days of the onset of the process[32].
Toxic shock syndrome
It is a severe acute febrile illness with multisystem involvement characterized by myalgia, vomiting, diarrhoea, pharyngitis, high fever, mucous membrane and conjunctival hyperaemia, and scarlatiniform rash and, in severe cases, with shock.
Although it is more common in adolescent females and young women, it can also affect males and children. It is mediated by a blood-borne toxins produced by Staphylococcus aureus at a focal site of infection. These include a phage group 1 S. aureus toxic shock syndrome toxin 1 (TSS-1) and staphylococcal enterotoxin B in most patients and less commonly other exotoxins such as enterotoxins A, C, D, E and H.
Most cases are related to tampon use during menstruation. However, they can also result from surgical wound infections, infections following sinusitis, nasal packing, ear piercing, deep and superficial abscesses, infected burns, herpes zoster, cellulitis, adenitis, bursitis, empyema, osteomyelitis, septic abortion, etc., The scarlatiniform rash typically desquamates, nails are shed and telogen effluvium may occur. Flaccid blisters with subepidermal fluid are seen in some infants with toxic shock syndrome[33,34].
Scarlet fever
Caused by group A beta-haemolytic Streptococcus (GABHS) and very rarely by group C or G streptococci. The streptococcal pyrogenic exotoxins (SPEs) and M protein are important virulence factors. Encountered more commonly in children, especially under 10 years of age. Transmission is by the aerosol route but may also spread by direct skin contact, and rarely can be food borne. Risk of scarlet fever has been shown to increase along with the degrees of air pollution.
The incubation period is about 2–3 days with a sudden onset of fever, headache, malaise, and evidence of tonsillitis and pharyngitis without rhinorrhoea or cough. Tender anterior cervical adenopathy is commonly present. The punctate erythematous rash, with characteristic ‘sandpaper’ feel, appears on the first or second day of illness on the groin, axilla, and trunk and then spreads to the extremities sparing the palms and soles. Pastia’s lines are seen in flexural creases. It may be difficult to identify the exanthem in darkly pigmented skin and may consist of only punctate papules resembling ‘goose flesh’.
Facial flushing with circumoral pallor is characteristic. Red papillae on a coated tongue (white strawberry tongue) are seen in the first few days of illness, which later becomes red and glistening studded with prominent papillae (red strawberry tongue) following resolution of the white coating. The exanthem usually resolves over 4–5 days followed by thick sheets of desquamation of the palms and soles, which typically begins on the fingertips at the free margin of the fingernails.
Complications include toxic and septic forms of scarlet fever with peritonsillar and retropharyngeal abscesses, acute rheumatic fever (RF), glomerulonephritis, bacteremia, pneumonia, endocarditis and meningitis. Rarely, hepatitis, gallbladder hydrops or splenomegaly may occur[35].
Vesiculobullous or Pustular Rash
Viral causes
Varicella zoster
It is transmitted by droplets or direct person-to-person contact. Incubation period is 10–14 days, and infective period starts 48 h prior to the onset of rash until the development of crusts. Clinical features include a prodrome of fever, chills, malaise, headache and arthralgias, followed by development of a generalized vesicular exanthem within 24–48 h.
These vesicles spread centrifugally from the scalp, face and trunk, and then to the extremities. Most lesions turn into crusts over a period of 1 week [Figure 6][36].
Figure 6.
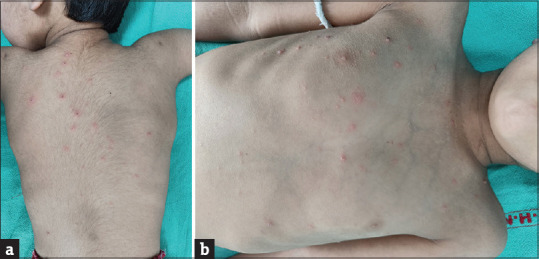
(a and b) Vesicular rash over trunk and back. Few lesions over the back show crusting, clinically diagnosed as varicella zoster infection
Complications include secondary bacterial infection of vesicles, pneumonia, meningitis, thrombocytopenia, glomerulonephritis and hepatitis. Breakthrough varicella is characterized by absent-to-low fever, less than 50 macules and papules and a shorter course of illness, and it can occur after a single dose of the varicella vaccine[37].
Herpangina
It is an enanthem characterized by fever, sore throat, and painful vesicles and erosions involving the soft palate, uvula and tonsillar pillars. It is typically caused by coxsackieviruses A, but other causes include coxsackie viruses B and echoviruses. Lesions are similar to herpetic gingivostomatitis, but more frequently affect the posterior oral cavity rather than the lips[38].
Drug-related causes
Stevens–Johnson syndrome—Toxic epidermal necrolysis (SJS–TEN)
They are severe mucocutaneous drug reactions presenting with acute onset fever, malaise, myalgia, headache, arthralgia and injection of conjunctivae. Oral ulceration and erosion of lips are early features. Bullae, usually haemorrhagic, develop extensively all over the body and subsequently rupture to leave large areas of erosions [Figure 7]. Cases with less than 10% epidermal involvement are classified as SJS; those with 30% or more are classified as toxic epidermal necrosis (TEN), and cases with 10%–30% involvement are considered overlap[39,40].
Figure 7.
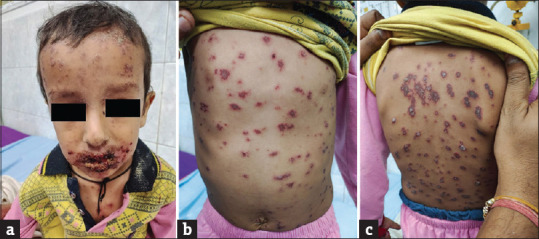
(a–c) Mucocutaneous involvement in a child with Stevens–John syndrome–toxic epidermal necrosis (SJS–TEN)
The drugs commonly implicated are penicillin, phenytoin, carbamazepine, barbiturates, NSAIDs, tetracyclines, etc.
Purpuric Rash
Henoch–Schonlein purpura
It is a small vessel vasculitis affecting the skin, joint, gastrointestinal tract and kidneys. Infections such as Group A streptococci, mycoplasma, EBV, varicella virus and Parvovirus B19 have been implicated. The triggers cause IgA immune complex deposition in the tissues. Children between the age of 4 and 8 years are commonly affected.
Crops of lesions occur chiefly on the lower extremities that start as palpable purpura. In 40% cases, the cutaneous lesions are preceded by mild fever, headache, joint pain and abdominal pain.
Gastrointestinal symptoms include nausea, vomiting, abdominal pain, diarrhoea, constipation and occasionally bleeding in stools. The most important manifestation is renal involvement, which produces proteinuria, haematuria and RBC casts.
It is self-limiting in most cases but may progress to renal failure, especially in adults. Pulmonary haemorrhage is rare but can be fatal[41,42].
Agents presenting with different morphologies of rash
COVID-19
More and more data on cutaneous presentations of COVID-19 are coming up. They are reported to be similar to other viral infections: an erythematous exanthema, acute urticaria, chickenpox-like vesicles, pseudo-chilblain lesions (referred to as COVID toes), retiform purpura or necrosis. In Italy, cutaneous manifestations were seen in 20% of patients, with most having non-specific viral rash. Often, it develops in already hospitalized patients and can possibly be misdiagnosed as drug reaction. In China, ischemic lesions suggesting coagulopathy and urticarial rash were seen. Dengue-like rash was observed in a few patients with COVID-19 infection in Singapore and Bangkok. In France, various skin rash observed were pseudo-frostbite and persistent painful redness of different parts of skin. Foot sore is another type of skin rash seen in some patients and termed as ‘COVID toes’[43,44,45].
In India, COVID-associated skin lesions are not very common. Amongst the reported cases, the most common are urticarial rash and maculopapular rash followed by vaso-occlusive lesions and COVID toes[46].
Monkeypox
Monkeypox virus is the most notable orthopoxvirus affecting humans since the eradication of smallpox. Human-to-human transmission occurs through droplet infection, direct contact and via fomites. The clinical features of human monkeypox virus resemble smallpox, with an incubation period of 7–17 days, an initial febrile prodromal period of 1–4 days and a rash period of 14–28 days. The characteristic clinical features include a prodrome of constitutional symptoms such as fever, headache, myalgia and lymphadenopathy, followed by generalized well-defined exanthem of typical centrifugal pattern that progresses through macular, papular, vesicular and pustular phases. An adequate clinical history (including that of travel, occupation and contact) as well as a laboratory diagnosis are required for diagnosis.[47]
The first case reported in India was from Kerala, reported on July 15, 2022. By mid-August, 2022, there were nine cases in India. On 1st August, India reported its first monkeypox death[47].
Conclusion
A wide variety of possibilities can come into mind when a child presents with fever and rash. Hence, a systematic approach based on morphology of rash is important to streamline our list of differential diagnoses and make a correct diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sarkar R, Mishra K, Garg VK. Fever with rash in a child in India. Indian J Dermatol Venereol Leprol. 2012;78:25–62. doi: 10.4103/0378-6323.95439. [DOI] [PubMed] [Google Scholar]
- 2.Tabak F, Murtezaoglu A, Tabak O, Ozaras R, Mete B, Kutlubay Z, et al. Clinical features and etiology of adult patients with fever and rash. Ann Dermatol. 2012;24:420–5. doi: 10.5021/ad.2012.24.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyer JA. Childhood viral exanthems. Pediatr Ann. 2007;36:21–9. doi: 10.3928/0090-4481-20070101-08. [DOI] [PubMed] [Google Scholar]
- 4.Mage V, Lipsker D, Barbarot S, Bessis D, Chosidow O, Del Giudice P, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62–9. doi: 10.1016/j.jaad.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Miguélez A, Dueñas J, Hervás D, Hervás JA, Salvá F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol. 2014;53:e583–5. doi: 10.1111/ijd.12557. [DOI] [PubMed] [Google Scholar]
- 6.Blauvelt A. Skin diseases associated with human herpesvirus 6, 7, and 8 infection. J Investig Dermatol Symp Proc. 2001;6:197–202. doi: 10.1046/j.0022-202x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 7.Outhred AC, Kok J, Dwyer DE. Viral arthritides. Expert Rev Anti Infect Ther. 2011;9:545–54. doi: 10.1586/eri.11.34. [DOI] [PubMed] [Google Scholar]
- 8.Fölster-Holst R, Kreth HW. Viral exanthems in childhood-infectious (direct) exanthems. Part 2: Other viral exanthems. J Dtsch Dermatol Ges. 2009;7:414–8. doi: 10.1111/j.1610-0387.2008.06869.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunner MJ, Rubin L, Dunlap F. A new papular erythema of childhood. Arch Dermatol. 1962;85:539–40. doi: 10.1001/archderm.1962.01590040103020. [DOI] [PubMed] [Google Scholar]
- 10.Gragasin FS, Metelitsa AI. Unilateral laterothoracic exanthem. CMAJ. 2012;184:322. doi: 10.1503/cmaj.110315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchani AJ. Exanthems in childhood: An update. Pediatr Ann. 1998;27:163–70. doi: 10.3928/0090-4481-19980301-10. [DOI] [PubMed] [Google Scholar]
- 12.Halsey ES, Williams M, Laguna-Torres VA, Vilcarromero S, Ocaña V, Kochel TJ, et al. Occurrence and correlates of symptom persistence following acute dengue fever in Peru. Am J Trop Med Hyg. 2014;90:449–56. doi: 10.4269/ajtmh.13-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carod-Artal FJ, Wichmann O, Farrar J, Gasco×n J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12:906–19. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 14.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359–64. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 15.Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24–8. [PubMed] [Google Scholar]
- 16.Padbidri VS, Gupta NP. Rickettsiosis in India: A review. J Indian Med Assoc. 1978;71:104–7. [PubMed] [Google Scholar]
- 17.D’Cruz DP. Systemic lupus erythematosus. BMJ. 2006;332:890–4. doi: 10.1136/bmj.332.7546.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JG, Jung JY, Yoon BY, Hahn YS. Clinical observations on juvenile rheumatoid arthritis: I. Systemic type. J Korean Rheum Assoc. 1994;1:175–82. [Google Scholar]
- 19.Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents: A 6 year series from Chandigarh, India. J Postgrad Med. 2001;47:95–9. [PubMed] [Google Scholar]
- 20.Sharma VK, Sethuraman G. Adverse cutaneous reactions to drugs: An overview. J Postgrad Med. 1998;42:15–22. [PubMed] [Google Scholar]
- 21.Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: Estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46:26–35. [PubMed] [Google Scholar]
- 22.Ravi V. Reemergence of chikungunya virus in India. Indian J Med Microbiol. 2006;24:83–4. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]
- 23.Feder HM, Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: A vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14:83–6. doi: 10.1016/S1473-3099(13)70264-0. [DOI] [PubMed] [Google Scholar]
- 24.Mathes EF, Oza V, Frieden IJ, Cordoro KM, Yagi S, Howard R, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:e149–57. doi: 10.1542/peds.2012-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubiche T, Schuffenecker I, Boralevi F, Léauté-Labrèze C, Bornebusch L, Chiaverini C, et al. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92–8. doi: 10.1097/INF.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 26.Puenpa J, Chieochansin T, Linsuwanon P, Korkong S, Thongkomplew S, Vichaiwattana P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641–3. doi: 10.3201/eid1904.121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montes M, Artieda J, Piñeiro LD, Gastesi M, Diez-Nieves I, Cilla G. Hand, foot, and mouth disease outbreak and coxsackievirus A6, northern Spain, 2011. Emerg Infect Dis. 2013;19:676–8. doi: 10.3201/eid1904.121589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biesbroeck L, Sidbury R. Viral exanthems: An update. Dermatol Ther. 2013;26:433–8. doi: 10.1111/dth.12107. [DOI] [PubMed] [Google Scholar]
- 29.Stock I. Streptococcus pyogenes--much more than the aetiological agent of scarlet fever. Med Monatsschr Pharm. 2009;32:408–16. [PubMed] [Google Scholar]
- 30.Feeney KT, Dowse GK, Keil AD, Mackaay C, McLellan D. Epidemiological features and control of an outbreak of scarlet fever in a Perth primary school. Commun Dis Intell. 2005;29:386–90. doi: 10.33321/cdi.2005.29.45. [DOI] [PubMed] [Google Scholar]
- 31.Manders SM. Toxin-mediated streptococcal and staphylococcal disease. J Am Acad Dermatol. 1998;39:383–98. doi: 10.1016/s0190-9622(98)70314-7. [DOI] [PubMed] [Google Scholar]
- 32.Mc Cornick JK. Toxic shock syndrome and bacterial superantigens: An update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Chi CY, Wang SM, Lin HC, Liu CC. A clinical and microbiological comparison of Staphylococcus aureus toxic shock and scalded skin syndromes in children. Clin Infect Dis. 2006;42:181–5. doi: 10.1086/498901. [DOI] [PubMed] [Google Scholar]
- 34.Gaensbauer JT, Birkholz M, Smit MA, Garcia R, Todd JK. Epidemiology and clinical relevance of toxic shock syndrome in US children. Pediatr Infect Dis J. 2018;37:1223–6. doi: 10.1097/INF.0000000000002002. [DOI] [PubMed] [Google Scholar]
- 35.Martin JM, Green M. Group A streptococcus. Semin Pediatr Infect Dis. 2006;17:140–8. doi: 10.1053/j.spid.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Paller AS, Mancini A. Hurwitz Clinical Pediatric Dermatology. 4th ed. Philadelphia: Saunders; 2011. Expert Consult. [Google Scholar]
- 37.Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev. 2013;26:728–43. doi: 10.1128/CMR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, et al. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One. 2014;9:e98888. doi: 10.1371/journal.pone.0098888. doi:10.1371/journal.pone.0098888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma VK, Sethuraman G, Minz A. Stevens Johnson syndrome, toxic epidermal necrolysis and SJS-TEN overlap: A retrospective study of causative drugs and clinical outcome. Indian J Dermatol Venereol Leprol. 2008;74:238–40. doi: 10.4103/0378-6323.41369. [DOI] [PubMed] [Google Scholar]
- 40.Guillaume JC, Roujeau JC, Revuz J, Pensol D, Touraine R. The culprit drugs in 87 cases of toxic epidermal necrolysis (Lyell's Syndrome) Arch Dermatol. 1987;123:1166–70. [PubMed] [Google Scholar]
- 41.Tizard EJ, Hamilton-Ayres MJ. Henoch Schonlein purpura. Arch Dis Child Educ Pract Ed. 2008;93:1–8. doi: 10.1136/adc.2004.066035. [DOI] [PubMed] [Google Scholar]
- 42.Saulsbury FT. Clinical update: Henoch-Schönlein purpura. Lancet. 2007;369:976–8. doi: 10.1016/S0140-6736(07)60474-7. [DOI] [PubMed] [Google Scholar]
- 43.Alexander Otto M. Skin manifestation are emerging in the Corona virus pandemic. Dermatology News;April 3, 2020 [Google Scholar]
- 44.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matar S, Oulès B, Sohier P, Chosidow O, Beylot-Barry M, Dupin N, et al. Cutaneous manifestations in SARS-CoV-2 infection (COVID-19): A French experience and a systematic review of the literature. J Eur Acad Dermatol Venereol. 2020;34:e686–9. doi: 10.1111/jdv.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: A worldwide review. JAAD Int. 2021;2:119–33. doi: 10.1016/j.jdin.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jindal R, Grover C, Sarkar R, Gupta LK. IADVL academy position statement on emerging dermatoses in India: Monkeypox. Indian Dermatol Online J. 2022;13:559–69. doi: 10.4103/idoj.idoj_437_22. [DOI] [PMC free article] [PubMed] [Google Scholar]