Abstract
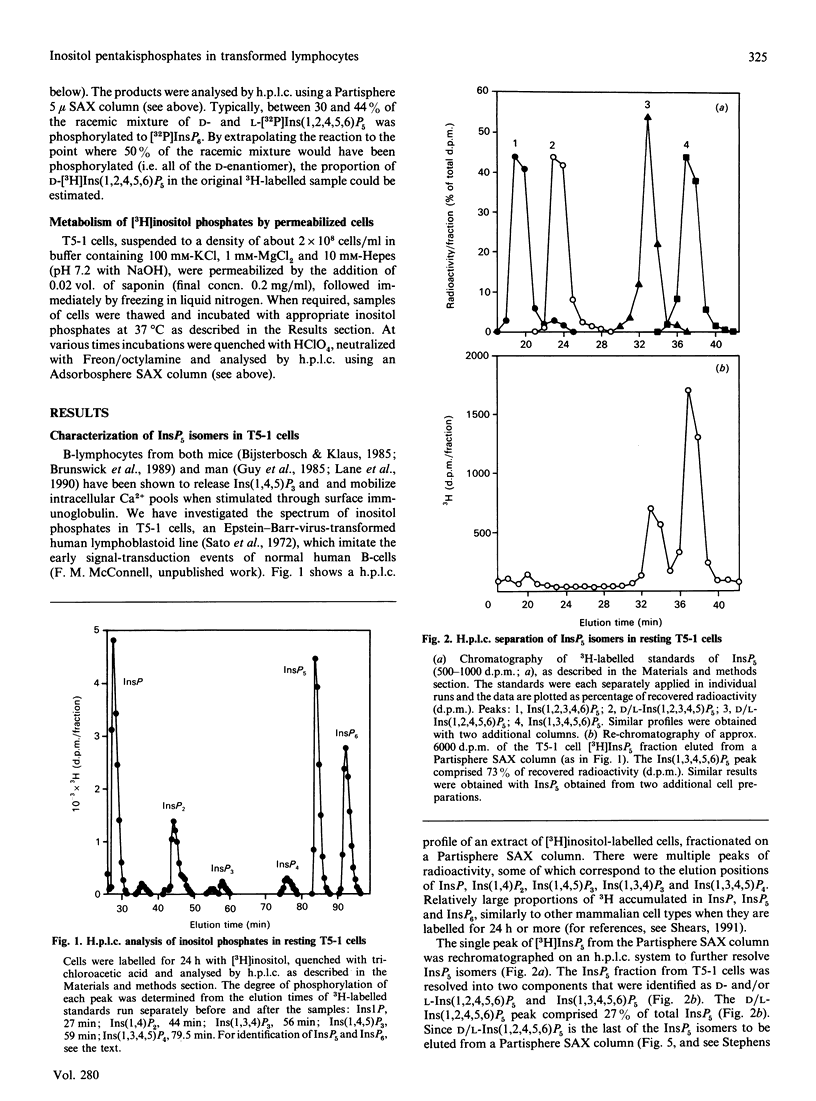
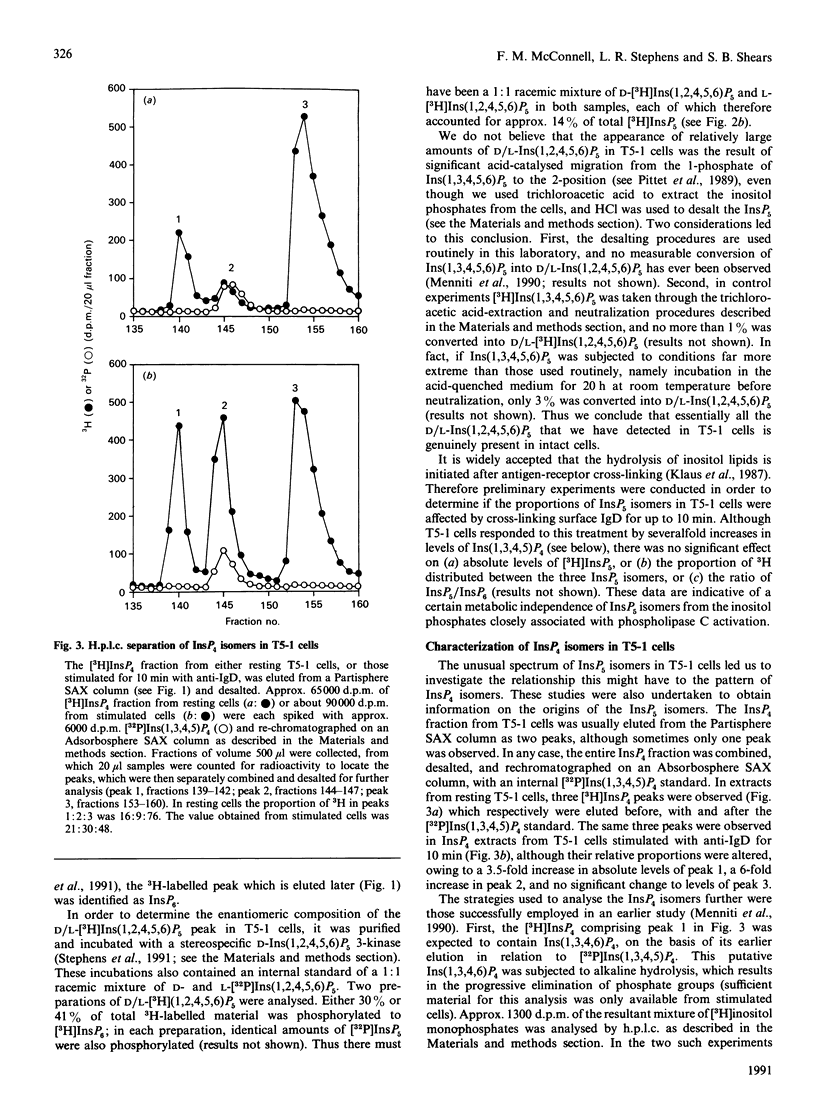
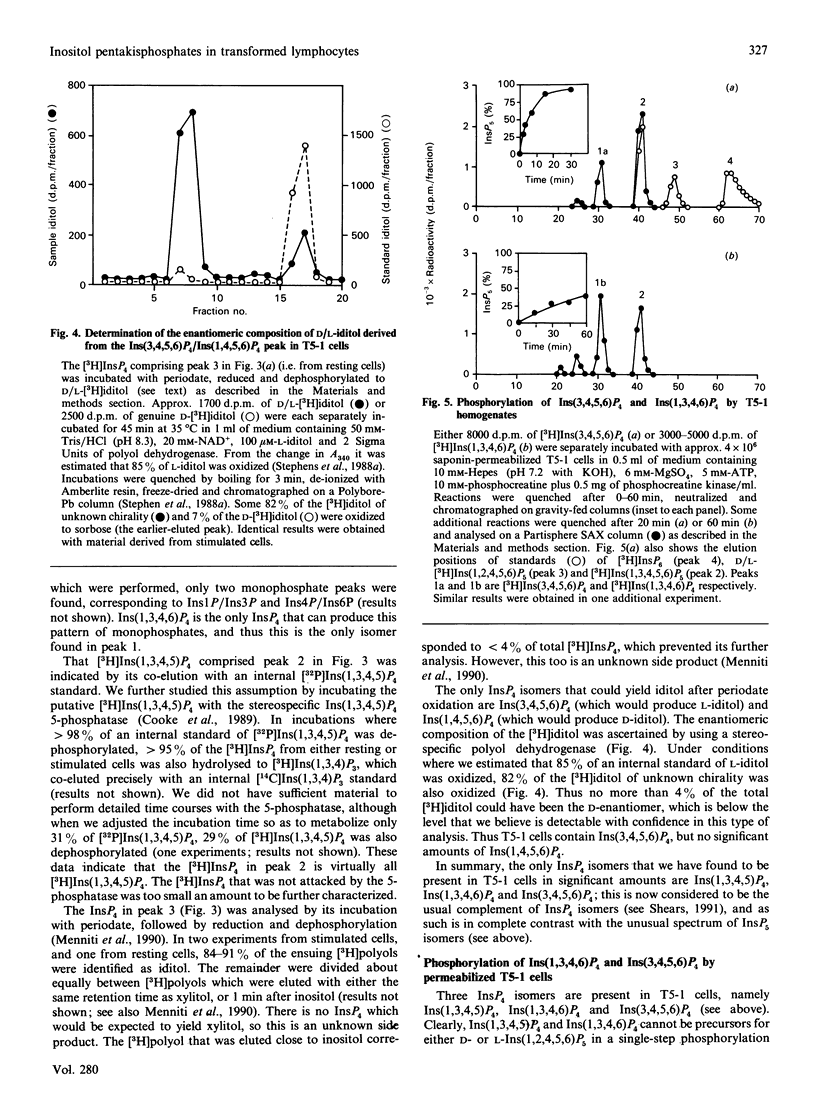
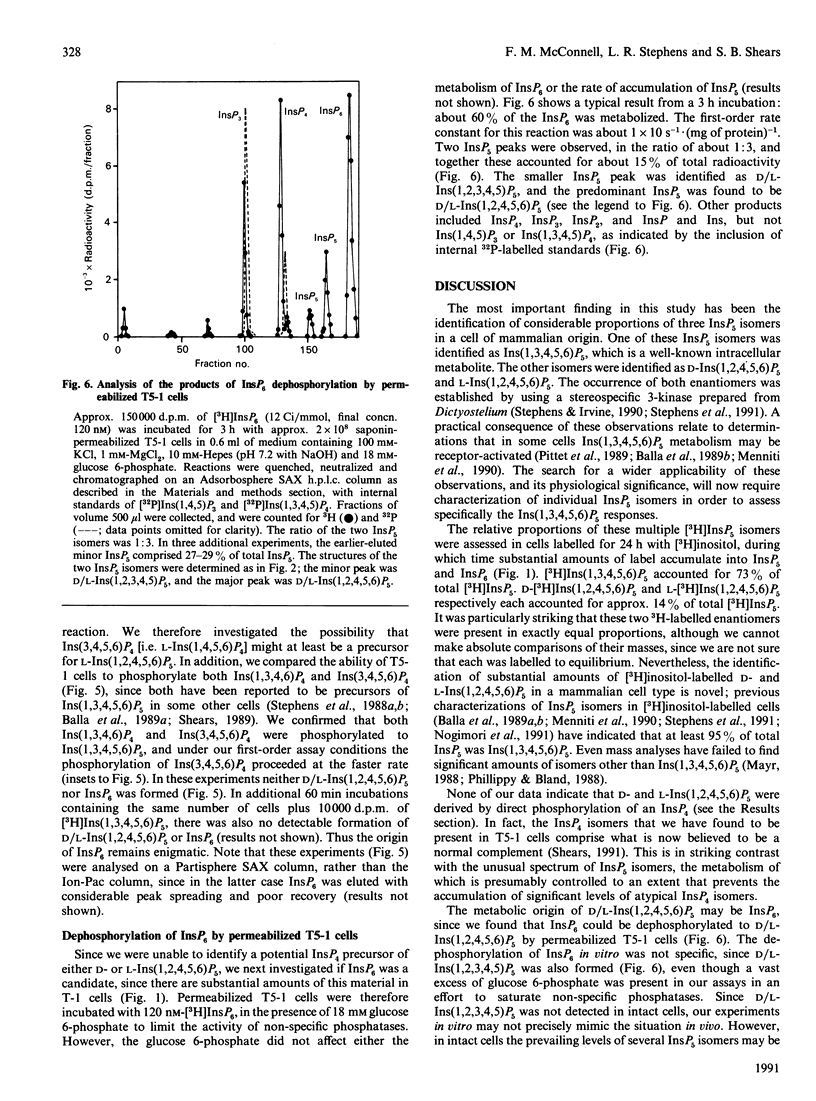
Substantial amounts of three [3H]InsP5 isomers were detected in [3H]inositol-labelled human lymphoblastoid (T5-1) cells. Their structures were determined by h.p.l.c. [Phillippy & Bland (1988) Anal. Biochem. 175, 162-166], and by utilizing a stereospecific D-inositol 1,2,4,5,6-pentakisphosphate 3-kinase from Dictyostelium discoideum [Stephens & Irvine (1990) Nature (London) 346, 580-583]. The structures were: inositol 1,3,4,5,6-pentakisphosphate, D-inositol 1,2,4,5,6-pentakisphosphate and L-inositol 1,2,4,5,6-pentakisphosphate. The relative proportions of these isomers (approx. 73:14:14 respectively) were unaffected by cross-linking anti-IgD receptors. The T5-1 cells also contained InsP6 and three Ins P4s, which were identified as the 1,3,4,5, 1,3,4,6 and 3,4,5,6 isomers. In incubations with permeabilized T5-1 cells, both 1,3,4,6 and 3,4,5,6 isomers of InsP4 were phosphorylated solely to Ins(1,3,4,5,6)P5. Permeabilized cells also dephosphorylated InsP6, even in the presence of a large excess of glucose 6-phosphate to saturate non-specific phosphatases. In the latter experiments the following isomers of InsP5 accumulated: D- and/or L-Ins(1,2,3,4,5)P5, plus D- and/or L-Ins(1,2,4,5,6)P5. This demonstration that multiple isomers of InsP5 may be formed in vivo and in vitro by a transformed lymphocyte cell line adds a new level of complexity to the study of inositol polyphosphate metabolism and function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla T., Baukal A. J., Hunyady L., Catt K. J. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem. 1989 Aug 15;264(23):13605–13611. [PubMed] [Google Scholar]
- Balla T., Hunyady L., Baukal A. J., Catt K. J. Structures and metabolism of inositol tetrakisphosphates and inositol pentakisphosphate in bovine adrenal glomerulosa cells. J Biol Chem. 1989 Jun 5;264(16):9386–9390. [PubMed] [Google Scholar]
- Barraco R. A., Phillis J. W., Simpson L. L. Cardiorespiratory effects of inositol hexakisphosphate following microinjections into the nucleus tractus solitarii. Eur J Pharmacol. 1989 Nov 28;173(1):75–84. doi: 10.1016/0014-2999(89)90010-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Klaus G. G. Crosslinking of surface immunoglobulin and Fc receptors on B lymphocytes inhibits stimulation of inositol phospholipid breakdown via the antigen receptors. J Exp Med. 1985 Dec 1;162(6):1825–1836. doi: 10.1084/jem.162.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick M., June C. H., Finkelman F. D., Mond J. J. Different patterns of inositol polyphosphate production are seen in B lymphocytes after cross-linking of sIg by anti-Ig antibody or by a multivalent anti-Ig antibody dextran conjugate. J Immunol. 1989 Sep 1;143(5):1414–1421. [PubMed] [Google Scholar]
- Cooke A. M., Nahorski S. R., Potter B. V. myo-inositol 1,4,5-trisphosphorothioate is a potent competitive inhibitor of human erythrocyte 5-phosphatase. FEBS Lett. 1989 Jan 2;242(2):373–377. doi: 10.1016/0014-5793(89)80504-6. [DOI] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Guy G. R., Gordon J., Michell R. H., Brown G. Synergism between diacylglycerols and calcium ionophore in the induction of human B cell proliferation mimics the inositol lipid polyphosphate breakdown signals induced by crosslinking surface immunoglobulin. Biochem Biophys Res Commun. 1985 Aug 30;131(1):484–491. doi: 10.1016/0006-291x(85)91828-5. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Johanson R. A., Williamson M. T., Williamson J. R. Purification and characterization of two types of soluble inositol phosphate 5-phosphomonoesterases from rat brain. J Biol Chem. 1987 Dec 25;262(36):17319–17326. [PubMed] [Google Scholar]
- Hughes P. J., Hughes A. R., Putney J. W., Jr, Shears S. B. The regulation of the phosphorylation of inositol 1,3,4-trisphosphate in cell-free preparations and its relevance to the formation of inositol 1,3,4,6-tetrakisphosphate in agonist-stimulated rat parotid acinar cells. J Biol Chem. 1989 Nov 25;264(33):19871–19878. [PubMed] [Google Scholar]
- Klaus G. G., Bijsterbosch M. K., O'Garra A., Harnett M. M., Rigley K. P. Receptor signalling and crosstalk in B lymphocytes. Immunol Rev. 1987 Oct;99:19–38. doi: 10.1111/j.1600-065x.1987.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Gathings W. E., Levitt D., Kearney J. F., Cooper M. D. Immunoglobulin isotype expression of normal pre-B cells as determined by immunofluorescence. J Clin Immunol. 1982 Oct;2(4):264–269. doi: 10.1007/BF00915065. [DOI] [PubMed] [Google Scholar]
- Lane P. J., McConnell F. M., Schieven G. L., Clark E. A., Ledbetter J. A. The role of class II molecules in human B cell activation. Association with phosphatidyl inositol turnover, protein tyrosine phosphorylation, and proliferation. J Immunol. 1990 May 15;144(10):3684–3692. [PubMed] [Google Scholar]
- Mayr G. W. A novel metal-dye detection system permits picomolar-range h.p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988 Sep 1;254(2):585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Nogimori K., Obie J. F., Shears S. B., Putney J. W., Jr Origins of myo-inositol tetrakisphosphates in agonist-stimulated rat pancreatoma cells. Stimulation by bombesin of myo-inositol 1,3,4,5,6-pentakisphosphate breakdown to myo-inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 1990 Jul 5;265(19):11167–11176. [PubMed] [Google Scholar]
- Nogimori K., Hughes P. J., Glennon M. C., Hodgson M. E., Putney J. W., Jr, Shears S. B. Purification of an inositol (1,3,4,5)-tetrakisphosphate 3-phosphatase activity from rat liver and the evaluation of its substrate specificity. J Biol Chem. 1991 Sep 5;266(25):16499–16506. [PubMed] [Google Scholar]
- Phillippy B. Q., Bland J. M. Gradient ion chromatography of inositol phosphates. Anal Biochem. 1988 Nov 15;175(1):162–166. doi: 10.1016/0003-2697(88)90374-0. [DOI] [PubMed] [Google Scholar]
- Pittet D., Schlegel W., Lew D. P., Monod A., Mayr G. W. Mass changes in inositol tetrakis- and pentakisphosphate isomers induced by chemotactic peptide stimulation in HL-60 cells. J Biol Chem. 1989 Nov 5;264(31):18489–18493. [PubMed] [Google Scholar]
- Sato K., Slesinski R. S., Littlefield J. W. Chemical mutagenesis at the phosphoribosyltransferase locus in cultured human lymphoblasts. Proc Natl Acad Sci U S A. 1972 May;69(5):1244–1248. doi: 10.1073/pnas.69.5.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Evans W. H., Kirk C. J., Michell R. H. Preferential localization of rat liver D-myo-inositol 1,4,5-trisphosphate/1,3,4,5-tetrakisphosphate 5-phosphatase in bile-canalicular plasma membrane and 'late' endosomal vesicles. Biochem J. 1988 Dec 1;256(2):363–369. doi: 10.1042/bj2560363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B. Regulation of the metabolism of 1,2-diacylglycerols and inositol phosphates that respond to receptor activation. Pharmacol Ther. 1991;49(1-2):79–104. doi: 10.1016/0163-7258(91)90023-f. [DOI] [PubMed] [Google Scholar]
- Shears S. B. The pathway of myo-inositol 1,3,4-trisphosphate phosphorylation in liver. Identification of myo-inositol 1,3,4-trisphosphate 6-kinase, myo-inositol 1,3,4-trisphosphate 5-kinase, and myo-inositol 1,3,4,6-tetrakisphosphate 5-kinase. J Biol Chem. 1989 Nov 25;264(33):19879–19886. [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Stanley A. F., Moore T., Poyner D. R., Morris P. J., Hanley M. R., Kay R. R., Irvine R. F. myo-inositol pentakisphosphates. Structure, biological occurrence and phosphorylation to myo-inositol hexakisphosphate. Biochem J. 1991 Apr 15;275(Pt 2):485–499. doi: 10.1042/bj2750485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Irvine R. F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990 Aug 9;346(6284):580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON R. V., BALLOU C. E. Complete characterization of the myo-inositol polyphosphates from beef brain phosphoinositide. J Biol Chem. 1961 Jul;236:1902–1906. [PubMed] [Google Scholar]
- Tilly B. C., van Paridon P. A., Verlaan I., Wirtz K. W., de Laat S. W., Moolenaar W. H. Inositol phosphate metabolism in bradykinin-stimulated human A431 carcinoma cells. Relationship to calcium signalling. Biochem J. 1987 May 15;244(1):129–135. doi: 10.1042/bj2440129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M., Jackson T., Lightman S., Hanley M. R. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987 Dec 17;330(6149):656–658. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]
- Wreggett K. A., Howe L. R., Moore J. P., Irvine R. F. Extraction and recovery of inositol phosphates from tissues. Biochem J. 1987 Aug 1;245(3):933–934. doi: 10.1042/bj2450933. [DOI] [PMC free article] [PubMed] [Google Scholar]


