Sir,
Anti-PD1 therapy in metastatic melanoma patients is associated with a high rate of cutaneous adverse events (AEs).[1] Atopic dermatitis (AD) is one of the AEs reported during therapy with pembrolizumab. Treatment of AD in cancer patients is challenging due to the limited availability of drugs. Dupilumab is a monoclonal antibody blocking IL-4 and IL-13 signalling.[2] Long-term treatment with dupilumab in patients with moderate-to-severe AD has been shown to be safe and effective.
We present the case of an 82-year-old Caucasic male who suffered from nodular melanoma (with regression, ulceration, and a 2.7-mm Breslow thickness). In October 2018, the patient underwent wide excision and sentinel lymph node biopsy, which tested positive for sub-capsular metastasis in one node of the left axilla. In December 2018, he presented with purplish cutaneous nodules in his left axilla, confirmed on histological examination as melanoma metastases. BRAF V600E gene mutation was identified. Considering the relevant disease burden and LDH serum level (390 U/L), the patient was started on 200 mg pembrolizumab treatment, administered intravenously every 3 weeks. In February 2019, clinical examination and PET/CT 18-FDG proved disease progression. In March 2019, the patient was switched to combined anti-BRAF/MEK targeted therapy with dabrafenib 75 mg four capsules daily and trametinib 2 mg daily. A follow-up PET/CT performed in May 2019 showed an almost complete metabolic response to therapy.
Since November 2019, the patient began to present AD symptoms such as diffuse eczematous lesions, skin xerosis, severe pruritus, and sleep disturbance [Figure 1]. The scoring Atopic Dermatitis (SCORAD) index was 58.2 (severe), Eczema Area and Severity Index (EASI) was 40, Pruritus-numerical rating scale (Pruritus-NRS) was 10/10, sleep-NRS was 9/10, and Dermatology Life Quality Index was 30. Laboratory tests showed a significant IgE serum level (827 kIU/L). In January 2020, the patient underwent an incisional biopsy of upper back skin lesions. Histological examination displayed mild spongiosis with hyperkeratosis, predominantly lymphocytic perivascular infiltrate, and fibrosis of the papillary dermis. According to the patient’s medical history and the above-mentioned results, he was diagnosed with AD. The patient was treated with topical therapies, including clobetasol 0.05% cream, tacrolimus 0.1% ointment, and systemic anti-histamines (cetirizine and rupatadine). However, pruritus and eczematous skin lesions were recalcitrant to this therapy. Systemic immunosuppressors were not considered because of safety concerns in cancer patients.[3] Thus, in October 2021, we decided to start dupilumab therapy, with a 600 mg induction dose, followed by 300 mg every 2 weeks. After 1 month of treatment, a noticeable clinical improvement and a decrease in pruritus severity (NRS 2/10) were observed, without side effects. After 16 weeks of dupilumab therapy, the patient showed further improvement with SCORAD 9, EASI 2.5, pruritus-NRS 0/10, and sleep-NRS 1/10. Only xerosis on the lower limbs was observed. To date, the patient is continuing dupilumab therapy for more than 6 months, and his clinical conditions are stable.
Figure 1.
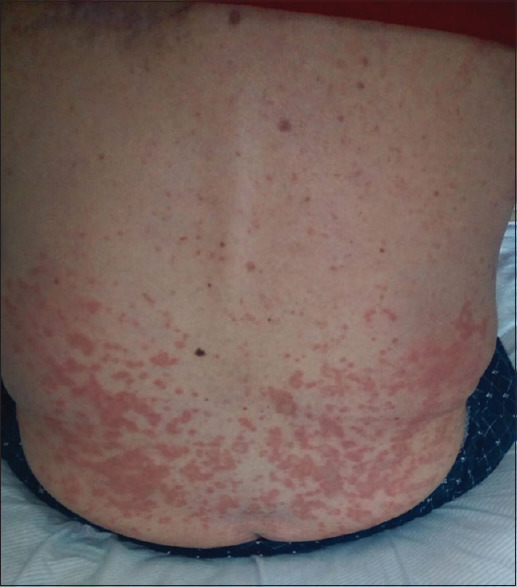
Multiple, confluent, erythematous-desquamative papules on the skin of the lumbar back
Cancer patients treated with anti-blastics frequently develop cutaneous AEs, with a very significant impact on the patient’s psychological state and quality of life. Moreover, managing AEs in cancer patients is particularly challenging due to the compromised general condition and the limited availability of drugs. In addition, the literature does not report an increase in malignancy recurrence in patients treated with dupilumab.[4,5] The case we presented here put in evidence, in a real-life setting, the safety and efficacy of dupilumab in the management of atopic symptoms in a patient treated with anti-PD1. However, further studies with larger cohorts and control groups, including patients with AD not treated with dupilumab, are needed to provide data on the long-term safety of dupilumab in cancer patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol. 2016;74:455–61.e1. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Deleuran M, Thaçi D, Beck LA, de Bruin-Weller M, Blauvelt A, Forman S, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377–88. doi: 10.1016/j.jaad.2019.07.074. [DOI] [PubMed] [Google Scholar]
- 3.Vial T, Descotes J. Immunosuppressive drugs and cancer. Toxicology. 2003;185:229–40. doi: 10.1016/s0300-483x(02)00612-1. [DOI] [PubMed] [Google Scholar]
- 4.Braddock M, Hanania NA, Sharafkhaneh A, Colice G, Carlsson M. Potential risks related to modulating interleukin-13 and interleukin-4 signalling: A systematic review. Drug Saf. 2018;41:489–509. doi: 10.1007/s40264-017-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siliquini N, Giura MT, Viola R, Ribero S, Panzone M, Dapavo P, et al. Atopic dermatitis, dupilumab and cancers: A case series. J Eur Acad Dermatol Venereol. 2021;35:e651–2. doi: 10.1111/jdv.17264. [DOI] [PubMed] [Google Scholar]


