Abstract
A variety of enzymic and non-enzymic methods to isolate epithelium from the small intestine have been previously published. Sequential fractionation of cells from the villus to the crypt has been reported in some of these papers, which allows the comparative study of terminally differentiated and proliferative cell phenotypes. However, these methods often involve the incubation of tissues at 37 degrees C, which may affect the structural and biochemical integrity of the cells. We have developed a rapid low-temperature (4 degrees C) method for isolating purified populations of crypt and villus cells from mouse and rat intestines. The fractionated cells have been partially characterized, and the potential value of the procedure has been indicated by the ability to analyse the comparative protein and mRNA expression along the crypt-villus axis.
Full text
PDF
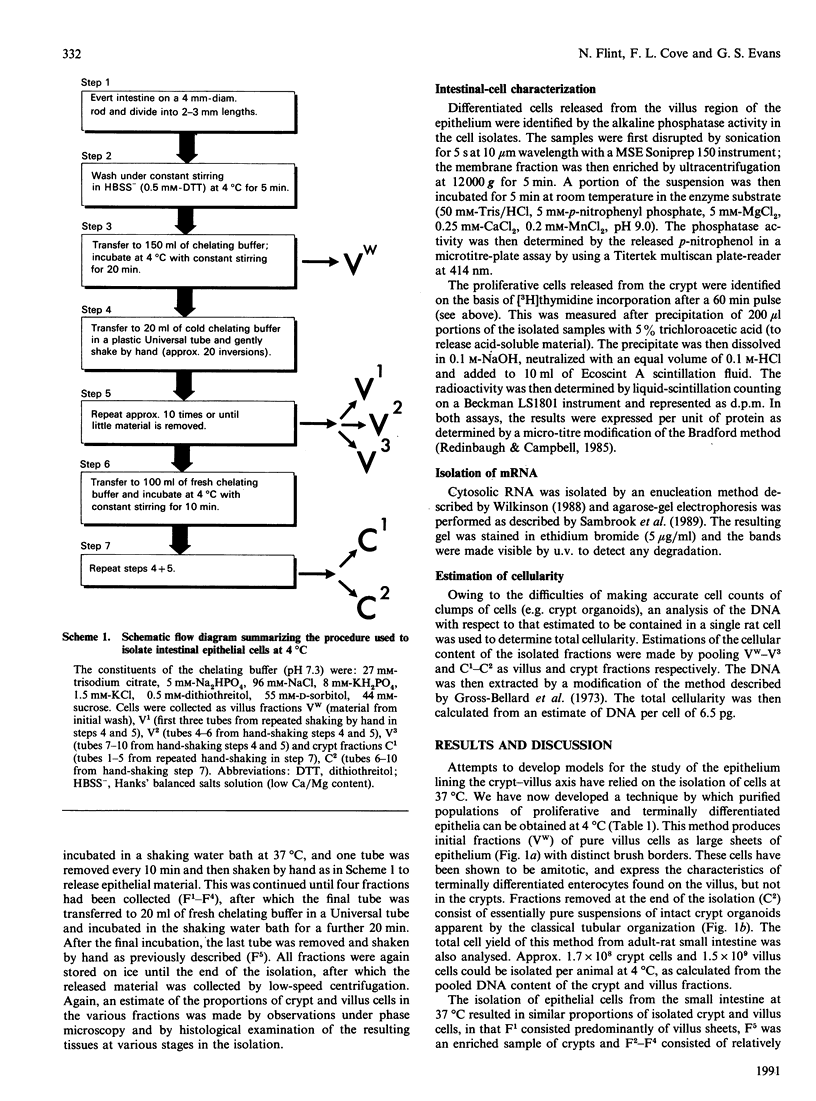
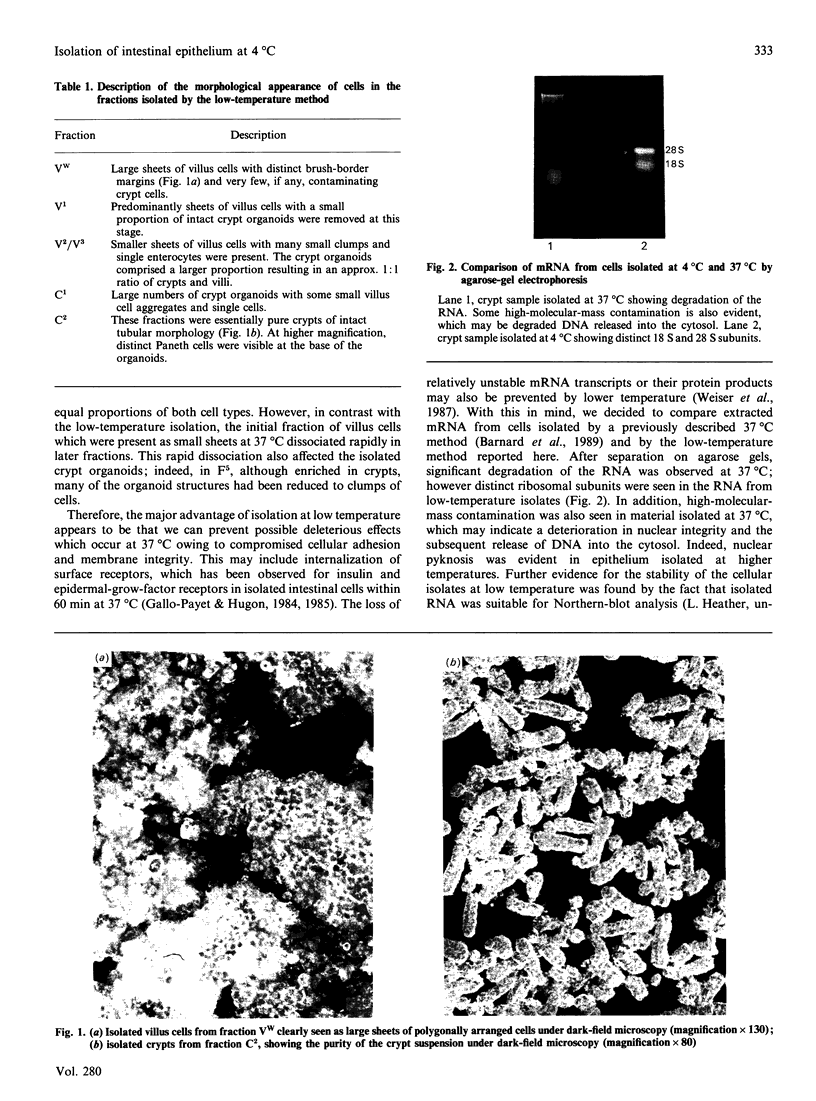
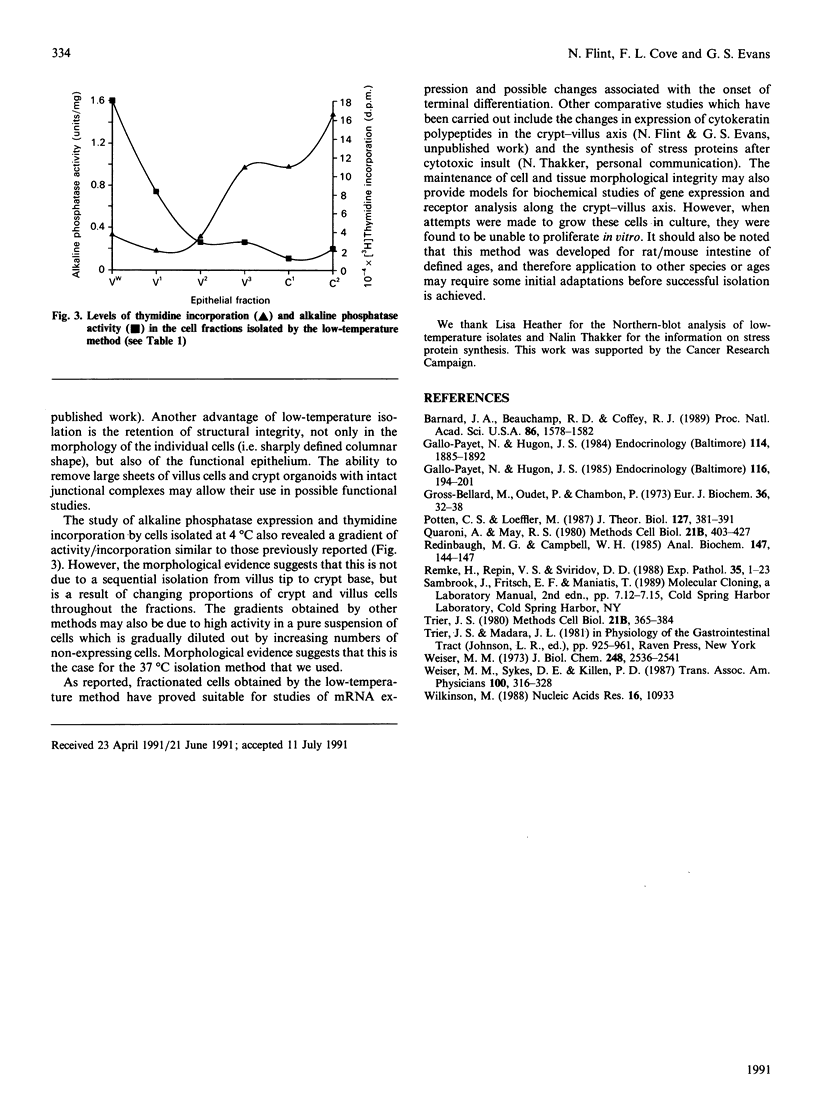
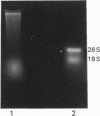
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard J. A., Beauchamp R. D., Coffey R. J., Moses H. L. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo-Payet N., Hugon J. S. Epidermal growth factor receptors in isolated adult mouse intestinal cells: studies in vivo and in organ culture. Endocrinology. 1985 Jan;116(1):194–201. doi: 10.1210/endo-116-1-194. [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N., Hugon J. S. Insulin receptors in isolated adult mouse intestinal cells: studies in vivo and in organ culture. Endocrinology. 1984 May;114(5):1885–1892. doi: 10.1210/endo-114-5-1885. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J Theor Biol. 1987 Aug 21;127(4):381–391. doi: 10.1016/s0022-5193(87)80136-4. [DOI] [PubMed] [Google Scholar]
- Quaroni A., May R. J. Establishment and characterizaton of intestinal epithelial cell cultures. Methods Cell Biol. 1980;21B:403–427. [PubMed] [Google Scholar]
- Redinbaugh M. G., Campbell W. H. Adaptation of the dye-binding protein assay to microtiter plates. Anal Biochem. 1985 May 15;147(1):144–147. doi: 10.1016/0003-2697(85)90020-x. [DOI] [PubMed] [Google Scholar]
- Remke H., Repin V. S., Sviridov D. D. Isolated cells in suspension for biological research--Part III. Structure and functional properties of enterocytes and adipocytes. Exp Pathol. 1988;35(1):1–23. doi: 10.1016/s0232-1513(88)80113-0. [DOI] [PubMed] [Google Scholar]
- Trier J. S. Organ culture of the mucosa of human small intestine. Methods Cell Biol. 1980;21B:365–384. doi: 10.1016/s0091-679x(08)60693-7. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Weiser M. M., Ryzowicz S., Soroka C. J., Albini B. In vitro translation of rat intestinal RNA prepared from isolated villus and crypt cells and from the epithelium-denuded intestine. Synthesis of intestinal basement membrane. Trans Assoc Am Physicians. 1987;100:316–328. [PubMed] [Google Scholar]
- Wilkinson M. RNA isolation: a mini-prep method. Nucleic Acids Res. 1988 Nov 25;16(22):10933–10933. doi: 10.1093/nar/16.22.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]