Abstract
Androgenetic alopecia (AGA) is defined as the alopecia induced by androgens in genetically predisposed individuals. AGA results in progressive miniaturization of the hair follicles leading to vellus transformation of terminal hair. The high prevalence and wide range of expressed phenotypes in AGA is a result of a polygenic inheritance mode. The androgen receptor (AR) gene located on the X chromosome at Xq11-12 is the first gene to show genetic association with AGA. Newer genetic associations with AGA are under study. In early-onset AGA, obesity, diabetes, hypertension, dyslipidaemia, insulin resistance, benign prostatic hyperplasia (BPH), prostate cancers and coronary artery disease (CAD) are associated with AGA. Screening of early-onset AGA patients and intervention for metabolic syndrome and insulin resistance can prevent the development of cardiovascular disease (CVD) at an early stage. As effective treatments continue to be topical minoxidil, systemic finasteride and hair transplantations, newer modalities are under investigation. Understanding the genetic factors involved in AGA and continued research into newer therapies, such as cell-based therapies, will lead to effective treatment and improve the quality of life in patients with AGA.
Keywords: Androgenic alopecia, androgenetic alopecia, androgen receptor gene, single nucleotide polymorphism
Introduction
Androgenetic alopecia (AGA), male pattern baldness (MPB) or male pattern hair loss (MPHL) is a genetically determined progressive condition that causes a gradual conversion of terminal hair into vellus hair.[1,2] “AGA is defined as the alopecia induced by androgens in genetically predisposed individuals.”[3] The characteristic ‘horseshoe’ pattern occurs in men, hair loss involving the temporal and vertex regions and sparing of the occipital region.[4] The type of hair loss is different in women, and the prevalence is lower than that in men.[4] The onset of AGA is highly variable and appears to be determined by sufficient circulating androgens and the degree of genetic predisposition.[5] AGA results in progressive miniaturization of the hair follicles leading to vellus transformation of terminal hair. Screening of early-onset AGA patients for identification and intervention for metabolic syndrome, insulin resistance and genetic risk factors can prevent the development of cardiovascular disease (CVD).
The androgen receptor (AR) gene plays a significant role in many studies showing mutations, single nucleotide polymorphisms (SNPs), and cytosine, adenine and guanine (CAG) repeats differences.[1,6] The morbidity in AGA is primarily psychological and differs from one person to another. Psychiatric disorders are more common in individuals with alopecia than the general population, suggesting that these individuals are at risk of getting an anxiety disorder, depressive episodes or social phobia. Oral finasteride and topical minoxidil are Food and Drug Administration (FDA)-approved for AGA. Hair transplant is the only available treatment for advanced AGA, but this needs donor occipital hairs. Future research targets new agents, such as gene therapy, angiogenic growth factors and prostaglandin analogues.[1] With increasing age, free-radical scavenging enzyme activity decreases, which adds to the harmful consequences of oxidative stress on the body. Reactive nitrogen species (NO) and other free radicals, such as reactive oxygen species (ROS), are essential for cellular damage.[2] High-density lipoproteins (HDLs) are linked to the liver-produced enzyme paraoxonase 1 (PON1), which prevents lipid peroxidation. Reduction in PON1 levels could increase with increasing grades of AGA.[7]
The risk factors of CAD, such as low high-density lipoprotein (HDL), high low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), triglycerides (TG), serum lipoprotein-a (SL-a) and serum homocysteine (SH), increase with increasing grades of AGA. Patients with AGA are at an increased risk of CAD, which increases with increasing grades of AGA.[8]
Screening of early-onset AGA patients and intervention for metabolic syndrome can prevent the development of CVD.[9] Carotid ultrasound in patients with early AGA helps detect the risk and start preventive measures against the development of CVD.[10] Insulin resistance is found to be associated with AGA. Insulin resistance hinders glucose disposal, resulting in a compensatory increase in beta-cell insulin production and hyperinsulinemia. AGA seems to connect with insulin resistance-linked diseases in men at an older age but not with the markers of insulin resistance. It remains unclear whether insulin resistance led to the development of the AGA (due to ageing) or there is a relationship between drugs (antihypertensive drugs, such as α- and β-channel blockers, angiotensin-converting enzyme (ACE) and calcium channel blockers).[11] There is a strong connection between coronary heart disease and hypertension with hyperinsulinemia due to insulin resistance.[12] Diseases, such as benign prostatic hyperplasia (BPH), prostate cancers and coronary artery disease (CAD), were found to be more in patients with AGA than in non-bald people.[13] People worldwide spend more money on hair products for AGA, but a proper cure remains questionable. Hence, in this study, we review all the important aspects of AGA, associated disorders, genetics and new treatment modalities.
Pathogenesis of AGA
AGA and the Hair Cycle
Follicles undergo cyclical phases of growth, involution, quiescence and regeneration. The growth phase is the anagen phase that lasts for 3 to 5 years. The duration of this growth phase determines the final hair length. At the end of anagen, the next stage (involutional), called catagen, occurs for a few weeks. They follow a period of hair follicle quiescence lasting approximately 3 months, known as telogen. In the first week of anagen, hair follicle regeneration occurs. After which, this phase continues until the hair reaches its final predetermined length. Molecular signals, such as cytokines, growth factors, intracellular signalling pathways and nuclear receptors, control the hair cycle.
Growth factors, such as keratinocyte growth factor, insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF) and hepatocyte growth factor, promote the anagen phase of the hair cycle. In AGA, a reduction in the duration of the anagen phase leads to shorter hair length, while an increase in the period of the telogen phase delays regeneration. An increased number of empty pores occurs due to the thinning and shortening of hairs, which fails to attain the needed length to reach the skin’s surface[1] [Figure 1]. The dysfunctional dermal papilla (DP) cell may cause follicle miniaturization in the case of AGA.[14]
Figure 1.
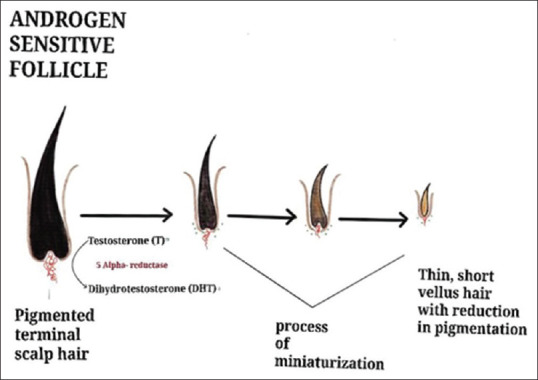
Pathogenesis of AGA
Metabolism of androgens in AGA
Androgen action in the follicles is on the bioavailability of locally produced androgens. In AGA men with normal circulating androgens, higher amounts of testosterone and dihydrotestosterone (DHT) are made locally. The skin contains the enzymes necessary for androgen metabolism. Skin is the peripheral organ that produces androgens through intracrine or paracrine actions.[15] This locally produced androgen leads to different responses occurring in the hair follicles.[16] Testosterone reaches the skin through capillaries and is converted to more potent androgen DHT by 5α-reductase (5αR) in the cytoplasm. Conversion of androgens occurs like dehydroepiandrosterone-sulphate (DHEA-S), dehydroepiandrosterone (DHEA), and androstenedione to DHT occurs in sebocytes, sweat glands and dermal papilla cells (DPCs).[16,17]
5αR metabolizes testosterone to DHT, and DHT binds strongly to the androgen receptors (ARs) to activate gene expression.[18,19] The androgen dependency of the hair follicles to have ARs is proved by the absence of body hair in people with androgen insensitivity[20] but the requirement for 5αR differs with follicle site. Patients with 5αR2 deficiency only produce female patterns of pubic and axillary hair growth. The androgen action in human follicles is presumably due to differential gene expression within individual hair follicles.[4]
Regulators of inflammation and energy metabolism, such as the nuclear peroxisome proliferator-activated receptors (PPARγ, α, β), are involved in hair loss. The epidermis and sebaceous glands in the skin express PPARγ. The mesenchymal DP cells, inner root sheath, epithelial cells of the outer root sheath (ORS) and matrix of the anagen human hair follicle express PPARγ.[21]
In the DP of the balding scalp of AGA, AR-mediated paracrine signalling, particularly TGF-β signalling, causes death of microvascular endothelial cells. This results in regression of vessels on DP of hair follicles.[22]
Androgens in genetically susceptible women and men, the thinning, begin between ages 12 and 40 years, and the inheritance pattern is polygenic[23] [Figures 1 and 2].
Figure 2.
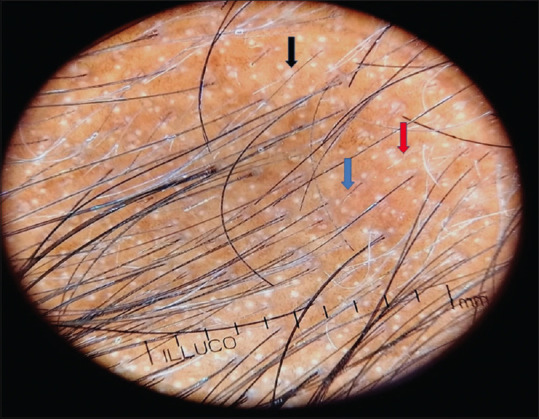
Trichoscopy findings in AGA showing vellus hair transformation (blue arrow), loss of hair follicles (red) and white peripilar sign (black arrow)
As lncRNAs can be effective in the formation of cardiotoxicity and the induction of CVD, they may also play a role in the development of CVD in AGA patients with hair follicle regeneration, proliferation, differentiation and apoptosis.[24]
Genetics
Baldness is known to be heritable. Identifying the genes linked to variation in baldness and building a genetic score to improve the prediction of hair loss are needed. About 80% of both early-onset and late-onset hair loss was attributed to genetic factors in twin studies. The high prevalence and wide range of expressed phenotypes in AGA result in a polygenic inheritance mode. Deoxyribonucleic acid (DNA) sequence variations can occur, such as SNPs, microsatellite repeats, insertion mutations, deletion mutations and copy number variations.[25] Senescence is a cell destiny that develops in response to damage in DNA, telomere and mitochondria as people age.[2] Newer molecular genetic methods have evaluated the SNP based on the common-variant heritability of baldness at around 50%. The AR decides the sensitivity of cells to androgens. Several studies have identified specific genetic variants linked to variations in baldness, usually with the AR gene showing the strongest association. Many published genome-wide association studies (GWAS) highlighted eight independent genetic loci linked to baldness, the top being an SNP in the AR gene. In a case-control study with ~600 per arm, a predictor based on 34,186 SNPs proved 4.5% of the variation on the liability scale.[26] Marcińska et al. used candidate genes in the study to build 5-SNP and 20-SNP polygenic predictors, which performed well in early-onset AGA but poorly when considering those with no baldness versus and those with severe baldness across all ages.[27]
AR Gene
The AR gene is situated on the X chromosome at Xq11-12.[9] The AR gene is a principal candidate for MPB. Hormonal and gene expression studies revealed that the predisposed scalp in balding men exhibits high levels of DHT[6,28,29] and enhanced expression of the AR.[19] The AR gene regulates the potency of androgens available to the hair follicles. Among the AR gene polymorphisms known, the Stu 1 polymorphism has the most significant association with AGA.[25] This was the first gene to be identified in AGA.
Differences in the DNA sequence of the gene encoding the AR might lead to differences in the activity of the receptor. Those differences increase sensitivity to DHT, leading to hair loss at an earlier age. A study discovered a significant single base change in the gene’s coding region (exon 1). This polymorphism does not alter the amino acid sequence of the protein and is non-functional.[5]
The CAG triplet repeats are seen on the amine end of the gene. The number of CAG repeats is polymorphic in the population. The variation in the CAG repeats (in the AR gene) results in different manifestations. The reduced number of CAG repeats increases CAD and prostate cancer. It may be associated with skin diseases, such as hirsutism, AGA and acne in men and women. In various studies, triple CAG repeats and a lower GGC (guanine, guanine and cytosine) are inversely related to the gene receptor’s protein levels and androgen transcriptional activity.
Recent investigations suggested that the shorter CAG repeat length in the first exon of the AR gene is associated with a higher rate of coronavirus disease 2019 (COVID-19) morbidity and mortality.[30]
Other Loci
A meta-analysis of the cohort GWAS studies discovered a novel locus on chromosome number 6. The study estimated the SNP-based heritability of late-onset baldness (42% (SE 23%) from the autosomes, 10% (SE 5%) from the X chromosome) and early-onset (56% (SE 22%) from the autosomes, 23% (SE 1.1%) from the X chromosome). The most significant gene set, GO: 0005667, was seen corresponding to the transcription factor complex gene set, including ALX4. ALX4 gene was found to be mutated in patients presenting with alopecia with frontonasal dysplasia. Out of the other genome-wide significant gene sets, ENSG00000141027 (NCOR1 subnetwork) includes the (HDAC) histone deacetylase family members.[29,31]
HDAC9 is associated with AGA (this paper and Liu et al.). GO:0003682 (transcription factor binding) comprises the murine gene Cux1 that is important for hair growth (among other things).[32] GO:0003712 (transcription cofactor activity) consists of the gene AIRE associated with alopecia. MP:0000097 and GO:0044212 (transcription regulatory region DNA binding) include the murine gene named Grhl1. Grhl1-null mice suffer a delay in coat growth and, at a later stage, hair loss.[33]
Four genome-wide significant risk loci for AGA were found on chromosomes 2q35, 3q25.1, 5q33.3 and 12p12.1. The strongest association was found in rs7349332 on chr2q35, located in WNT10A. The risk variant rs7349332 for AGA had a regulatory effect on WNT10A expression in the hair follicle. This effect might be of relevance in regulating hair cycle dynamics functionally, as Wnt signalling has been implicated in several human disorders.[34,35]
Aromatase is encoded by the CYP19A1 gene, which is located on chromosome 15q21.1. The CYP19A1 gene plays a crucial role in regulating androgen metabolism. The rs6493497 and rs7176005 SNPs of the CYP19A1 gene may be genetic markers that influence the risk of female pattern hair loss in this Chinese population.[36] Xq12 loci, comprising AR/EDA2R and the region on 20p11, are major determinants of AGA in European populations. Multiple DNA variants in these two regions have a major positive influence on the performance of tests designed to predict MPB. The three additional genes of EBF1, TARDBP and HDAC9 showed weaker association with AGA but achieved statistical significance. The combination of 20 SNPs from 10 known AGA-associated loci enabled a test with good predictive performance for MPB in Europeans. rs5919324 near AR, rs1998076 in 20p11 region, rs929626 in EBF1, rs12565727 in TARDBP and rs756853 in HDAC9 are few SNPs found in a European population study.[27]
The seven included SNPs rs4827528 of the AR gene, rs7680591 of the FGF5 gene and rs1042028, rs1042157, rs78-8068 and rs6839 of the SULT1A1 gene, as well as rs775746 of the CYP3A5 gene, may be related to the medication metabolism utilized in AGA treatment in a study conducted in Mexican population.[6]
Three main groups discovered in a study from Edinburgh genes linked to Wnt signalling (LGR4, RSPO2, WNT3, WNT10A, SOX13, DKK2, TWIST1, TWIST2, IQGAP1 and PRKD1), genes involved in apoptosis (BCL2, DFFA, TOP1, IRF4 and MAPT) and a third more heterogeneous group, including the AR and TGF-β pathways (RUNX2, RUNX3, PTHLH, ALPL, AR, RUNX1, PDGFA, SRD5A2, FGF5 and PAX3).[27,35]
Genes associated with the hypoxia-inducible factor-1 pathway (EGLN1 and EGLN3) and Wnt pathway inhibitors (SERPINF1 and SFRP2) may be crucial in the development of AGA.[35]
Epigenetics
Epigenetic modifications, such as X chromosome inactivation, hypermethylation and hypomethylation of DNA, can occur. The two significant genetic risk loci are the X chromosome AR/EDA2R locus and the PAX1/FOX A2 locus on chromosome 20.19. Many studies are in progress to find out genetic association in AGA to make it a diagnostic tool in preventing the complications caused by AGA. DNA methylation of the AR promoter in the occipital scalp is increased compared to the vertex AGA scalp. Increased AR methylation, resulting in reduced AR expression, protects occipital hairs from miniaturization and hair loss. In DPCs from androgen-sensitive sites, such as AGA and beard, Hic-5/ARA55, a TGF-β1-inducible AR coactivator has expressed highly. Hic-5/ARA55 scaffold protein stabilizes chromatin-modifying coactivator complexes to the target promoters. Eighteen Hic-5/ARA55 levels correlate with AR levels from different sites. The pre-receptor 5αR, ARs and post-receptor androgen coactivators regulate the sensitivity of hair follicles to androgen[4] [Table 1].
Table 1.
Genomes and SNPs involved with Androgenic alopecia in different studies
| GENOME | SNPs | POSITION/DESIGN ID |
|---|---|---|
| Chr2q35 located in WNT10A[17] | rs7349332 | chr2:218891661 (GRCh38.p13) |
| CYP19A1 gene | rs6493497 | Chr15:51338638 (GRCh38.p13) |
| CHINESE POPULATION[21] | rs7176005 | Chr15:51339082 (GRCh38.p13) |
| Near AR | rs5919324 | chrX: 67210798 |
| 20p11 | rs1998076 | chr20:21899407 |
| EBF1 | rs929626 | chr5:158883623 |
| TARDBP | rs12565727 | chr1:10973025 |
| HDAC9 | rs756853 | chr7:18850377 |
| EUROPEAN POPULATION[20] | ||
| C1ORF127 | rs7542354 | 11040385 |
| NA | rs12745121 | 25467880 |
| NA | rs10919382 | 170361164 |
| MEMO2/DPY30 | rs13021718 | 32181424 |
| AC144525.1 | rs11684254 | 239695893 |
| MRPS22 | rs7642536 | 139032333 |
| FGF5 | rs7680591 | 81197949 |
| EBF1 | rs1422798 | 158320877 |
| IRF4 | rs12203592 | 396321 |
| NA | rs9357047 | 9327556 |
| HDAC9 | rs71530654 | 18896988 |
| NA | rs939963 | 68587797 |
| AP001331.1 | rs79206101 | 109145555 |
| MAPT | rs112385572 | 44066172 |
| NSF | rs538628 | 44787313 |
| SLC14A2 | rs8085664 | 42814156 |
| NA | rs6035986 | 21894764 |
| LOC100270679/RP11-125P18.1 | rs201593 | 22033819 |
| NA | rs7362397 | 22100070 |
| NA[26] | rs7362398 | 22100072 |
| AR | rs4827528 | Hs.GT.rs4827528.G.1 |
| FGF5 | rs7680591 | Hs.GT.rs7680591.A.1 |
| CYP3A5 | rs776746 | Hs.ADME.rs776746.C.1 |
| SULT1A1[37] | rs1042028 | CD.GT.CGFP1420.1 |
| rs1042157 | CD.GT.NQFT9529.1 | |
| rs6839 | CD.GT.NTVL6110.1 | |
| rs788068 | CD.GT.VXLQ2918.1 |
Treatment
As we know, the already existing treatments, such as oral and topical minoxidil, oral finasteride, oral dutasteride, antiandrogens, platelet-rich plasma, growth factor therapy, low-level laser light therapy, camouflage techniques and hair transplantation, are conducted.[37]
Increased androgen, senescent cell burden and oxidative stress-induced senescence mechanisms in ageing may be initial targets to improve AGA.[2]
Others
-
1)
Promotion of hair regrowth by activation of the DPs (Ginkgo biloba, millet seeds, aloe vera, hibiscus, iron and zinc supplements, retinoids, cyclosporine).
-
2)
Perifollicular vascularization (prostaglandin analogues, such as aminexil, mesotherapy, benzyl nicotinate, latanoprost and bimatoprost).
-
3)
Inhibition of 5-alpha-reductase (green tea, polysorbate, biotin and Serenoa repens).
-
4)
Anti-inflammatory (corticosteroids, zinc pyrithione, Nigella sativa and Canadian willowherb).
-
5)
Improving nutrition (vitamin supplements, such as vitamin D, trace elements).
Other techniques, such as acupuncture to increase circulation, stimulate hair follicles and decrease inflammatory infiltrate, can be tried. Other topicals without proper evidence include carpronium caffeine, chloride, t-flavanone, melatonin, capsaicin, adenosine, curcumin, garlic gel, azelaic acid, procyanidin, cystopurin/pentadecane and cepharanthine.[27]
Many new therapies, such as topical antiandrogen (clascoterone 1%, fluridil), botulinum toxin, topical cetirizine, alfatradiol, melatonin, stem cell therapy, scalp threading and exosome therapy, give promising results, and many studies are under trial.
Through lowering mitochondria-associated ER membrane (MAM) production and mitochondrial malfunction, cyanidin 3-O-arabinoside is a promising natural drug for AGA therapies against DHT-induced DPC senescence.[38] Given the benefits of using a nanoparticulated method for intrafollicular drug administration, it is anticipated that a drug-loaded nanocarrier will soon be available on prescription for the treatment of AGA.[37]
Challenges
In patients as early as the 20s, AGA causes stress. The person compares themselves with their peers, social teasing and worries about ageing, which gradually leads to a lack of self-esteem. Such people can also spend much time and money on hair loss treatment. The increased psychological stress has been reflected in the increased demands for medical therapies for hair loss and hair transplantation. But once the patient starts to live this condition for longer periods, they tend to cope up with this hair loss and have less stress compared to patients in early stages.
At present, there is no reliable marker to determine the early differences in the outcome and severity of the disease. So, further studies are required to make a new biomarker before the differences observed in the phenotypical changes of AGA. There are various promising biomarkers involved in the pathophysiological mechanism underlying the damage and repair mechanism of AGA. There are no biomarkers for early detection and to predict the progression of severity along with long-term comorbidities for AGA, as there is no gold standard available.
In the Indian setup, there are no accreditations for conducting hair transplantation. So, validation of the whole protocol is questionable as non-trained people/clinical personals have been used for the complete protocol.
We need to understand the pathophysiology in more detail; so, genetic detection can be used, such as GWAS analysis of different populations to understand more about this disease.
Future prospectives
Recent developments/advancement in the field of OMICS (genomics, transcriptomics and proteomics), genome editing (CRISPR) and bioinformatics tools helps to understand the pathophysiology of AGA and also integrate with all available tools to help elucidate the mechanism of androgenic alopecia. So, we need the identification of a specific novel and reliable biomarkers for the progression of this condition.
Assessment of multiple biomarkers instead of a single biomarker in one platform will be a reasonable approach to detect and predict the progression of this condition. And at the same time, the existing marker should be affordable/inexpensive to the patients.
The treatment should be affordable to the patients, commonly available and specific, and the surgeries should be conducted by trained clinicians.
Therefore, the recent advancement in molecular techniques could enable us to develop high-throughput screening approaches with effective treatment modalities for the patients suffering from AGA.
Conclusion
Men and women both experience AGA, but hair loss is usually slightly more common in men. Blood investigations may be needed to evaluate other causes of hair loss, such as hormonal changes, iron levels (anaemia) or thyroid disorders. The psychosocial impact can be severe for people with hair loss, especially women, as there is less acceptance or less understanding of AGA. Providing false hope may do more psychological harm to the patients.
Multiple studies elucidate the pathogenesis, genetic susceptibility, the association of serious medical diseases and their relation with increased risk of CVD, which emphasizes the importance of medical screening in patients with AGA.
AR gene study, newer SNPs and other locus involvement in AGA are under research. A better understanding of the genetic factors can lead to newer therapies, including cell-based therapies. As effective therapeutic options are limited, AGA remains an area of expanding research, developing more information regarding pathogenesis and newer therapeutic options.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Blume-Peytavi U, editor. Hair growth and disorders. Berlin: Springer; 2008. p. 564. [Google Scholar]
- 2.Deng Y, Wang M, He Y, Liu F, Chen L, Xiong X. Cellular Senescence: Ageing and Androgenetic Alopecia. Dermatology. 2023;239:533–41. doi: 10.1159/000530681. [DOI] [PubMed] [Google Scholar]
- 3.Sehgal VN, Kak R, Aggarwal A, Srivastava G, Rajput P. Male pattern androgenetic alopecia in an Indian context:a perspective study. J Eur Acad Dermatol Venereol JEADV. 2007;21:473–9. doi: 10.1111/j.1468-3083.2006.01920.x. [DOI] [PubMed] [Google Scholar]
- 4.Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, et al. Androgenetic alopecia: A review. Endocrine. 2017;57:9–17. doi: 10.1007/s12020-017-1280-y. [DOI] [PubMed] [Google Scholar]
- 5.Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia:pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11. doi: 10.1017/S1462399402005112. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Chapoy D, Cruz-Arroyo FJ, Ancer-Leal FD, Rodriguez-Leal RA, Camacho-Zamora BD, Guzman-Sanchez DA, et al. Pilot study:genetic distribution of AR, FGF5, SULT1A1 and CYP3A5 polymorphisms in male Mexican population with androgenetic alopecia. Int J Mol Epidemiol Genet. 2022;13:32–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Tantawy M, Khabir A, Mahsoub N, Zohdy M. Serum paroxonase 1 level may be an indicator and predictor of the severity of androgenetic alopecia. Int J Trichology. 2021;13:26. doi: 10.4103/ijt.ijt_128_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma L, Dubey A, Gupta PR, Agrawal A. Androgenetic alopecia and risk of coronary artery disease. Indian Dermatol Online J. 2013;4:283–7. doi: 10.4103/2229-5178.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharam Kumar KC, Kishan Kumar YH, Neladimmanahally V. Association of Androgenetic Alopecia with Metabolic Syndrome: A Case-control Study on 100 Patients in a Tertiary Care Hospital in South India. Indian J Endocrinol Metab. 2018;22:196–9. doi: 10.4103/ijem.IJEM_650_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women:a comparative study. J Am Acad Dermatol. 2010;63:420–9. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Hirsso P, Laakso M, Matilainen V, Hiltunen L, Rajala U, Jokelainen J, et al. Association of insulin resistance linked diseases and hair loss in elderly men. Finnish population-based study. Cent Eur J Public Health. 2006;14:78–81. doi: 10.21101/cejph.b0045. [DOI] [PubMed] [Google Scholar]
- 12.González-González JG, Mancillas-Adame LG, Fernández-Reyes M, Gómez-Flores M, Lavalle-González FJ, Ocampo-Candiani J, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol (Oxf) 2009 Oct;71(4):494–9. doi: 10.1111/j.1365-2265.2008.03508.x. [DOI] [PubMed] [Google Scholar]
- 13.Agamia NF, Youssif TA, El-Hadidy A, El-Abd A. Benign prostatic hyperplasia, metabolic syndrome and androgenic alopecia: Is there a possible relationship? Arab J Urol. 2016;14:157–62. doi: 10.1016/j.aju.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen A. Comprehensive Review of Keloid Formation. J Clin Res Dermatol. 2017;4:1–18. [Google Scholar]
- 15.Zouboulis CC. The human skin as a hormone target and an endocrine gland. Horm Athens Greece. 2004;3:9–26. doi: 10.14310/horm.2002.11109. [DOI] [PubMed] [Google Scholar]
- 16.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116:793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 17.Inui S, Itami S. Androgen actions on the human hair follicle:perspectives. Exp Dermatol. 2013;22:168–71. doi: 10.1111/exd.12024. [DOI] [PubMed] [Google Scholar]
- 18.Randall VA. Role of 5 alpha-reductase in health and disease. Baillieres Clin Endocrinol Metab. 1994;8:405–31. doi: 10.1016/s0950-351x(05)80259-9. [DOI] [PubMed] [Google Scholar]
- 19.Gianfrilli D, Pierotti S, Pofi R, Leonardo C, Ciccariello M, Barbagallo F. Sex Steroid Metabolism in Benign and Malignant Intact Prostate Biopsies: Individual Profiling of Prostate Intracrinology. BioMed Res Int. 2014;2014:1–8. doi: 10.1155/2014/464869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPhaul MJ. Androgen receptor mutations and androgen insensitivity. Mol Cell Endocrinol. 2002;198:61–7. doi: 10.1016/s0303-7207(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 21.Ho BSY, Vaz C, Ramasamy S, Chew EGY, Mohamed JS, Jaffar H, et al. Progressive expression of PPARGC1? is associated with hair miniaturization in androgenetic alopecia. Sci Rep. 2019;9:8771. doi: 10.1038/s41598-019-43998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Z, Chen M, Liu F, Wang Y, Xu S, Sha K, et al. Androgen Receptor-Mediated Paracrine Signaling Induces Regression of Blood Vessels in the Dermal Papilla in Androgenetic Alopecia. J Invest Dermatol. 2022;142:2088–2099.e9. doi: 10.1016/j.jid.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Price VH. Androgenetic alopecia in women. J Investig Dermatol Symp Proc. 2003;8:24–7. doi: 10.1046/j.1523-1747.2003.12168.x. [DOI] [PubMed] [Google Scholar]
- 24.Roohaninasab M, Yavari SF, Babazadeh M, Hagh RA, Pazoki M, Amrovani M. Evaluating the Role of lncRNAs in the Incidence of Cardiovascular Diseases in Androgenetic Alopecia Patients. Cardiovasc Toxicol. 2022;22:603–19. doi: 10.1007/s12012-022-09742-w. [DOI] [PubMed] [Google Scholar]
- 25.Kaliyadan F, Nambiar A, Vijayaraghavan S. Androgenetic alopecia: An update. Indian J Dermatol Venereol Leprol. 2013;79:613–25. doi: 10.4103/0378-6323.116730. [DOI] [PubMed] [Google Scholar]
- 26.Endo C, Johnson TA, Morino R, Nakazono K, Kamitsuji S, Akita M, et al. Genome-wide association study in Japanese females identifies fifteen novel skin-related trait associations. Sci Rep. 2018;8:8974. doi: 10.1038/s41598-018-27145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcińska M, Pośpiech E, Abidi S, Andersen JD, Van Den Berge M, Carracedo Á, et al. Evaluation of DNA Variants Associated with Androgenetic Alopecia and Their Potential to Predict Male Pattern Baldness. Prokunina-Olsson L, editor. PLOS ONE. 2015;10:e0127852. doi: 10.1371/journal.pone.0127852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones. II. Androstenedione metabolism in isolated hairs. J Clin Endocrinol Metab. 1974;39:1012–9. doi: 10.1210/jcem-39-6-1012. [DOI] [PubMed] [Google Scholar]
- 29.Heilmann-Heimbach S, Hochfeld LM, Henne SK, Nöthen MM. Hormonal regulation in male androgenetic alopecia—Sex hormones and beyond: Evidence from recent genetic studies. Exp Dermatol. 2020;29:814–27. doi: 10.1111/exd.14130. [DOI] [PubMed] [Google Scholar]
- 30.McCoy J, Wambier CG, Herrera S, Vaño-Galván S, Gioia F, Comeche B, et al. Androgen receptor genetic variant predicts COVID-19 disease severity:a prospective longitudinal study of hospitalized COVID-19 male patients. [Last accessed on 2024 Jun 12];J Eur Acad Dermatol Venereol [Internet. 2021 35 doi: 10.1111/jdv.16956. Available from:https://onlinelibrary.wiley.com/doi/10.1111/jdv.16956 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orii N, Ganapathiraju MK. Wiki-pi: A web-server of annotated human protein-protein interactions to aid in discovery of protein function. PloS One. 2012;7:e49029. doi: 10.1371/journal.pone.0049029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi A, Anzalone A, Fortuna MC, Caro G, Garelli V, Pranteda G, et al. Multi-therapies in androgenetic alopecia:review and clinical experiences: An Update and Future Potential Treatments. Dermatol Ther. 2016;29:424–32. doi: 10.1111/dth.12390. [DOI] [PubMed] [Google Scholar]
- 33.Hagenaars SP, Hill WD, Harris SE, Ritchie SJ, Davies G, Liewald DC, et al. Genetic prediction of male pattern baldness. PLoS Genet. 2017;13:e1006594. doi: 10.1371/journal.pgen.1006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heilmann S, Kiefer AK, Fricker N, Drichel D, Hillmer AM, Herold C, et al. Androgenetic Alopecia: Identification of Four Genetic Risk Loci and Evidence for the Contribution of WNT Signaling to Its Etiology. J Invest Dermatol. 2013;133:1489–96. doi: 10.1038/jid.2013.43. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Tang Y, Huang Y, Wang J, Yang K, Zhang Y, et al. Insights into male androgenetic alopecia using comparative transcriptome profiling:hypoxia-inducible factor-1 and Wnt/?-catenin signalling pathways. Br J Dermatol. 2022;187:936–47. doi: 10.1111/bjd.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rui W, Sheng Y, Hu R, Miao Y, Han Y, Guo X, et al. Association of Single Nucleotide Polymorphisms in the CYP19A1 Gene with Female Pattern Hair Loss in a Chinese Population. Dermatology. 2015;231:239–44. doi: 10.1159/000433597. [DOI] [PubMed] [Google Scholar]
- 37.Katzer T, Leite Junior A, Beck R, Da Silva C. Physiopathology and current treatments of androgenetic alopecia: Going beyond androgens and anti-androgens. [Last accessed on 2024 Jun 12];Dermatol Ther [Internet. 2019 32 doi: 10.1111/dth.13059. Available from:https://onlinelibrary.wiley.com/doi/10.1111/dth.13059 . [DOI] [PubMed] [Google Scholar]
- 38.Jung YH, Chae CW, Choi GE, Shin HC, Lim JR, Chang HS, et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J Biomed Sci. 2022;29:17. doi: 10.1186/s12929-022-00800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


