Abstract
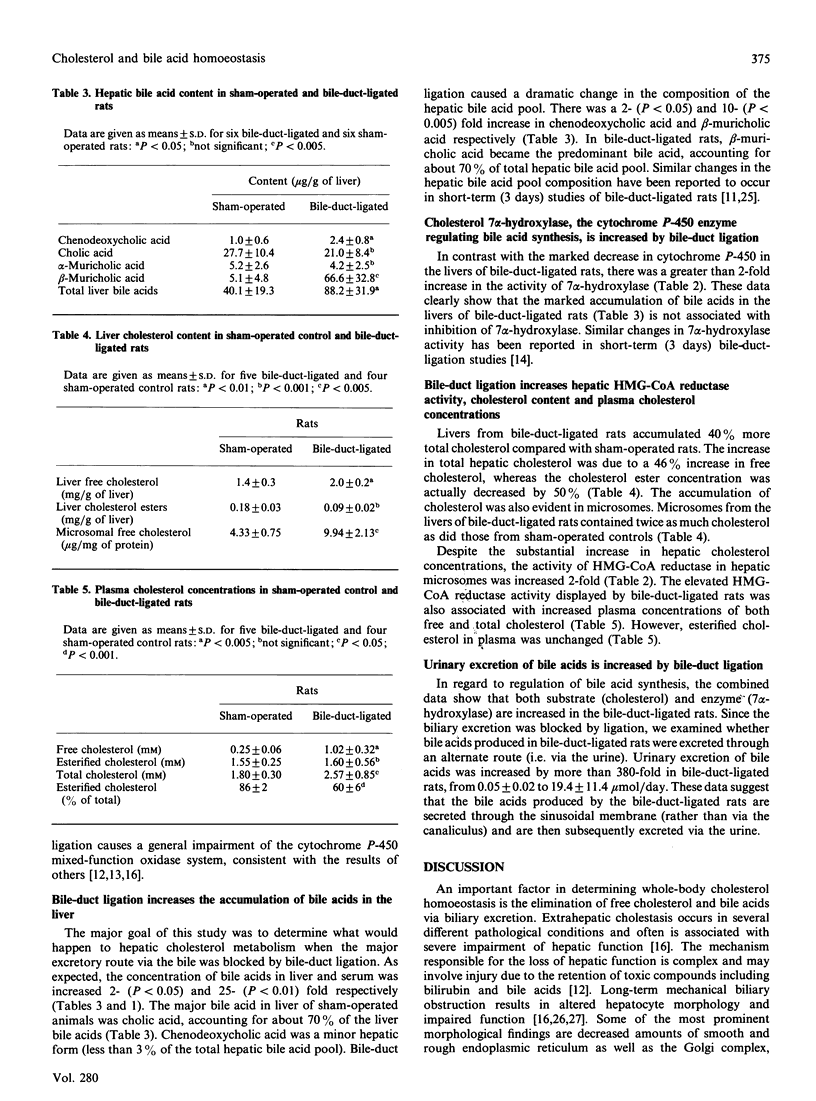
We examined how total blockage of biliary excretion, the major pathway through which cholesterol and bile acids are removed from the body, affects liver function, cholesterol and bile acid metabolism and homoeostasis. After 4 weeks of bile-duct ligation, rats showed impaired liver function, as documented by elevations in serum bilirubin and alkaline phosphatase activity. Moreover, bile-duct ligation decreased by about 30% both the amount of microsomal cytochrome P-450 in the liver and the elimination of aminopyrine in vivo, a reliable index in vivo of microsomal mixed-function oxidase activity. Cholesterol and bile acid contents in livers of bile-duct-ligated rats were doubled compared with sham-operated controls. Despite the increase in the contents of cholesterol and bile acids in liver, activities of the respective rate-limiting enzymes, 3-hydroxy-3-methylglutaryl-CoA reductase and cholesterol 7 alpha-hydroxylase, were doubled. Serum concentrations of bile acids and free cholesterol increased 25- and 4-fold respectively. The large increase in serum bile acids was associated with a 380-fold increase in the urinary excretion of bile acids. Although there is a general decrease in cytochrome P-450 content and drug metabolism involving cytochrome P-450-containing hydroxylases, the activity of cholesterol 7 alpha-hydroxylase, also a cytochrome P-450-containing enzyme, is actually increased. These data show that complete obstruction of the bile duct results in the selective impairment of microsomal cytochrome P-450. Increased activity of 7 alpha-hydroxylase, bile acid synthesis and urinary excretion provides an alternative excretory pathway that helps to maintain cholesterol homoeostasis when the biliary excretory pathway is eliminated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J., Tephly T. R. Effect of 3-amino-1,2,4-triazole on the stimulation of hepatic microsomal heme synthesis and induction of hepatic microsomal oxidases produced by phenobarbital. Mol Pharmacol. 1969 Jan;5(1):10–20. [PubMed] [Google Scholar]
- Cohen B. I., Raicht R. F., Mosbach E. H. Sterol metabolism studies in the rat. Effects of primary bile acids (sodium taurochenodeoxycholate and sodium taurocholate) on sterol metabolism. J Lipid Res. 1977 Mar;18(2):223–231. [PubMed] [Google Scholar]
- Cooper A. D., Jones A. L., Koldinger R. E., Ockner R. K. Selective biliary obstruction: a model for the study of lipid metabolism in cholestasis. Gastroenterology. 1974 Apr;66(4):574–585. [PubMed] [Google Scholar]
- Cooper A. D., Ockner R. K. Studies of hepatic cholesterol synthesis in experimental acute biliary obstruction. Gastroenterology. 1974 Apr;66(4):586–595. [PubMed] [Google Scholar]
- Danielsson H. Effect of biliary obstruction on formation and metabolism of bile acids in rat. Steroids. 1973 Oct;22(4):567–579. doi: 10.1016/0039-128x(73)90013-5. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Highsmith W. E., McNeal M. M., Schexnayder J. A., Kuan J. C. Bile acid synthesis by cultured hepatocytes. Inhibition by mevinolin, but not by bile acids. J Biol Chem. 1983 Apr 10;258(7):4079–4082. [PubMed] [Google Scholar]
- Davis R. A., Kern F., Jr, Showalter R., Sutherland E., Sinensky M., Simon F. R. Alterations of hepatic Na+,K+-atpase and bile flow by estrogen: effects on liver surface membrane lipid structure and function. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4130–4134. doi: 10.1073/pnas.75.9.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Musso C. A., Malone-McNeal M., Lattier G. R., Hyde P. M., Archambault-Schexnayder J., Straka M. Examination of bile acid negative feedback regulation in rats. J Lipid Res. 1988 Feb;29(2):202–211. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- Erickson S. K., Jaeckle S., Lear S. R., Brady S. M., Havel R. J. Regulation of hepatic cholesterol and lipoprotein metabolism in ethinyl estradiol-treated rats. J Lipid Res. 1989 Nov;30(11):1763–1771. [PubMed] [Google Scholar]
- Everson G. T., Polokoff M. A. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986 Feb 15;261(5):2197–2201. [PubMed] [Google Scholar]
- Galeazzi R., Javitt N. B. Bile acid excretion: the alternate pathway in the hamster. J Clin Invest. 1977 Sep;60(3):693–701. doi: 10.1172/JCI108821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greim H., Trülzsch D., Roboz J., Dressler K., Czygan P., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972 Nov;63(5):837–845. [PubMed] [Google Scholar]
- Gross J. B., Jr, Reichen J., Zeltner T. B., Zimmermann A. The evolution of changes in quantitative liver function tests in a rat model of biliary cirrhosis: correlation with morphometric measurement of hepatocyte mass. Hepatology. 1987 May-Jun;7(3):457–463. doi: 10.1002/hep.1840070308. [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Sexton R. C., Rudney H. Differential regulation of low density lipoprotein suppression of HMG-CoA reductase activity in cultured cells by inhibitors of cholesterol biosynthesis. J Lipid Res. 1990 Feb;31(2):203–215. [PubMed] [Google Scholar]
- Gupta A., Sexton R. C., Rudney H. Modulation of regulatory oxysterol formation and low density lipoprotein suppression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity by ketoconazole. A role for cytochrome P-450 in the regulation of HMG-CoA reductase in rat intestinal epithelial cells. J Biol Chem. 1986 Jun 25;261(18):8348–8356. [PubMed] [Google Scholar]
- HURLOCK B., TALALAY P. Principles of the enzymatic measurement of steroids. J Biol Chem. 1957 Jul;227(1):37–52. [PubMed] [Google Scholar]
- Harry D. S., Dini M., McIntyre N. Effect of cholesterol feeding and biliary obstruction on hepatic cholesterol biosynthesis in the rat. Biochim Biophys Acta. 1973 Jan 19;296(1):209–220. doi: 10.1016/0005-2760(73)90061-1. [DOI] [PubMed] [Google Scholar]
- Heuman D. M., Vlahcevic Z. R., Bailey M. L., Hylemon P. B. Regulation of bile acid synthesis. II. Effect of bile acid feeding on enzymes regulating hepatic cholesterol and bile acid synthesis in the rat. Hepatology. 1988 Jul-Aug;8(4):892–897. doi: 10.1002/hep.1840080431. [DOI] [PubMed] [Google Scholar]
- Hutterer F., Denk H., Bacchin P. G., Sckenkman J. B., Schaffner F., Popper H. Mechanism of cholestasis. 1. Effect of bile acids on microsomal cytochrome P-450 dependent biotransformation system in vitro. Life Sci II. 1970 Aug 8;9(15):877–887. doi: 10.1016/0024-3205(70)90057-3. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Andersson S., Slaughter C. A., Russell D. W. Cloning and regulation of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem. 1990 May 15;265(14):8190–8197. [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Mooney J. S., Adler R. D., Ockner R. K. Morphometric analysis of rat hepatocytes after total billary obstruction. Gastroenterology. 1976 Dec;71(6):1050–1060. [PubMed] [Google Scholar]
- Junker L. H., Davis R. A. Receptor-mediated uptake of low density lipoprotein stimulates bile acid synthesis by cultured rat hepatocytes. J Lipid Res. 1989 Dec;30(12):1933–1941. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- Krähenbühl S., Reichen J. Canalicular bile flow and bile salt secretion are maintained in rats with liver cirrhosis. Further evidence for the intact cell hypothesis. J Hepatol. 1988 Aug;7(1):63–71. doi: 10.1016/s0168-8278(88)80507-5. [DOI] [PubMed] [Google Scholar]
- Kubaska W. M., Gurley E. C., Hylemon P. B., Guzelian P. S., Vlahcevic Z. R. Absence of negative feedback control of bile acid biosynthesis in cultured rat hepatocytes. J Biol Chem. 1985 Nov 5;260(25):13459–13463. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauterburg B. H., Bircher J. Expiratory measurement of maximal amino-pyrine demethylation in vivo: effect of phenobarbital, partial hepatectomy, protacaval shunt and bile duct ligation in the rat. J Pharmacol Exp Ther. 1976 Feb;196(2):501–509. [PubMed] [Google Scholar]
- Mackinnon A. M., Drevon C. A., Sand T. M., Davis R. A. Regulation of bile acid synthesis in cultured rat hepatocytes: stimulation by apoE-rich high density lipoproteins. J Lipid Res. 1987 Jul;28(7):847–855. [PubMed] [Google Scholar]
- Mackinnon A. M., Simon F. R. Reduced synthesis of hepatic microsomal cytochroma P450 in the bile duct ligated rat. Biochem Biophys Res Commun. 1974 Jan 23;56(2):437–443. doi: 10.1016/0006-291x(74)90861-4. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Myant N. B. The effect of interruption of the enterophecatic circulation of bile acids and of cholesterol feeding on cholesterol 7 alpha-hydroxylase in relation to the diurnal rhythm in its activity. Biochim Biophys Acta. 1973 Dec 20;326(3):428–438. doi: 10.1016/0005-2760(73)90143-4. [DOI] [PubMed] [Google Scholar]
- Myant N. B., Mitropoulos K. A. Cholesterol 7 alpha-hydroxylase. J Lipid Res. 1977 Mar;18(2):135–153. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Panini S. R., Sexton R. C., Rudney H. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by oxysterol by-products of cholesterol biosynthesis. Possible mediators of low density lipoprotein action. J Biol Chem. 1984 Jun 25;259(12):7767–7771. [PubMed] [Google Scholar]
- Phillips M. J., Poucell S., Oda M. Mechanisms of cholestasis. Lab Invest. 1986 Jun;54(6):593–608. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., JAYKO M. E., CHAIKOFF I. L., DAUBEN W. G. Nature of the metabolic products of C14-cholesterol excreted in bile and feces. Proc Soc Exp Biol Med. 1952 Dec;81(3):720–724. doi: 10.3181/00379727-81-19999. [DOI] [PubMed] [Google Scholar]
- STRAND O. EFFECTS OF D- AND L-TRIIODOTHYRONINE AND OF PROPYLTHIOURACIL ON THE PRODUCTION OF BILE ACIDS IN THE RAT. J Lipid Res. 1963 Jul;4:305–311. [PubMed] [Google Scholar]
- Schwartz C. C., Halloran L. G., Vlahcevic Z. R., Gregory D. H., Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978 Apr 7;200(4337):62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- Shefer S., Nguyen L., Salen G., Batta A. K., Brooker D., Zaki F. G., Rani I., Tint G. S. Feedback regulation of bile-acid synthesis in the rat. Differing effects of taurocholate and tauroursocholate. J Clin Invest. 1990 Apr;85(4):1191–1198. doi: 10.1172/JCI114552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka M. S., Junker L. H., Zacarro L., Zogg D. L., Dueland S., Everson G. T., Davis R. A. Substrate stimulation of 7 alpha-hydroxylase, an enzyme located in the cholesterol-poor endoplasmic reticulum. J Biol Chem. 1990 May 5;265(13):7145–7149. [PubMed] [Google Scholar]
- Takita M., Ikawa S., Ogura Y. Effect of bile duct ligation on bile acid and cholesterol metabolism in rats. J Biochem. 1988 May;103(5):778–786. doi: 10.1093/oxfordjournals.jbchem.a122346. [DOI] [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Presence of an intact cholesterol feedback mechanism in the liver in biliary stasis. Gastroenterology. 1971 Jul;61(1):77–84. [PubMed] [Google Scholar]


