Abstract
Background.
In 2014, a nationwide outbreak of severe respiratory illness occurred in the United States, primarily associated with enterovirus D68 (EV-D68). A proportion of illness was associated with rhinoviruses (RVs) and other enteroviruses (EVs), which we aimed to characterize further.
Methods.
Respiratory specimens from pediatric and adult patients with respiratory illness were submitted to the Centers for Disease Control and Prevention during August 2014–November 2014. While initial laboratory testing focused on identification of EV-D68, the negative specimens were typed by molecular sequencing to identify additional EV and RV types. Testing for other pathogens was not conducted. We compared available clinical and epidemiologic characteristics among patients with EV-D68 and RV species A–C identified.
Results.
Among 2629 typed specimens, 1012 were EV-D68 (39%) and 81 (3.1%) represented 24 other EV types; 968 were RVs (37%) covering 114 types and grouped into 3 human RV species (RV-A, 446; RV-B, 133; RV-C, 389); and 568 (22%) had no RV or EV detected. EV-D68 was more frequently identified in patients who presented earlier in the investigation period. Among patients with EV-D68, RV-A, RV-B, or RV-C, the age distributions markedly differed. Clinical syndromes and intensive care unit admissions by age were largely similar.
Conclusions.
RVs were commonly associated with severe respiratory illness during a nationwide outbreak of EV-D68, and most clinical. Characteristics were similar between groups. A better understanding of the epidemiology of RVs and EVs is needed to help inform development and use of diagnostic tests, therapeutics, and preventive measures.
Keywords: respiratory disease, outbreak, rhinovirus, enterovirus, epidemiology
The Enterovirus genus is currently composed of 13 species, including 4 enterovirus (EV) species that are found in humans (EV A–D), and 3 human rhinovirus (RV) species (RV A–C). These 7 EV and RV species include 283 distinct types. These viruses are ubiquitous in humans, and infections are frequently mild or asymptomatic [1].
EV infections in humans can exhibit a wide range of clinical manifestations. EVs typically cause illnesses that involve the gastrointestinal tract but can also affect the central nervous, cardiac, hepatic, and, less typically, respiratory systems. EV-D68 differs from most other EV types in that it is primarily associated with respiratory illness and, unlike all other EV A–D, is biologically similar to RVs [2, 3]. RV infections in humans are primarily limited to the respiratory tract. Although frequently detected in asymptomatic individuals, RVs are a common cause of acute upper respiratory infections (eg, common cold, acute otitis media, rhinosinusitis) and have also been associated with lower respiratory infections (eg, bronchiolitis, community-acquired pneumonia) and exacerbations of chronic pulmonary diseases (eg, asthma, chronic obstructive pulmonary disease, cystic fibrosis) [1].
Commercial qualitative multiplex molecular assays currently available for EV/RV diagnosis using respiratory specimens in clinical laboratories do not reliably distinguish between the species of EVs and RVs, and sequencing all or selected regions of the Enterovirus capsid is necessary to determine the specific types. The epidemiology and clinical features of respiratory infections associated with specific EVs and RVs have not been fully described, in part due to the complexity of EV/RV differentiation in the clinical setting.
During August 2014–November 2014, an unexpected increase in severe respiratory illness occurred in the United States [4]. As part of the emergency public health response, the Centers for Disease Control and Prevention (CDC) accepted clinical specimens from across the country for EV/RV detection and typing [5]; most had already tested EV/RV-positive in clinical laboratories or in state/local public health department laboratories. CDC initially prioritized specimens from severely ill patients but subsequently encouraged submission of specimens from states that had not yet reported an EV/RV-positive specimen and from new patient populations and settings such as adults in long-term care facilities. While EV-D68 was the predominant pathogen detected, a notable proportion of specimens were positive for RVs or other EVs [5].
In this investigation, we aimed to further characterize EV-D68–negative specimens through additional testing to identify other EV and RV types. Additionally, we aimed to better understand the contribution of RV infection to this outbreak and to compare the epidemiologic and clinical characteristics of patients infected with EV-D68 and RV-A, -B, and -C.
METHODS
Virologic Analysis
At CDC, upper and lower respiratory tract clinical specimens underwent RNA extraction (QIAamp Viral RNA Mini Kit spin protocol, Qiagen, Inc., Valencia, California) and screening with a panEV real-time reverse-transcription polymerase chain reaction (RT-PCR) assay designed to detect EV species A–D [6]. However, as with all panEV A–D assays that target the 5ʹ nontranslated region (5ʹNTR), this 5ʹNTR panEV test also detects an undefined subset of RV A–C. Specimens with positive EV/RV detections then had typing attempted using 1 of the following 2 methods: an EV-D68–specific real-time RT-PCR assay [7] that was developed in September during the outbreak and used on specimens received in mid-September forward or, prior to introduction of the EV-D68 real-time assay, an additional panEV RT-PCR assay that targets the amino terminal portion of the viral protein 1 (VP1) region. This VP1 panEV test was designed to amplify EV species A–J [8]; however, it also amplifies an undefined subset of RV A–C. The RT-PCR amplicon from the assay was Sanger sequenced; bioinformatic analyses of the sequences were used to determine EV or RV type [8].
Patient specimens that were 5ʹNTR panEV/RV negative or 5ʹNTR panEV/RV positive but VP1 panEV negative were considered to be possible RV A–C and subjected to the following 2 additional assays: a real-time panRV RT-PCR assay designed to detect RV species A–C [9, 10] (this assay cross-reacts with an undefined subset of high tittered EV A–D [5 × 105 copies and above per reaction] and, for panRV-positive specimens, Sanger sequencing of the RV/EV viral protein 4/viral protein 2 (VP4/VP2) region for panRV-positive specimens; bioinformatic analyses of the sequences were used to determine RV or EV type [11].
Epidemiologic Analysis
As previously described, we requested completion of a brief case report form for collection of basic clinical and epidemiologic data for all specimens received [12]. To compare the clinical and epidemiologic characteristics of severely ill patients with respiratory conditions with RV species identified to those with EV-D68, we limited our analysis to hospitalized patients infected with EV-D68 or RV-A, -B, and -C with an onset of illness or specimen collection date from 1 August 2014 to 30 November 2014. For patients whose date of hospital admission was reported, we excluded those with specimen collection dates that were more than 7 days before or after admission. When the specimen collection date was unknown, we applied the same exclusion rule but used illness onset date as a proxy for specimen collection date. A patient was considered to have had fever if indicated on the data collection form or if temperature was reported as >100.4°F. We included a single laboratory result per patient. In addition, we excluded data from patients who had specimens with multiple EV and/or RV detected at CDC and any specimen noted by the submitting laboratories to have had any other pathogen detected.
Statistical Analyses
Clinical and epidemiologic characteristics of patients with EV-D68, RV-A, RV-B, and RV-C were compared. Markers of clinical severity included admission to an intensive care unit (ICU), the need for ventilator support (ie, noninvasive ventilation, defined as continuous positive airway pressure and bilevel positive airway pressure or intubation), or extracorporeal membrane oxygenation. To account for potential age-related differences in response to infection, such as fever, and in underlying conditions, such as prematurity, as well as the lower likelihood of asthma or reactive airway disease (asthma/RAD) diagnosis among children, we also assessed clinical factors stratified by age groups of <5 years and ≥5 years. χ2 tests and P values were calculated along with Wilcoxon rank sum tests for comparison of the mean number of days to hospitalization. In addition, due to the nonnormal distribution of age in this population, a Kruskal-Wallis test was used to assess age distribution differences between the virus groups. Due to the multiple comparisons conducted, the significance of apparent differences should be interpreted with caution. Therefore, we note comparisons with P values of both <.05 and <.01 in Tables 1 and 2. We conducted descriptive and statistical analyses using SAS 9.3 (SAS Institute, Cary, North Carolina).
Table 1.
Demographic and Clinical Description of Hospitalized Patients Aged <5 Years With Enterovirus D68 and Rhinovirus Species A, B, and C Identified (N = 395) 1 August 2014–30 November 2014
| Characteristic | Enterovirus D68 (n = 199) |
RV-A (n = 57) |
RV-B (n = 18) |
RV-C (n = 121) |
Comparisona (P Value) |
|---|---|---|---|---|---|
| Gender—male, n(%) | 114(57.3) | 32(56.1) | 12(66.7) | 75(62.0) | |
| Symptom, n(%) | |||||
| Cough | 153(76.9) | 34(59.6) | 7(38.9) | 91(75.2) | A, B, E, F |
| Shortness of breath | 151(75.9) | 35(61.4) | 10(55.6) | 81(66.9) | A |
| Wheezing | 124(62.3) | 22(38.6) | 5(27.8) | 69(57.0) | A, B, E, F |
| Retractions | 99(49.7) | 12(21.1) | 6(33.3) | 47(38.8) | A, E |
| Tachypnea | 94(47.2) | 17(29.8) | 6(33.3) | 48(39.7) | A |
| Fever | 92(46.2) | 25(43.9) | 5(27.8) | 57(47.1) | |
| Runny nose | 77(38.7) | 21(36.8) | 5(27.8) | 45(37.2) | |
| Vomiting | 45(22.6) | 11(19.3) | 4(22.2) | 16(13.2) | C |
| Lethargy | 13(6.5) | 11(19.3) | 5(27.8) | 11(9.1) | A, B, F |
| Cyanosis | 11(5.5) | 4(7.0) | 5(27.8) | 6(5.0) | B, D, F |
| Sore throat | 9(4.5) | 2(3.5) | 0 | 1(0.8) | |
| Comorbidity, n(%) | |||||
| History of asthma or reactive airway disease | 67(33.7) | 14(24.6) | 2(11.1) | 37(30.6) | B |
| Cardiac disease | 8(4.0) | 4(7.0) | 2(11.1) | 8(6.6) | |
| Immunocompromised | 3(1.5) | 1(1.8) | 0 | 0 | |
| Prematurity | 15(7.5) | 10(17.5) | 1(5.6) | 15(12.4) | A |
| Clinical course, n(%) | |||||
| Number of days to hospitalization, meanb | 2.2 | 2.5 | 1.9 | 2.4 | |
| Abnormal chest X ray or computed tomography | 85(42.7) | 21(36.8) | 5(27.8) | 38(31.4) | C |
| Ventilation supportc | 32(16.1) | 19(33.3) | 4(22.2) | 28(23.1) | A |
| Intubated | 7(3.5) | 12(21.1) | 3(16.7) | 15(12.4) | A, B, C, E |
| Admitted to intensive care unit | 102(51.3) | 29(50.9) | 11(61.1) | 58(47.9) | |
| Extracorporeal membrane oxygenation | 2(1.0) | 0 | 1(5.6) | 0 | F |
| Death | 2(1.0) | 2(3.5) | 0 | 3(2.5) | A |
Abbreviation: RV, rhinovirus.
χ2 comparisons with P values < .05 are indicated with letters A–F as follows: A (enterovirus [EV] D68 vs RV-A), B (EV-D68 vs RV-B), C (EV-D68 vs RV-C), D (RV-A vs RV-B), E (RV-A vs RV-C), F (RV-B vs RV-C). The same letters in bold indicate P values < .01. Comparisons of P values for the mean number of days to hospitalization used Wilcoxon rank sum tests.
Difference between date of hospitalization and symptom onset date. Due to missing values, denominators for the 4 groups are 169, 45, 12, and 97, respectively.
Ventilation support means noninvasive ventilation, including continuous positive airway pressure and bilevel positive airway pressure or intubation.
Table 2.
Demographic and Clinical Description of Hospitalized Patients Aged ≥5 Years With Enterovirus D68 and Rhinovirus Species A, B, and C Identified (N = 489) 1 August 2014–30 November 2014
| Characteristic | Enterovirus D68 (n = 312) |
RV-A (n = 90) |
RV-B (n = 27) |
RV-C (n = 60) |
Comparisona (P Value) |
|---|---|---|---|---|---|
| Gender—male, n(%) | 167(53.5) | 48(53.3) | 12(44.4) | 31(51.7) | |
| Symptom, n(%) | |||||
| Cough | 258(82.7) | 66(73.3) | 19(70.4) | 49(81.7) | A |
| Shortness of breath | 261(83.7) | 77(85.6) | 23(85.2) | 50(83.3) | |
| Wheezing | 205(65.7) | 36(40.0) | 13(48.1) | 33(55.0) | A |
| Retractions | 116(37.2) | 12(13.3) | 3(11.1) | 14(23.3) | A, B, C |
| Tachypnea | 152(48.7) | 24(26.7) | 11(40.7) | 23(38.3) | A |
| Fever | 137(43.9) | 41(45.6) | 6(22.2) | 19(31.7) | B, D |
| Runny nose | 103(33.0) | 27(30.0) | 2(7.4) | 16(26.7) | B, D, F |
| Vomiting | 62(19.9) | 12(13.3) | 2(7.4) | 5(8.3) | C |
| Lethargy | 32(10.3) | 9(10.0) | 7(25.9) | 5(8.3) | B, D, F |
| Cyanosis | 6(1.9) | 3(3.3) | 1(3.7) | 6(10.0) | C |
| Sore throat | 60(19.2) | 18(20.0) | 1(3.7) | 5(8.3) | B, C, D |
| Diarrhea | 10(3.2) | 10(11.1) | 3(11.1) | 1(1.7) | A, B, E |
| Chills | 11(3.5) | 9(10.0) | 6(22.2) | 2(3.3) | A, B, F |
| Comorbidity, n(%) | |||||
| History of asthma or reactive airway disease | 190(60.9) | 45(50.0) | 10(37.0) | 33(55.0) | B |
| Cardiac disease | 4(1.3) | 11(12.2) | 2(7.4) | 2(3.3) | A, B |
| Immunocompromised | 5(1.6) | 7(7.8) | 2(7.4) | 1(1.7) | A, B |
| Clinical course, n(%) | |||||
| Number of days to hospitalization, meanb | 2.1 | 3.4 | 4.6 | 2.1 | A, B, F |
| Abnormal chest X ray or computed tomography | 136(43.6) | 44(48.9) | 15(55.6) | 27(45.0) | |
| Ventilation supportc | 85(27.2) | 30(33.3) | 11(40.7) | 18(30.0) | |
| Intubated | 21(6.7) | 18(20.0) | 7(25.9) | 8(13.3) | A, B |
| Admitted to intensive care unit | 196(62.8) | 45(50.0) | 15(55.6) | 33(55.0) | A |
| Extracorporeal membrane oxygenation | 2(0.6) | 1(1.1) | 0 | 0 | |
| Death | 1(0.3) | 3(3.3) | 0 | 2(3.3) | A, C |
Abbreviation: RV, rhinovirus.
χ2 comparisons with P values < .05 are indicated with letters A–F as follows: A (enterovirus [EV] D68 vs RV-A), B (EV-D68 vs RV-B), C (EV-D68 vs RV-C), D (RV-A vs RV-B), E (RV-A vs RV-C), F (RV-B vs RV-C). The same letters in bold indicate P values < .01. Comparisons of P values for the mean number of days to hospitalization used Wilcoxon rank sum tests.
Difference between date of hospitalization and symptom onset date. Due to missing values, denominators for the 4 groups are 270, 76, 24, and 48, respectively.
Ventilation support means noninvasive ventilation, including continuous positive airway pressure and bilevel positive airway pressure or intubation.
CDC determined these activities to be public health response nonresearch, thus investigational review board review was not required.
RESULTS
During the investigation period, specimens were submitted from the District of Columbia and 47 states, and 2629 patients (inpatient and outpatient) had a specimen that underwent EV/RV testing and typing at CDC. EV-D68 was identified in specimens from 46 jurisdictions and RV in all 48 jurisdictions. We identified 25 distinct EV types and 114 RV types (Supplementary Figures S1 and S2). Although most specimens were nasopharyngeal swabs (83%), other upper and lower respiratory specimens (sputum and a variety of washes and aspirates) were also included. Among the 2629 patients, EV-D68 was identified in 1012 (38.5%) and was the predominant type among all EV or RV types during the investigation period. Additionally, 81 (3.1%) patients had another EV type, representing 24 additional EV types (Figure 1). The 3 most commonly identified types among these 81 were coxsackievirus (CV) B3 (n = 15, 18.5%), CV-A6 (n = 12, 14.8%), and echovirus (E) 18 (n = 6, 7.4%).
Figure 1.
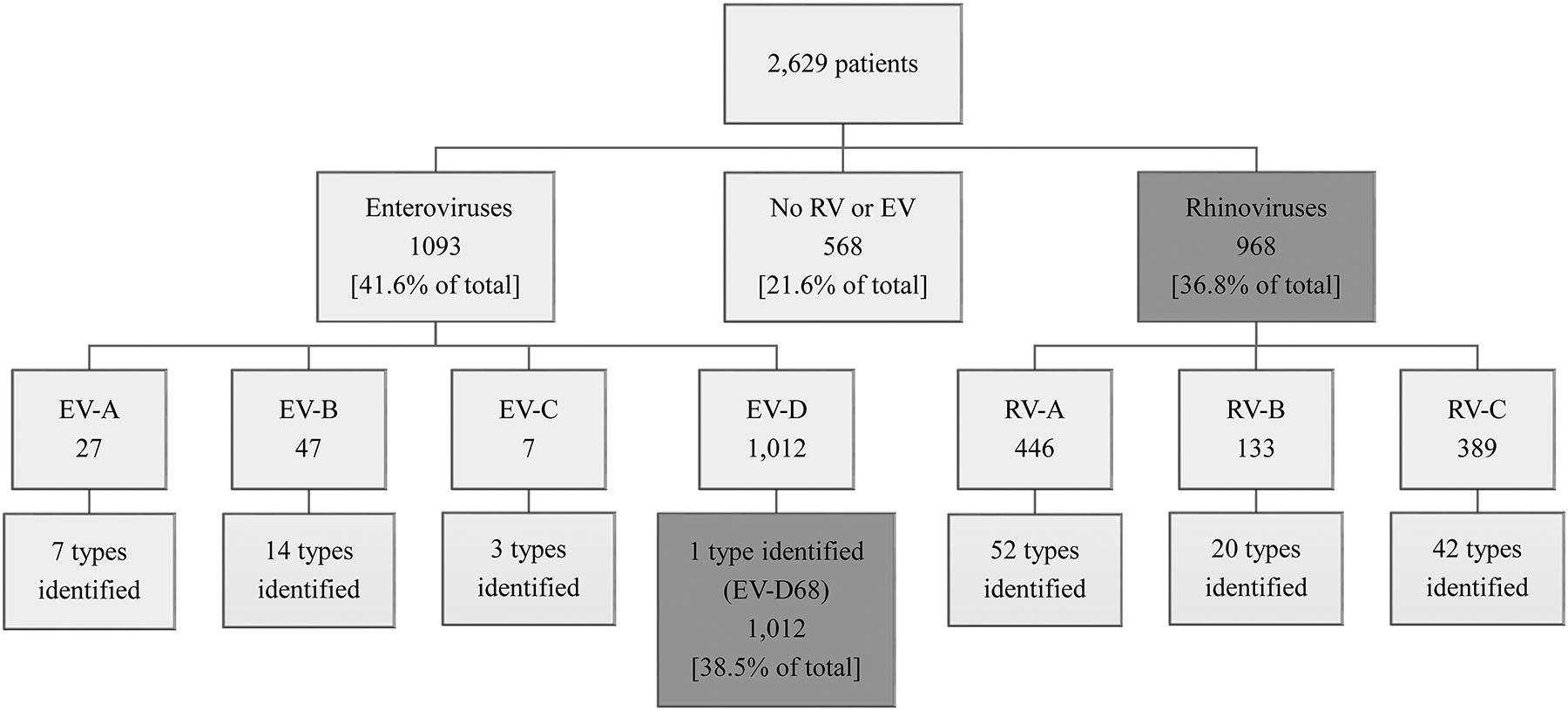
Enteroviruses and rhinoviruses identified among patients with respiratory specimens submitted to the Centers for Disease Control and Prevention for testing, August 2014–November 2014. Specimens with codetections were excluded. Abbreviations: EV, enterovirus; RV, rhinovirus.
RVs were identified in 968 (36.8%) specimens: RV-A (n = 446), RV-B (n = 133), and RV-C (n = 389); among these, we found 114 RV types. The 6 most common types were RV-A101 (n = 63, 6.5%), RV-C40 (n = 55, 5.7%), RV-C18 (n = 40, 4.1%), RV-C6 (n = 40, 4.1%), RV-A29 (n = 36, 3.7%), and RV-C43 (n = 36, 3.7%). The nucleotide sequences for RV and EV types identified by VP4/VP2 or VP1 sequencing have been deposited in the GenBank nonredundant nucleotide sequence database, accession numbers MF159576-MF160250 and MF160251-MF161068. No RV or EV was detected for 568 (21.6%) specimens (Figure 1).
The proportion of samples positive for EV-D68 and RV A–C among specimens tested, by week (when more than 20 specimens were tested per week), is shown in Figure 2. The number of specimens tested peaked during September. Early in the investigation period, the number of EV-D68 positives was 10%–20% higher than the number for all 3 RV species combined. As the outbreak progressed, the proportion of specimens that were EV-D68 positive decreased, while the proportion of RV A–C specimens increased. After the week ending 20 September 2014, RV was identified more frequently than EV-D68 for the remainder of the outbreak period.
Figure 2.
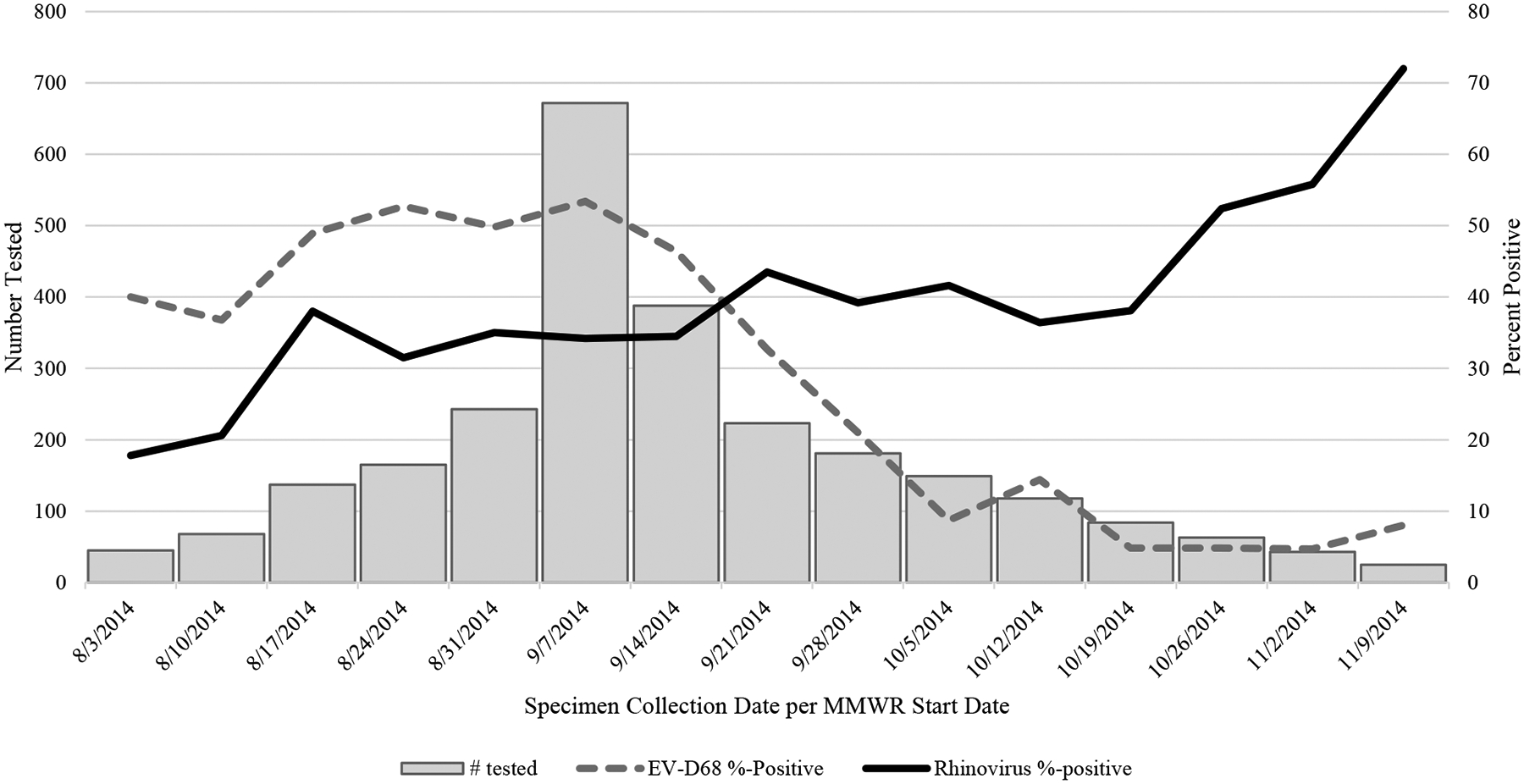
Number of specimens tested at the Centers for Disease Control and Prevention (N = 2629) by week and comparison of enterovirus D68 (dotted line) and rhinovirus (solid line) percent positive, 3 August 2014–15 November 2014. Weeks shown are limited to those with >20 specimens tested. Abbreviation: EV, enterovirus.
Demographic and Clinical Data
Among the 2629 patients with a specimen tested at CDC, 1002 (38.1%) were hospitalized with severe respiratory illness and had sufficient demographic and clinical data available for further analysis. Of these 1002, 511 (51.0%) were EV-D68 positive, 373 (37.2%) were RV A–C (RV-A, 147; RV-B, 45; RV-C, 181), and 29 (2.9%) had another EV identified. No RV or EV was detected by any test for 89 (8.9%) patients. The remainder of our analysis describes the 884 patients with severe respiratory illness with available clinical data and with either EV-D68 or RV A-C identified.
Overall, patient age ranged from <1 month to 92 years. The age distribution varied significantly across pathogens (P < .0001; Figure 3). Relative to RVs, EV-D68 was more commonly identified in children aged 1–17 years than in infants (aged <1 year) or adults (aged ≥18 years). Among those with EV-D68, 86.9% were aged 1–17 years, whereas 46.3%, 24.4%, and 68.0% were aged 1–17 years among those with RV-A, -B, or -C, respectively. This difference was also notable for the narrower range of 5–11 years; the percentage of these school-aged children was 44.2% among those with EV-D68 compared to 17.7%, 8.9%, and 20.4% among those with RV-A, -B, or -C, respectively. RV A–C were more common than EV-D68 among infants; the frequency of children aged <1 year was 19.0%, 28.9%, and 24.9% among those infected with RV-A, -B, and -C, respectively, vs 6.3% for EV-D68. Among the RV species, RV-A (34.7%) and RV-B (46.7%) were more likely to be found in adults than was RV-C (7.2%). Similarly, RV-C was more common than RV-A (19.7%) or RV-B (11.1%) among children aged 1–4 years (42.0%).
Figure 3.
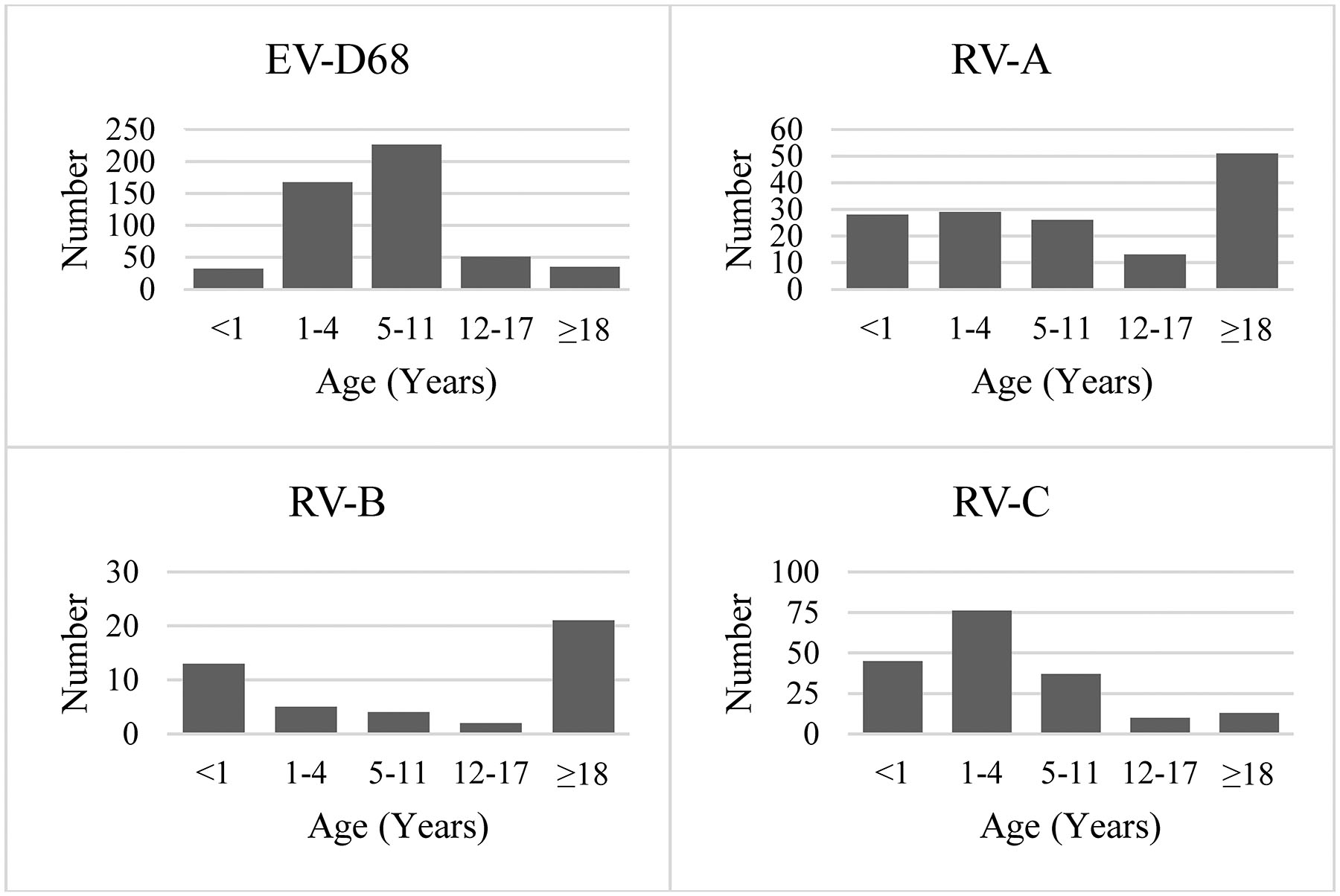
Distribution of enterovirus D68 and rhinovirus species (A, B, C) by age group among hospitalized patients, August 2014 –November 2014 (N = 884). Abbreviations: EV, enterovirus; RV, rhinovirus.
For EV-D68 and the 3 RV species, cough and shortness of breath were the most commonly reported symptoms for patients of all ages (Tables 1 and 2). Regardless of age, fever was reported in almost half of the patients with EV-D68, RV-A, or RV-C but in only about a quarter of patients with RV-B. Among patients aged <5 years (Table 1), cough and wheezing were more commonly reported among those with either EV-D68 or RV-C than among those with RV-A or RV-B, and cyanosis was more commonly reported among those with RV-B (all P values < .05). For patients aged ≥5 years (Table 2), retractions were more commonly reported among those with EV-D68, and lethargy was more common among those with RV-B (all P values < .05).
For all patient/virus groups under comparison, the most common underlying condition reported was a history of asthma/RAD. Among those aged <5 years, 33.7%, 24.6%, and 30.6% of patients with EV-D68, RV-A, and RV-C, respectively, had a previous history of asthma/RAD compared to 11.1% of those with RV-B. Among patients aged ≥5 years, more than half with EV-D68, RV-A, or RV-C reported a history of asthma/RAD compared to 37% of those with RV-B. In both age strata, this difference was only statistically significant when EV-D68 was compared to RV-B (P < .05). Cardiac disease and immuno-compromising conditions were infrequently reported.
The frequency of ICU admission was similar among all groups being compared, ranging from 48% to 63% (Tables 1 and 2). Patients with EV-D68 were less likely than those with RV species to require intubation, and this difference was more pronounced among those aged <5 years. The mean length of time between onset of symptoms and hospitalization for patients aged ≥5 years was 4.6 days for RV-B vs 2.1 days for EV-D68 (P = .017), 3.4 days for RV-A (P = .264), and 2.1 days for RV-C (P = .048).
DISCUSSION
We identified a high degree of cocirculation between EV-D68 and RVs during the 2014 outbreak of respiratory illness across the United States and demonstrate that RVs can be associated with widespread severe illness. To date, diagnostic challenges and the lack of clinical treatments have limited the full characterization of RV illness. However, this nationwide event provided a unique opportunity to explore the epidemiologic and clinical characteristics of different RV species. We observed differences in the age distribution and several clinical factors among those who were infected with these viruses and hospitalized during this time period. To our knowledge, this investigation provides the largest comparison of severe illness associated with RV A–C and EV-D68.
During the investigation period, 25 distinct EV types and 114 RV types were identified from 2629 patients with respiratory illness. Although the predominant pathogen among this group was EV-D68 (n = 1012, 38.5%), a similar proportion were RVs (n = 968, 36.8%). Throughout August and the first half of September, the percentage of weekly EV-D68 identifications was consistently greater than that of RV-A, -B, and -C combined. However, the frequency of weekly RV identifications surpassed that of EV-D68 during the week beginning 21 September 2014 and remained higher over the next 2 months. Our results demonstrate not only the broad array of circulating respiratory Enterovirus during this 4-month period but also the frequent association of RVs with severe respiratory disease.
Among the 3 RV species, differences in signs and symptoms, illness severity, and patient outcomes have been suggested but not firmly established [13–21]. RV-C infection has been found to be more common in children than adults [22], and RV-A infection in adults has previously been associated with greater clinical severity than RV-B [23]. RV-B, however, has been less frequently identified within surveillance populations [14, 24, 25].
When we compared clinical and epidemiologic features among those infected with EV-D68 and each of the 3 RV species, the age distributions varied substantially, with a bimodal distribution for RV-A and RV-B. That factor, combined with age-related differences in clinical factors such as fever and underlying conditions, necessitated careful comparisons by age, including some stratification by age groups.
Clinical syndromes, including illness severity, were largely similar among EV-D68 and RV species in our investigation, a finding noted in previous comparisons of EV/RV-positive patients with and without EV-D68 [26–29]. Also consistent with prior studies [27–29], among patients of all ages infected with EV-D68 or any of the RV species, the percentage of patients with a history of asthma/RAD (ranging from 11% to 61%), was notably higher than the 8.4% observed in the general population in 2010 [30]. However, some differences were noted. For children aged <5 years, EV-D68 and RV-C were more likely to be associated with cough and wheezing. Overall, we found that patients with EV-D68 were less likely than those with RV species to require intubation, a finding consistent with a prior study of children admitted to an ICU [31], and that RV-A and RV-B were more common in adults.
We also found differences with illness associated with RV-B, which has not previously been well characterized. Among patients aged ≥5 years, RV-B was less likely to present with fever than was EV-D68 or RV-A. The mean length of time between onset of symptoms and hospitalization was also longer among patients in this age group for RV-B vs EV-D68, RV-A, and RV-C, a finding possibly associated with the relative infrequency of fever among those with RV-B identified. Although this is the largest clinical description of RV-B to date to our knowledge, RV-B was still relatively infrequent in our investigation (n = 45, 4.5%).
Our investigation is subject to several limitations. First, our data were collected as part of an emergency public health response, and the criteria for collection and submission of specimens varied over time and between states and facilities, limiting our ability to understand rates or burden of disease. As such, frequencies of reporting of specific viruses over time may not reflect their true seasonality. Second, asymptomatic detection of RVs and EVs is common, and detection of a particular virus in a clinical specimen does not necessarily indicate that it caused any or all symptoms present at the time of detection. No controls were available for comparison. Third, data completeness was not uniform, and some variables such as race, ethnicity, and detection of other pathogens may have been reported less consistently. Last, an age-specific selection bias might have been introduced since most reporting facilities were children’s hospitals; samples from adults were sought and tested, but awareness might have been increased in pediatric settings.
EVs and RVs were frequently detected in association with severe respiratory illness during a large outbreak in 2014, allowing a more detailed characterization of their epidemiology and clinical impact. In addition to specific and rapid diagnostic testing to more accurately determine etiology during outbreaks of respiratory illness, increased clinical awareness of RVs and EVs and a better understanding of their epidemiology are needed to help facilitate the development or use of diagnostic tests, therapeutics, and preventive measures.
Supplementary Material
Acknowledgments.
A special thanks to Christina Castro (Centers for Disease Control and Prevention/Division of Viral Diseases) for organizing, annotating, and submitting rhinovirus (RV) and enterovirus (EV) sequences to GenBank. The authors also acknowledge the treating clinicians and the staff in state and local health departments who facilitated the submission of specimens to CDC for testing and the collection of related data during the 2014 respiratory illness outbreak.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC. Although not directly related to this work, the CDC does receive royalties from ZeptoMetrix Corporation for the licensing of EV and parechovirus strains for redistribution as reagents and controls.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
References
- 1.Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier/Saunders, 2015. [Google Scholar]
- 2.Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–41. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist S, Savolainen C, Råman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. Morb Mortal Wkly Rep 2014; 63:798–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Midgley CM, Watson JT, Nix WA, et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med 2015; 3:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberste MS, Peñaranda S, Rogers SL, Henderson E, Nix WA. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol 2010; 49:73–4. [DOI] [PubMed] [Google Scholar]
- 7.Real time EV-D68 RT-PCR. Available at: http://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM446784.pdf. Accessed 28 October 2014.
- 8.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 2006; 44:2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Holloway B, Dare RK, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol 2008; 46:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. Morb Mortal Wkly Rep 2011; 60:1301–4. [PubMed] [Google Scholar]
- 11.Lu X, Schneider E, Jain S, et al. Rhinovirus viremia in patients hospitalized with community-acquired pneumonia. J Infect Dis 2017; 216:1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EV-D68 Patient Summary Form. Available at: http://www.cdc.gov/non-po-lio-enterovirus/downloads/EV68-PatientSummaryForm.pdf. Accessed 28 October 2014.
- 13.Royston L, Tapparel C. Rhinoviruses and respiratory enteroviruses: not as simple as ABC. Viruses 2016; 8:doi: 10.3390/v8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis 2011; 204:1702–10. [DOI] [PubMed] [Google Scholar]
- 15.Aponte FE, Taboada B, Espinoza MA, et al. Rhinovirus is an important pathogen in upper and lower respiratory tract infections in Mexican children. Virol J 2015; 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SH, Hong SB, Kim T, et al. Clinical and molecular characterization of rhinoviruses A, B, and C in adult patients with pneumonia. J Clin Virol 2015; 63:70–5. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Yuan XH, Xie ZP, et al. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol 2009; 47:2895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WM, Lemanske RF Jr, Evans MD, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 2012; 186:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller EK, Edwards KM, Weinberg GA, et al. ; New Vaccine Surveillance Network. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 2009; 123:98–104.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang Z, Gonzalez R, Xie Z, et al. Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J Clin Virol 2010; 49:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauinger IL, Bible JM, Halligan EP, et al. Patient characteristics and severity of human rhinovirus infections in children. J Clin Virol 2013; 58:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piralla A, Rovida F, Campanini G, et al. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol 2009; 45:311–7. [DOI] [PubMed] [Google Scholar]
- 23.Chen WJ, Arnold JC, Fairchok MP, et al. Epidemiologic, clinical, and virologic characteristics of human rhinovirus infection among otherwise healthy children and adults: rhinovirus among adults and children. J Clin Virol 2015; 64:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Linden L, Bruning AH, Thomas XV, et al. A molecular epidemiological perspective of rhinovirus types circulating in Amsterdam from 2007 to 2012. Clin Microbiol Infect 2016; 22:1002.e9–.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard LM, Johnson M, Gil AI, et al. Molecular epidemiology of rhinovirus detections in young children. Open Forum Infect Dis 2016; 3:ofw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orvedahl A, Padhye A, Barton K, et al. Clinical characterization of children presenting to the hospital with enterovirus D68 infection during the 2014 outbreak in St. Louis. Pediatr Infect Dis J 2016; 35:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertz D, Alawfi A, Pernica JM, Rutherford C, Luinstra K, Smieja M. Clinical severity of pediatric respiratory illness with enterovirus D68 compared with rhinovirus or other enterovirus genotypes. CMAJ 2015; 187:1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster JE, Miller JO, Selvarangan R, et al. Severe enterovirus 68 respiratory illness in children requiring intensive care management. J Clin Virol 2015; 70:77–82. [DOI] [PubMed] [Google Scholar]
- 29.Launes C, Armero G, Anton A, et al. Molecular epidemiology of severe respiratory disease by human rhinoviruses and enteroviruses at a tertiary paediatric hospital in Barcelona, Spain. Clin Microbiol Infect 2015; 21:799.e5–7. [DOI] [PubMed] [Google Scholar]
- 30.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3 2012; 1–58. [PubMed] [Google Scholar]
- 31.Rao S, Messacar K, Torok MR, et al. Enterovirus D68 in critically ill children: a comparison with pandemic H1N1 influenza. Pediatr Crit Care Med 2016; 17:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


