Abstract
Background:
Standard linear echoendoscopes have a large distal tip and bending radius, which can preclude adequate examination in some patients.
Objective:
We examined the impact of having available slim linear echoendoscopes (SLE) on our endoscopic ultrasound (EUS) practice.
Materials and Methods:
As a quality improvement measure, data on the need for the use of SLE were documented in 2000 consecutive procedures performed over a 10-month period from February to November 2022. When examination using a standard size echoendoscope failed due to technical limitations, the procedure was reattempted in the same session using a SLE. The main outcome was the impact of SLE, which was defined as the establishment of a new diagnosis or if findings altered treatment plan.
Results:
A complete EUS examination failed in 23 of 2000 procedures (1.15%, 95% CI, 0.73-1.72%) performed using standard size echoendoscope (14 male, median age 73 y [IQR 66 to 79]). The examination was technically successful when using SLE in 22 of 23 (95.6%) patients. SLE impacted clinical management in all 22 patients (100%) by establishing tissue diagnosis in 19 and/or altering subsequent treatment plan in 5. Adverse event of transient hypoxia was observed in one patient (4.3%).
Conclusions:
A very experienced EUS team required SLE in 1.1% of consecutive examinations. Our findings suggest that when used, 95% of patients benefitted as it had a significant impact on their clinical management.
Key Words: slim linear echoendoscope, endoscopic ultrasound, tissue acquisition
Endoscopic ultrasound (EUS) is an essential procedure for the diagnosis and staging of gastrointestinal malignancies. EUS-guided fine needle aspiration and biopsy (FNB) is standard practice for tissue acquisition from pancreatic masses, lymph nodes, subepithelial lesions, and other lesions adjacent to the gastrointestinal (GI) tract. EUS is the most sensitive imaging modality for the detection of pancreatic masses and is particularly useful when the results of other cross-sectional imaging modalities are inconclusive.1,2 More recently, the role of EUS has expanded from a diagnostic to a therapeutic modality. As with all endoscopic procedures, adverse events can be encountered at EUS, which may be directly related to the echoendoscope itself. Although available data is limited, perforation is more common with upper GI EUS than with EGD.3 The increased risk is likely due to the echoendoscope design, which combines oblique or side-view optics with a relatively long rigid tip that extends well beyond the lens. Therefore, the tip of the echoendoscope may cause luminal perforation during advancement, particularly in areas of tight angulation (oropharynx or the duodenal bulb), stenosis (esophageal cancer), blind pouch (pharyngeal or esophageal diverticula), or altered surgical anatomy (Roux-en-Y gastric bypass). The presence of the ultrasound transducer increases the diameter of the scope tip and adversely affects the bending radius; these 2 factors limit flexibility and maneuverability. Consequently, to minimize adverse events in patients with luminal stenoses or acute angulation, the EUS procedure may have to be aborted to avoid an adverse event.
To overcome some of these technical drawbacks, a slim linear echoendoscope (EG34-J10U, Pentax Medical Americas, Montvale, New Jersey, USA) with a probe tip diameter of 12.9 mm and a tighter bending radius was developed. The objective of this study was to evaluate the impact of this slim linear echoendoscope (SLE) on the practice of EUS in real-life clinical settings.
MATERIALS AND METHODS
Patients and Settings
As part of the quality assessment and improvement initiative, data on the need for the use of SLE was documented prospectively in 2000 consecutive patients aged ≥18 years who underwent EUS over a 10-month period from February to November 2022 at Orlando Health. Included were patients in whom a standard curvilinear echoendoscope could not be successfully advanced to a required position due to anatomic circumstances. In the same procedure, the SLE instrument was used. Excluded were patients who were pregnant, children, those with abnormal coagulation parameters, and those on anticoagulation or antiplatelet therapy. Written informed consent was obtained from all patients undergoing EUS procedures. As data were collected for quality improvement, after administrative review of the data collection tools (Supplemental File 1, Supplemental Digital Content 1, http://links.lww.com/JCG/B22), the Institutional Review Board waived the requirement for informed consent and ethics approval was obtained for data analysis (Approval notice no. 1941652). All authors had full access to study data and have reviewed and approved the final manuscript.
Procedures
All EUS procedures were performed using standard curvilinear echoendoscopes (GF-UCT180, Olympus; EG38-J10UT, Pentax; EG-580UT, Fujifilm) with patients in the left lateral position under monitored anesthesia care using propofol or general anesthesia by 4 expert endosonographers (J.Y.B., U.N., R.H., and S.V.) with lifetime experience of 5000 to 30,000 procedures. When the echoendoscope could not be advanced, dilation of stricture to 15 mm was attempted using Savary-Gilliard dilators (Cook Medical, Bloomington, Indiana) for esophageal strictures or with radial expansion balloons (CRE Boston Scientific Corp., Marlborough, Massachusetts) for other strictures. However, if resistance was encountered during echoendoscope passage or if dilation up to 15 mm could not be achieved successfully, then the procedure was performed using a SLE (EG34-J10U, Pentax) in the same endoscopy session. Characteristics and differences between the standard and slim curvilinear echoendoscopes are shown in Table 1.
TABLE 1.
Technical Specifications of All Commercially Available Latest Generation Echoendoscopes and the Slim Linear Echoendoscope
| Echoendoscope | Pentax slim linear | Pentax linear | Olympus linear | Fujifilm linear |
|---|---|---|---|---|
| Model | EG34-J10U | EG38-J10UT | GF-UCT189 | EG-58OUT |
| Distal end width (mm) | 12.9 | 14.3 | 14.6 | 13.9 |
| Insertion tube width (mm) | 11.6 | 12.8 | 12.6 | 124 |
| Channel width (mm) | 2.8 | 4.0 | 3.7 | 3.8 |
| Field of view (°) | 120 | 120 | 100 | 140 |
| Angulation up/down (°) | 160/130 | 160/130 | 130/90 | 150/150 |
| Angulation right/left (°) | 120/120 | 120/120 | 90/90 | 120/120 |
Tissue acquisition, when indicated, was performed using third-generation FNB needles (Acquire, Boston Scientific; SharkCore, Medtronic, Minneapolis, Minnesota) and cyst aspirations using 19 or 22-gauge fine needle aspiration needles (Expect, Boston Scientific). As the instrument channel diameter of SLE is only 2.8 mm, therapeutic interventions were not included in this cohort. Rapid onsite evaluation was undertaken per institutional standard of care in all patients undergoing tissue acquisition procedures.
Follow-up
Procedural indications, reasons for technical failure, and clinical outcomes were prospectively documented in all patients following each procedure and transcribed to an electronic database. All outpatients were discharged home the same day if they met discharge criteria. Per standard of care, all patients were contacted by telephone call by endoscopy staff 1 week postprocedure to assess for adverse events. Hospital records were reviewed in patients who were admitted for management of adverse events.
Outcome Measures
The primary outcome measure was to evaluate the impact of SLE, which was defined as the establishment of a new diagnosis or if findings at EUS altered subsequent treatment plans. The secondary outcome measure was adverse events, which were graded per established criteria.3
Statistical Analysis
A descriptive analysis of patients undergoing EUS examination using SLE and overall procedural impact was reported. Continuous data were summarized as medians with interquartile range, and categorical data were summarized as frequencies with percentages and 95% confidence intervals. Statistical analyses were performed using Stata 17 (Stata Corp, College Station, Texas).
RESULTS
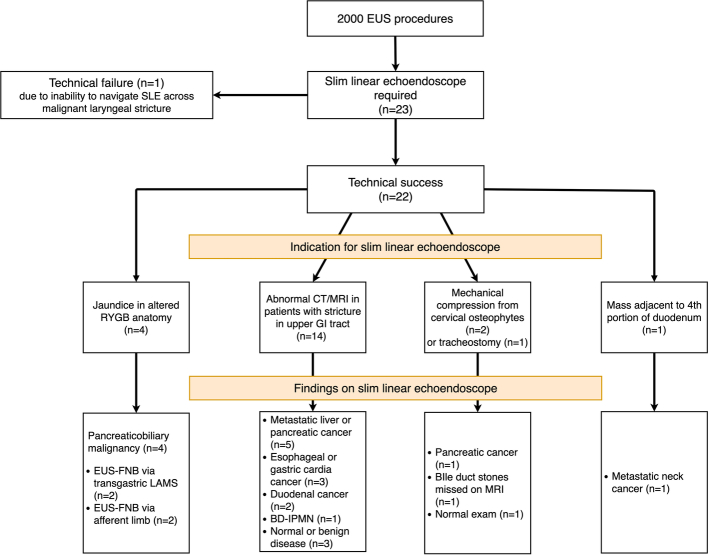
Of 2000 consecutive EUS procedures performed over a 10-month period, examination using a standard curvilinear echoendoscope failed in 23 patients (1.15%, 95% CI, 0.73-1.72%) who then subsequently underwent EUS using the SLE. The majority of patients were male (n=14, 60.9%) and the median age of the study cohort was 73 years (IQR, 66 to 79). The flow diagram of patients who failed EUS using standard echoendoscopes and required examination using SLE is shown in Figure 1. Procedural indications for using SLE were broadly categorized into four main groups:
FIGURE 1.
Flow diagram of patients who failed EUS using standard echoendoscopes and required examination using SLE. CT indicates computed tomography; EUS, endoscopic ultrasound; FNB, fine needle biopsy; GI, gastrointestinal; IPMN, intraductal papillary mucinous neoplasm; LAMS, lumen-apposing metal stents; MRI, magnetic resonance imaging; SLE, slim linear echoendoscopes.
Esophageal Stricture or Gastric Outlet Obstruction
Fifteen patients required evaluation of abnormal computed tomogram (CT), magnetic resonance imaging, or positron emission tomography findings in the presence of tight esophageal stricture or gastric outlet obstruction that precluded passage of a standard curvilinear echoendoscope. Three patients with tight malignant esophageal strictures were found to have metastasis to the liver or peri-hepatic lymph nodes (Video 1, Supplemental Digital Content 2, http://links.lww.com/JCG/B23) and 2 with gastric outlet obstruction and eosinophilic esophagitis had pancreatic head cancer on EUS-guided FNB. Likewise, 3 patients were proven to have underlying malignancy in the setting of postradiation mid-esophageal stricture, linitis plastica-type gastric cancer, and gastric cardia cancer that were nondiagnostic on prior endoscopic biopsies and in whom tissue acquisition using standard size linear echoendoscope was unsuccessful. Two other patients with tight duodenal strictures who had nondiagnostic endoscopic and standard-size echoendoscope-guided biopsies were proven to have primary duodenal adenocarcinoma on EUS-guided FNB (Video 2, Supplemental Digital Content 3, http://links.lww.com/JCG/B24). In 1 patient with laryngeal cancer and gastric outlet obstruction, it was not possible to advance the SLE across the larynx as mechanical compression induced hypoxia. The procedure was aborted, and at long-term follow-up, the patient was diagnosed to have duodenal malignancy.
Four patients with abnormal cross-sectional imaging were proven at EUS to have benign disease; 1 patient with esophageal peptic stricture and suspected pancreatic tail mass had 21 mm side-branch type intraductal papillary mucinous neoplasm (IPMN), 1 patient with normal bile duct and elevated liver tests in the setting of eosinophilic esophagitis had a bile duct stone that was subsequently extracted at endoscopic retrograde cholangiopancreatography (ERCP), and 2 patients with esophageal strictures that were strongly positive on positron emission tomography had benign esophageal disease on FNB that was confirmed at 12-month follow-up.
Pancreaticobiliary Mass in Roux-en-Y gastric Bypass
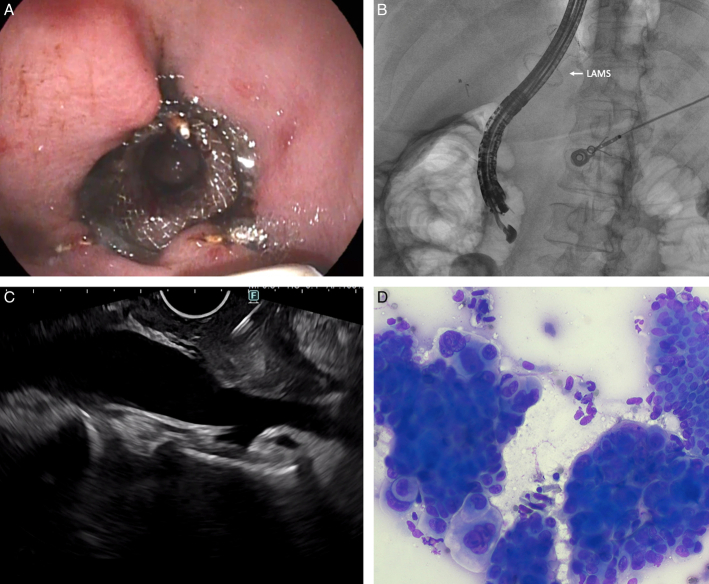
One patient with mildly elevated liver tests and normal bile duct on CT was diagnosed to have cholangiocarcinoma (Video 3, Supplemental Digital Content 4, http://links.lww.com/JCG/B25) and another patient with abnormal CT was diagnosed to have a 2 cm pancreatic head neuroendocrine tumor. The distal bile duct and pancreatic head region could be visualized by advancing the SLE deep into the afferent limb, which otherwise was not possible using a standard size curvilinear echoendoscope. Two patients with obstructive jaundice underwent gastroenterostomy by placement of 20 mm lumen-apposing metal stents (LAMS) in the gastric remnant. Despite dilation of the endoprosthesis to 18 mm, it was not possible to advance the standard curvilinear echoendoscope through the LAMS as the transducer tip was entrapped repeatedly in the proximal flange. In both patients, the SLE could be advanced through the LAMS without any resistance. EUS-guided FNB revealed cholangiocarcinoma (Video 4, Supplemental Digital Content 5, http://links.lww.com/JCG/B26; Fig. 2A-D) in one patient and biliary IPMN in another. Both patients also underwent ERCP with biliary stent placement in the same setting.
FIGURE 2.
The slim linear echoendoscope being advanced through the lumen-apposing metal stent on an endoscopic view (A), corresponding fluoroscopic image (B), sampling of pancreatic head mass (C), and rapid onsite assessment revealing adenocarcinoma (Diff-Quik staining, ×200) (D).
Extrinsic Mechanical Compression
In 2 patients with enlarged osteophytes that caused cervical compression, the use of SLE enabled the diagnosis of pancreatic cancer by FNB in one patient, and in another patient with suspected chronic pancreatitis, the EUS examination was normal. In an 80-year-old patient with lung cancer and tracheostomy who presented with obstructive jaundice, the magnetic resonance imaging was normal. Standard EUS examination failed as the tracheostomy mechanically impeded the passage of the echoendoscope. The patient was proven to have an embedded stone in the major duodenal papilla on a slim echoendoscope examination and subsequently underwent ERCP for ductal clearance.
Distant Mass
In an 81-year-old patient with a history of squamous cell cancer of the tongue, CT imaging revealed a mass adjacent to the fourth portion of the duodenum. This lesion could only be imaged and sampled using a SLE, as the standard echoendoscope could not be advanced past the third portion of the duodenum. Rapid onsite evaluation revealed metastatic squamous cell cancer.
Outcomes
Endoscopic ultrasound examination was technically successful when using SLE in 22 of 23 (95.6%) patients with a median procedural duration of 12 mins (IQR 9 to 18). Failure in 1 patient with gastric outlet obstruction was due to the inability to navigate the SLE across a malignant laryngeal stricture. At long-term follow-up, this patient was found to have duodenal cancer. SLE impacted clinical management in all 22 patients in whom the procedure was technically successful by establishing tissue diagnosis in 19 (malignancy 17; benign 2) and altering the treatment plan in 5 (excluding malignancy by diagnosing IPMN in 2, establishing diagnosis of bile duct stones in 2, and excluding chronic pancreatitis in 1). An adverse event was observed in 1 patient with malignant laryngeal stricture who developed transient airway compromise on passage of the SLE, and the procedure had to be aborted. A case capsule of all 23 patients who underwent EUS examination using the SLE is shown in Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JCG/B22.
DISCUSSION
The present study demonstrates the utility of the slim linear echoendoscope in 1.1% of EUS examinations when standard echoendoscopes failed. The novel design helped to overcome anatomic challenges associated with echoendoscope passage at the level of the cervical spine, navigation across postsurgical gastric bypass anatomy, maneuverability to reach the distal duodenum or across endoprosthesis for tissue sampling and traversing complex strictures resistant to dilation. It enabled adequate visualization of the region of interest, and the sonographic image quality was optimal. In clinical practice, we were able to visualize and sample all lesions with optimal clinical outcomes. While the 2.8 mm working channel diameter precluded the performance of therapeutic interventions, it was technically feasible to perform diagnostic procedures such as tissue acquisition without limitations.
A salient feature worth reporting is the safety profile of SLE. Despite being selectively used only in challenging cases, its technical performance was excellent and without major adverse events. The reported incidence of gastrointestinal perforation during EUS ranges from 0% to 0.4%.4,5 In 1 prospective study of 4894 patients who underwent upper EUS, 3 patients experienced cervical esophageal perforations.6 Underlying risk factors for cervical perforation were identified to be age >65 years, history of swallowing difficulties, known history of cervical osteophytes, and kyphosis of the spine. In the present report, both patients with prominent osteophytes in whom a standard echoendoscope could not be advanced beyond the cervical esophagus were also aged >65 years.
Approximately 15 to 45% of patients with esophageal cancer have a nontraversable obstructing tumor.7 Some investigators discourage routine dilation of obstructing tumors because of the risk of perforation and the fact that a tight, circumferential esophageal cancer is uniformly at least T3N1. However, others report that valuable information, such as distant lymphadenopathy or metastasis, can be obtained by performing a complete examination that can alter the treatment plan in 10% to 40% of cases8,9 In 2 reports, dilation allowed immediate passage of an echoendoscope beyond the tumor in 75% to 85% of cases.8,9 However, caution is necessary when semi-circumferential tumor infiltration is present because the normal (and hence thinner) esophageal wall may be at increased risk of tearing in this setting. In the present study, completing an EUS examination using SLE enabled the detection of distant metastases in 3 patients, thereby altering subsequent treatment plan. In some patients with tight esophageal strictures, particularly postradiation, establishing a tissue diagnosis can be challenging as the tumor is buried deep within the distorted esophageal wall layers. Unlike standard curvilinear echoendoscopes, the thin transducer tip of the SLE could be better wedged within the esophageal lumen for deep tissue sampling.
While data on EUS-related duodenal perforation is limited, in a retrospective study of 8504 procedures, perforation was observed in 0.15% of patients.10 This is more commonly encountered in patients with long-segment duodenal strictures in whom the elongated transducer tip precludes adequate visualization. However, when blind endoscopic biopsies are nondiagnostic, the clinical/surgical management can be challenging, particularly in high-risk surgical candidates. In the present report, 2 patients with malignant appearing duodenal strictures had several nondiagnostic upper GI endoscopic biopsies, and the standard echoendoscope could not be navigated adequately. By being able to wedge the small transducer of the SLE within the stricture, we could sample the deeper wall layers and establish a tissue diagnosis of primary duodenal adenocarcinoma in both patients.
Diagnostic evaluation of patients with suspected pancreaticobiliary malignancy in the setting of Roux-en-Y gastric bypass can be challenging. Endoscopic perforation is not infrequent in these patients, particularly when advancing the curvilinear echoendoscopes, which are less flexible and more rigid. In our experience, most pancreatic head and biliary malignancies can be identified by advancing the SLE through the afferent limb and tracing the bile duct, which is usually visualized inferior to the portal vein (Video 3, Supplemental Digital Content 4, http://links.lww.com/JCG/B25). Also, in the case of EUS-guided gastro-gastrostomy with LAMS, while dilation of the lumen facilitates easy advancement of duodenoscopes, the long transducer tip inherent to curvilinear echoendoscopes makes passage challenging. In contrast, the distal end of the SLE could be advanced with minimal resistance, and hence, we were able to establish the diagnosis of cholangiocarcinoma and biliary IPMN in 2 patients who presented with obstructive jaundice.
What lessons can we learn from this quality assessment audit that can advance our knowledge? One, the SLE by virtue of its design, can be maneuvered more easily across strictures and difficult anatomy. This is particularly relevant when encountering patients with significant comorbidities who are unlikely to tolerate major adverse events such as perforation. Two, the SLE has unique advantages when evaluating patients with gastric bypass anatomy who are now being seen with increasing frequency. The ability to navigate the SLE into the afferent limb and perform tissue sampling through the lumen of the LAMS provides access to difficult-to-reach areas in the GI tract. Three, it is important to note that while the SLE was required in only 1.15% of 2000 consecutive examinations, it could theoretically be used for performing almost all EUS procedures except in those patients requiring therapeutic interventions. Although its technical attributes were uniquely advantageous only for a small patient cohort, the clinical impact was significant. In more than 95% of patients, the information yielded altered treatment plans, thereby obviating the need for additional investigations and possible delay in patient care. As all patients in this study underwent EUS using a slim echoendoscope at the same session following a failed examination using a standard echoendoscope, we did not perform a cost-effectiveness analysis. Consequently, a comparison of clinical or cost-effectiveness with alternative modalities, such as image-guided or surgical techniques, was not performed. Finally, we captured data only in patients who failed examination using standard echoendoscopes and therefore required SLE for successful procedure completion. In most patients with luminal strictures or altered surgical anatomy, following dilation, an EUS examination could be completed successfully using standard echoendoscopes. Therefore, the inclusion of SLE in the endoscopy armamentarium would be particularly relevant to large-volume quaternary referral centers treating patients with complex and varied pathology who have failed procedures at outside facilities.
Supplementary Material
Footnotes
Podium Presentation at ESGE Days, April 2023, Dublin, Ireland.
J.Y.B.: Study design, endoscopist performing procedures in the study, acquisition of data, and analysis and interpretation of data, statistical analysis, drafting of the manuscript, critical revision of manuscript. S.V.: Study concept and design, endoscopist performing procedures in the study, interpretation of data, drafting and critical revision of the manuscript. U.N., R.H.: Endoscopists performing procedures in the study and critical revision of the manuscript. P.W.: Critical revision of the manuscript, video editing and compilation of figures
J.Y.B.: Consultant for Olympus America Inc. and Boston Scientific Corporation. S.V.: Consultant for Boston Scientific Corporation, Olympus America Inc, Medtronic. R.H.: Consultant for Fujifilm, GIE Medical Inc, Olympus America Inc. U.N.: Consultant for Janssen, Pfizer, Takeda, AbbVie, Bristol Myers Squibb and GIE Medical Inc. P.W. declares that there is nothing to disclose.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
Contributor Information
Ji Young Bang, Email: jybang213@gmail.com.
Philippe Willems, Email: philippe.willems@umontreal.ca.
Udayakumar Navaneethan, Email: udhaykumar81@gmail.com.
Robert Hawes, Email: robert.hawesmd@gmail.com.
Shyam Varadarajulu, Email: svaradarajulu@yahoo.com.
REFERENCES
- 1.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–1439. [DOI] [PubMed] [Google Scholar]
- 2.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. [DOI] [PubMed] [Google Scholar]
- 3.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endo- scopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. [DOI] [PubMed] [Google Scholar]
- 4.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. [DOI] [PubMed] [Google Scholar]
- 5.O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–474. [DOI] [PubMed] [Google Scholar]
- 6.Eloubeidi MA, Tamhane A, Lopes TL, et al. Cervical esophageal perforations at the time of endoscopic ultrasound: a prospective evaluation of frequency, outcomes, and patient management. Am J Gastroenterol. 2009;104:53–56. [DOI] [PubMed] [Google Scholar]
- 7.Bang JY, Ramesh J, Hasan M, et al. Endoscopic ultrasonography is not required for staging malignant esophageal strictures that preclude the passage of a diagnostic gastroscope. Dig Endosc. 2016;28:650–656. [DOI] [PubMed] [Google Scholar]
- 8.Pfau PR, Ginsberg GG, Lew RJ, et al. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol. 2000;95:2813–2815. [DOI] [PubMed] [Google Scholar]
- 9.Wallace MB, Hawes RH, Sahai AV, et al. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest Endosc. 2000;51:309–313. [DOI] [PubMed] [Google Scholar]
- 10.El Bacha H, Prat F. Endoscopic management of iatrogenic EUS-related duodenal perforations with over-the-scope clips. Endosc Int Open. 2020;8:E59–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.