Abstract
Introduction
The phase III IMbrave150 study established atezolizumab + bevacizumab as the global standard of care in patients with unresectable hepatocellular carcinoma (HCC). This exploratory analysis examined the impact of bevacizumab interruption due to bevacizumab adverse events of special interest (AESIs).
Methods
Patients in IMbrave150 who were randomized to atezolizumab + bevacizumab and received treatment for ≥6 months (to reduce immortal time bias) were included in group A-1 if bevacizumab had ever been skipped due to bevacizumab AESIs or to group A-2 otherwise. Efficacy analyses included overall survival (OS) and progression-free survival (PFS) by whether bevacizumab was skipped (group A-1 vs. A-2). PFS was evaluated per independent review facility (IRF)-assessed Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 and HCC-modified RECIST (IRF-HCC mRECIST). Safety was also evaluated.
Results
Of the 210 patients who received ≥6 months of atezolizumab + bevacizumab, 69 were assigned to group A-1 and 141 to A-2. At data cutoff (August 20, 2020), hazard ratio (HR) for OS was 1.04 (95% CI: 0.64, 1.69) for group A-1 versus A-2. HR for PFS was 1.07 (95% CI: 0.74, 1.55) per IRF-assessed RECIST 1.1 and 1.10 (95% CI: 0.76, 1.59; 15.5 vs. 9.7 months) per IRF-HCC mRECIST for group A-1 versus A-2. Safety profiles for atezolizumab and bevacizumab were largely similar between groups. More group A-1 patients had grade 3/4 adverse events. A separate analysis investigating the impact of immortal time bias in patients who received ≥3 months of atezolizumab + bevacizumab supported the appropriateness of the ≥6-month landmark analysis.
Discussion/Conclusion
Efficacy was similar between patients who skipped bevacizumab due to bevacizumab AESIs and those who did not. Although this comparison was nonrandomized and exploratory, results suggest that skipping bevacizumab due to bevacizumab AESIs did not considerably impact the efficacy and safety of atezolizumab + bevacizumab.
Keywords: Atezolizumab, Bevacizumab, Unresectable hepatocellular carcinoma, Adverse events of special interest, Skipped bevacizumab
Introduction
Liver cancer, including hepatocellular carcinoma (HCC), is a significant contributor to global cancer-related mortality [1]. After its approval in 2007, sorafenib was the standard-of-care first-line (1L) treatment for patients with advanced, unresectable HCC for over a decade, having demonstrated a median overall survival (OS) of 10.7 months in phase III trials [2]. The combination of atezolizumab and bevacizumab (atezolizumab + bevacizumab) has now emerged as the standard of care for the 1L treatment of patients with unresectable HCC [3, 4].
Atezolizumab enhances antitumor immunity through the selective inhibition of programmed death ligand-1 and its binding to programmed death-1, thus reversing T-cell suppression [5]. The addition of an anti-vascular endothelial growth factor therapy such as bevacizumab can augment responses to programmed death ligand-1 inhibition by atezolizumab [6]. Bevacizumab reduces vascular endothelial growth factor-mediated immunosuppression in the tumor microenvironment and enhances T-cell infiltration into the tumor; this promotes an immune-permissive tumor microenvironment [7].
Data from the phase Ib GO30140 study (NCT02715531) demonstrated significantly longer progression-free survival (PFS) with atezolizumab + bevacizumab versus atezolizumab monotherapy (5.6 vs. 3.4 months; hazard ratio [HR], 0.55 [80% CI: 0.40, 0.74]). These findings illustrated the clinically relevant contribution of bevacizumab in terms of immune enhancement and normalization of tumor blood vessels [6].
Results from the primary analysis of the global phase III IMbrave150 trial (NCT03434379) demonstrated statistically significant and clinically meaningful improvements with atezolizumab + bevacizumab versus sorafenib [8]. At the 12-month updated analysis, median OS was 19.2 months with atezolizumab + bevacizumab versus 13.4 months with sorafenib after a median follow-up duration of 15.6 months [9]. This was the longest median OS seen with a 1L treatment in a phase III study of patients with unresectable HCC.
In IMbrave150, 17% of patients in the atezolizumab + bevacizumab arm experienced an adverse event of special interest (AESI) defined for bevacizumab that led to bevacizumab interruption [8, 10]. Previous studies in colorectal cancer have suggested that bevacizumab interruption can accelerate tumor regrowth and revascularization and increase tumor resistance [11, 12]. Therefore, it is important to better understand the impact of bevacizumab interruption in patients with HCC treated with atezolizumab + bevacizumab in IMbrave150. Managing bevacizumab AESIs remains a challenge in clinical practice. The first data addressing the impact of bevacizumab interruption were obtained during an exploratory analysis conducted in the Taiwanese subpopulation of the IMbrave150 and GO30140 studies [13]. This exploratory analysis suggested that bevacizumab interruption did not appear to impact efficacy in patients who received atezolizumab + bevacizumab for ≥6 months. Here, we report an exploratory analysis of the IMbrave150 intention-to-treat population that examined outcomes in patients treated with atezolizumab + bevacizumab for ≥6 months who skipped bevacizumab due to bevacizumab AESIs versus those who did not.
Materials and Methods
Participants
Detailed eligibility criteria of the global, multicenter, open-label, randomized, phase III IMbrave150 study (ClinicalTrials.gov, NCT03434379) were previously reported [8, 9]. Patients aged 18 years or older who had locally advanced or metastatic and/or unresectable HCC, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, Child Pugh Class A liver function, and had not previously received systemic therapy for liver cancer were included in the study [8]. Key exclusion criteria included a history of autoimmune disease, coinfection with hepatitis B virus and hepatitis C virus, and untreated or incompletely treated esophageal or gastric varices with bleeding or high risk of bleeding.
Study Design
The study design and outcome measures of the open-label IMbrave150 study were previously reported [3, 4] and are summarized in Figure S1 (for all online suppl. material, see https://doi.org/10.1159/000535501). Patients were randomized in a 2:1 ratio to receive either 1,200 mg of atezolizumab plus 15 mg/kg of bevacizumab intravenously every 3 weeks (Arm A) or 400 mg of sorafenib orally twice daily (Arm B). Randomization was stratified by geographic region (Asia excluding Japan vs. the rest of the world), macrovascular invasion or extrahepatic spread of disease (presence vs. absence), baseline alpha-fetoprotein level (<400 vs. ≥400 ng/mL), and ECOG performance status (0 vs. 1).
Per the IMbrave150 protocol, treatment was given until loss of clinical benefit as determined by the investigator after an integrated assessment of radiographic and biochemical data and clinical status (e.g., symptomatic deterioration such as pain secondary to disease) or unacceptable toxicity [8]. Treatment could be continued after disease progression if the investigator assessed that there was a clinical benefit. If patients transiently or permanently discontinued either atezolizumab or bevacizumab due to an adverse event, single-agent therapy was allowed if the patient was experiencing clinical benefit. This exploratory analysis of IMbrave150 evaluated the impact of skipping bevacizumab due to bevacizumab AESIs in patients treated with atezolizumab + bevacizumab for ≥6 months.
Patients from arm A of IMbrave150 who received atezolizumab + bevacizumab for ≥6 months were assigned to group A-1 if bevacizumab was ever skipped due to bevacizumab AESIs (online suppl. Fig. S1). Bevacizumab interruption was defined as either skipping bevacizumab until the next treatment cycle or delaying the administration date of the next cycle (online suppl. Fig. S1) as defined in a previous analysis of bevacizumab interruption in IMbrave150 and GO30140 [13]. Group A-1 consisted of patients who transiently skipped bevacizumab, including those who subsequently withdrew from bevacizumab for any reason. Patients were assigned to group A-2 if they were not included in group A-1. AESIs were defined by the sponsor and reported regardless of causality. For bevacizumab, AESIs were defined based on known adverse drug reactions with bevacizumab, including bleeding, congestive heart failure, fistula, hypertension, wound healing complications, proteinuria, and thromboembolic events, according to the IMbrave150 protocol and a previously reported analysis of AESIs [10].
Outcomes
The coprimary endpoints of IMbrave150 were OS (defined as time from randomization to death from any cause) and PFS (defined as time from randomization to disease progression per independent review facility-assessed Response Evaluation Criteria in Solid Tumours [IRF-RECIST] version 1.1 or death from any cause, whichever occurred first) [8]. Secondary efficacy endpoints included PFS per IRF-assessed HCC-modified RECIST.
Tumors were assessed by computed tomography or magnetic resonance imaging at baseline and every 6 weeks until week 54 and then every 9 weeks thereafter. Safety was continuously evaluated by recording vital signs and clinical laboratory test results and assessing the incidence and severity of adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [8].
Statistical Analysis
To minimize immortal time bias, landmark analyses of OS and PFS were performed in patients who received atezolizumab + bevacizumab for ≥6 months, including those who experienced the first instance of disease progression within the first 6 months of treatment. To investigate the comparability of groups A-1 and A-2, baseline patient characteristics were summarized for each group. Separate analyses of OS and PFS were performed by whether bevacizumab was skipped due to bevacizumab AESIs in patients who received atezolizumab + bevacizumab for ≥3 months to explore the impact of immortal time bias.
To further evaluate the robustness of the primary ≥6-month landmark analysis, time-dependent analyses using a stratified Cox proportional model were performed in patients who were treated with atezolizumab + bevacizumab for any length of time. In this model, the first occurrence of bevacizumab AESIs leading to bevacizumab interruption was included as the time-dependent variable.
The Kaplan-Meier method was used to estimate OS and PFS. HRs were estimated with a stratified Cox proportional-hazards model. Stratification factors for efficacy analyses included all the randomization stratification factors except ECOG performance status.
Results
Patients
In IMbrave150, 336 patients were randomized to the atezolizumab + bevacizumab arm, of whom 210 patients (63%) received the combination for ≥6 months and were included in this exploratory analysis (online suppl. Fig. S2). Of these patients, 69 (33%) skipped bevacizumab due to an AESI for bevacizumab and were included in group A-1. The remaining 141 patients (67%) had never skipped bevacizumab due to an AESI for bevacizumab and were included in group A-2.
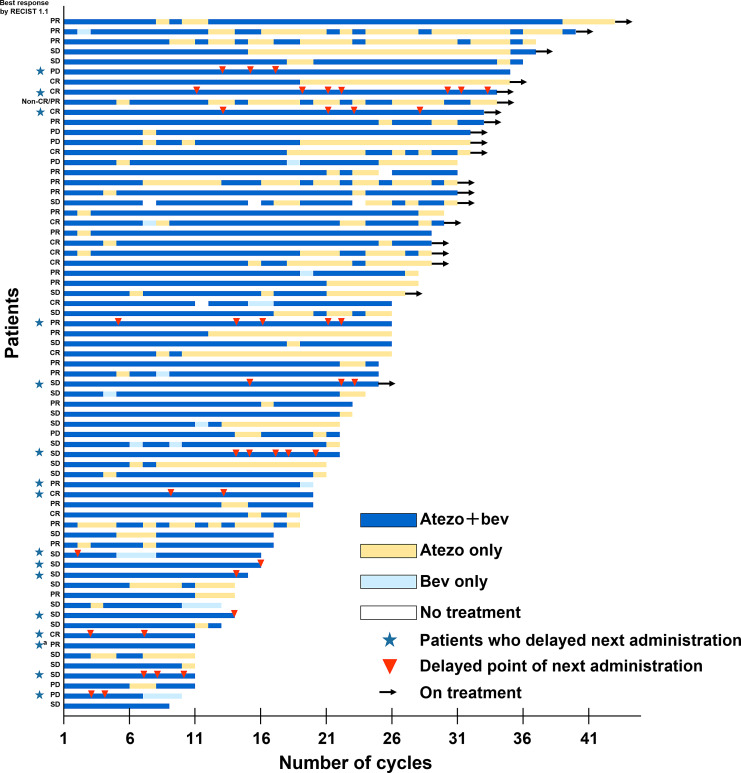
Of the 69 patients who skipped bevacizumab due to a bevacizumab AESI, 53 (77%) skipped a treatment cycle, and 16 (23%) delayed the administration date of the next cycle (Fig. 1). The rate of bevacizumab reintroduction was 78% (n = 54). After skipping bevacizumab due to AESIs, 49 patients (71%) in group A-1 withdrew from bevacizumab, including 25 (36%) due to progressive disease, 9 (13%) due to death, and 7 (10%) due to adverse events. A total of 105 patients (74%) in group A-2 withdrew from bevacizumab, including 50 (35%) due to progressive disease, 39 (28%) due to adverse events, and 8 (6%) due to withdrawal by the patient.
Fig. 1.
Bevacizumab interruption due to bevacizumab AESIs by patient (n = 69) in those treated with atezolizumab + bevacizumab for ≥6 months. AESI, adverse event of special interest; atezo, atezolizumab; bev, bevacizumab; CR, complete response; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease. aOne patient was assessed to have bevacizumab interruption for the next cycle but withdrew from the study before skipping.
No obvious differences between groups were observed in the distribution of baseline characteristics, apart from the presence of varices (33% [n = 23] in group A-1 versus 22% [n = 31] in group A-2), age ≥65 years (57% [n = 39]) versus 48% [n = 67]), and an ECOG performance status of 0 (72% [n = 50] versus 63% [n = 89]; Table 1). Median age (range) was 66 (40–88) and 63 (29–87) years in groups A-1 and A-2, respectively. Of the patients in group A-1, 75% (n = 52) had Barcelona Clinic Liver Cancer stage C disease, and 77% (n = 53) had Child-Pugh class A5 disease. Among patients in group A-2, 80% (n = 113) had Barcelona Clinic Liver Cancer stage C HCC, and 77% (n = 107) had Child-Pugh class A5 HCC.
Table 1.
Patient demographics by whether bevacizumab was skipped due to bevacizumab AESIs in patients treated with atezolizumab + bevacizumab for ≥6 months
| Group A-1 (n = 69) | Group A-2 (n = 141) | |
|---|---|---|
| Age, median (range), years | 66 (40–88) | 63 (29–87) |
| ≥65 years, n (%) | 39 (57) | 67 (48) |
| Male sex, n (%) | 58 (84) | 116 (82) |
| Geographic region, n (%)a | ||
| Asia, excluding Japan | 28 (41) | 56 (40) |
| Rest of world, including Japanb | 41 (59) | 85 (60) |
| ECOG PS, n (%)a | ||
| 0 | 50 (72) | 89 (63) |
| 1 | 19 (28) | 52 (37) |
| Child-Pugh class, n (%)c | ||
| A5 | 53 (77) | 107 (77) |
| A6 | 16 (23) | 32 (23) |
| BCLC stage, n (%) | ||
| A | 2 (3) | 4 (3) |
| B | 15 (22) | 24 (17) |
| C | 52 (75) | 113 (80) |
| AFP ≥400 ng/mL, n (%)a | 25 (36) | 42 (30) |
| Presence of EHS, MVI, or both, n (%)a | 46 (67) | 106 (75) |
| EHS | 37 (54) | 84 (60) |
| MVI | 26 (38) | 50 (35) |
| Varices, n (%) | ||
| Present | 23 (33) | 31 (22) |
| Treated | 12 (17) | 12 (9) |
| HCC etiology, n (%) | ||
| HBV | 32 (46) | 66 (47) |
| HCV | 18 (26) | 30 (21) |
| Nonviral | 19 (28) | 45 (32) |
| Prior local therapy for HCC, n (%) | 36 (52) | 62 (44) |
AESI, adverse event of special interest; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MVI, macrovascular invasion.
aPer electronic case report form not interactive voice/web response system.
bRest of the world includes the USA, Australia, and Japan.
c n = 139 for group A-2. Data were not available for 2 patients in group A-2.
Efficacy
Main Analysis
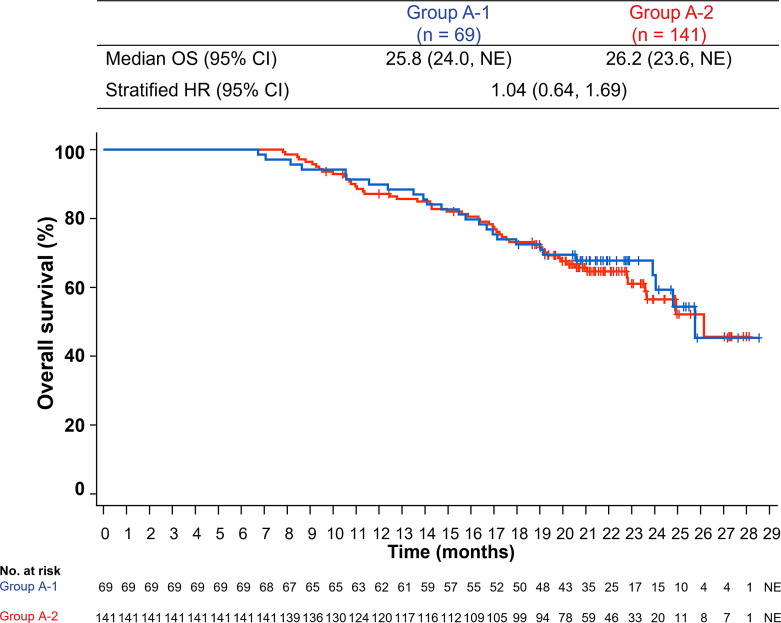
At the data cutoff of August 20, 2020, the median follow-up duration was 21.0 months in group A-1 (interquartile range, 6.7–28.6) and 20.4 months in group A-2 (interquartile range, 7.8–28.1). Among patients who received atezolizumab + bevacizumab for ≥6 months, the HR for OS was 1.04 (95% CI: 0.64, 1.69) (Fig. 2).
Fig. 2.
Kaplan-Meier estimates of OS in patients treated with atezolizumab + bevacizumab for ≥6 months by whether bevacizumab was skipped due to bevacizumab AESIs. AESI, adverse event of special interest; HR, hazard ratio; NE, not estimable; OS, overall survival.
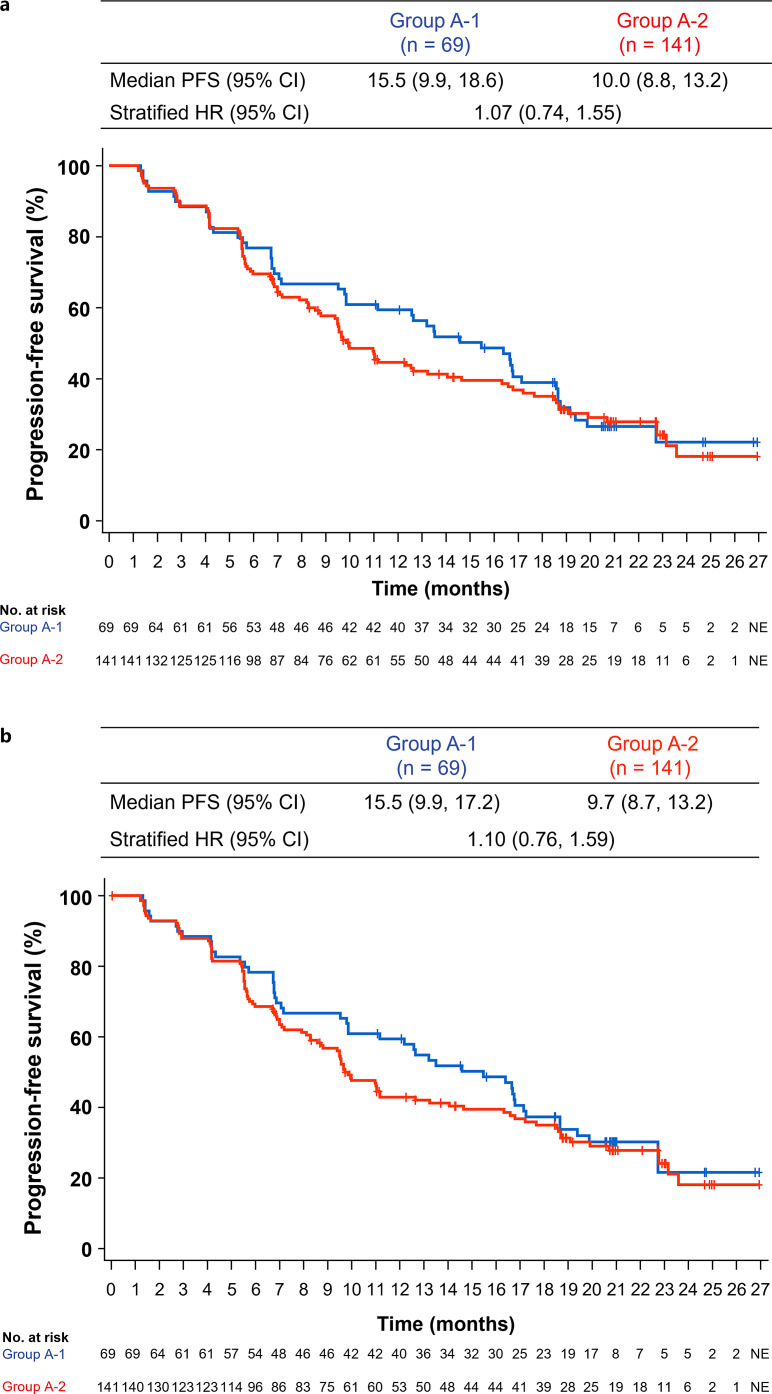
The HR for PFS per IRF-RECIST 1.1 was 1.07 (95% CI: 0.74, 1.55) (Fig. 3a). For PFS per IRF-assessed HCC-modified RECIST, the HR was 1.10 (95% CI: 0.76, 1.59; Fig. 3b). Follow-up systemic therapy was received by 33% of patients (n = 23) in group A-1 versus 33% (n = 47) in group A-2, with 23% (n = 16) versus 30% (n = 42) receiving second-line therapy and 10% (n = 7) versus 10% (n = 14) receiving third-line therapy, respectively (online suppl. Table S1). The most common follow-up regimen was tyrosine kinase inhibitors, which were administered to 29% (n = 20) of patients in group A-1 and 30% (n = 43) in group A-2. Data on follow-up local therapies are presented in online suppl. Table S1.
Fig. 3.
Kaplan-Meier estimates of PFS in patients treated with atezolizumab + bevacizumab for ≥6 months per IRF-RECIST 1.1 (a) or IRF-HCC mRECIST (b) by whether bevacizumab was skipped due to bevacizumab AESIs. AESI, adverse event of special interest; HCC, hepatocellular carcinoma; HR, hazard ratio; IRF, independent review facility; mRECIST, modified RECIST for HCC; RECIST, Response Evaluation Criteria in Solid Tumours; NE, not estimable; PFS, progression-free survival.
Separate Analysis
To investigate the impact of immortal bias in patients who received atezolizumab + bevacizumab for ≥3 months, separate analyses of OS and PFS per RECIST 1.1 were conducted by whether bevacizumab was skipped due to bevacizumab AESIs (online suppl. Fig. S3). OS HR was 1.29 (95% CI: 0.85, 1.95), and PFS HR was 1.26 (95% CI: 0.90, 1.75) in patients who skipped bevacizumab due to bevacizumab AESIs versus those who did not. In time-dependent analyses conducted with the first occurrence of bevacizumab AESIs leading to bevacizumab interruption as a time-dependent variable, HRs for OS and PFS were 0.92 (95% CI: 0.62, 1.36) and 1.11 (95% CI: 0.77, 1.61), respectively.
Safety
The median duration of atezolizumab treatment was 18.2 months (range, 5–28) and 12.9 months (range, 1–27) in groups A-1 and A-2, respectively (Table 2). Bevacizumab was administered for a median duration of 15.3 months (range, 3–28) in group A-1 and 11.8 months (range, 1–27) in group A-2. The median time to bevacizumab interruption due to bevacizumab AESIs was 6.7 months (95% CI: 4.1, 9.3).
Table 2.
Safety summary in patients treated with atezolizumab + bevacizumab for ≥6 months by whether bevacizumab was skipped due to bevacizumab AESIs
| Group A-1 (n = 69)a | Group A-2 (n = 141)a | |
|---|---|---|
| Treatment duration, median (range), months | atezolizumab: 18.2 (5–28); bevacizumab: 15.3 (3–28) | atezolizumab; 12.9 (1–27); bevacizumab: 11.8 (1–27) |
| Patients with ≥1 all-grade AE of any cause, n (%) | 69 (100) | 141 (100) |
| Treatment-related all-grade AE | 66 (96) | 125 (89) |
| Patients with ≥1 grade 3/4 AE, n (%)b | 54 (78) | 87 (62) |
| Treatment-related grade 3/4 AEb | 44 (64) | 57 (40) |
| Serious AE, n (%) | 39 (57) | 72 (51) |
| Treatment-related serious AE | 16 (23) | 34 (24) |
| Grade 5 AE, n (%) | 7 (10) | 7 (5) |
| Treatment-related grade 5 AE | 0 | 1 (1) |
| AE leading to withdrawal from any study treatment, n (%) | 7 (10) | 42 (30) |
| AE leading to withdrawal from atezolizumab | 1 (1) | 20 (14) |
| AE leading to withdrawal from bevacizumab | 7 (10) | 39 (28) |
| AE leading to dose interruption of any study treatment, n (%) | 69 (100) | 82 (58) |
AE, adverse event; AESI, AE of special interest.
aSafety-evaluable population.
bHighest grade experienced.
In both groups, all patients experienced an all-grade adverse event. Any-grade treatment-related adverse events (TRAEs) occurred in 66 patients (96%) in group A-1 and 125 patients (89%) in group A-2 (Table 2). These TRAEs were of grade 3/4 severity in 44 patients (64%) in group A-1 and 57 (40%) in group A-2. A grade 5 TRAE of pneumonia occurred in 1 patient in group A-2.
The most common adverse events with ≥10% incidence in groups A-1 and A-2 were proteinuria (67% vs. 26%), hypertension (54% vs. 39%), and fatigue (33% vs. 23%; Table 3). The major AESIs that led to bevacizumab interruption in group A-1 were proteinuria in 49% of patients (n = 34) and hypertension in 23% (n = 16) (online suppl. Table S2). The most common AESI leading to bevacizumab interruption was hypertension <180 days after treatment and proteinuria throughout the treatment period.
Table 3.
Adverse events occurring in ≥10% of patients in either group who were treated with atezolizumab + bevacizumab for ≥6 months
| Patients with ≥1 adverse event, n (%) | Group A-1 (n = 69) | Group A-2 (n = 141) |
|---|---|---|
| Proteinuria | 46 (67) | 37 (26) |
| Hypertension | 37 (54) | 55 (39) |
| Fatigue | 23 (33) | 33 (23) |
| Pruritus | 21 (30) | 31 (22) |
| Aspartate aminotransferase increased | 20 (29) | 32 (23) |
| Diarrhea | 19 (28) | 34 (24) |
| Decreased appetite | 19 (28) | 20 (14) |
| Platelet count decreased | 19 (28) | 21 (15) |
| Blood bilirubin increased | 15 (22) | 30 (21) |
| Pyrexia | 15 (22) | 29 (21) |
| Constipation | 15 (22) | 22 (16) |
| Alanine aminotransferase increased | 14 (20) | 26 (18) |
| Rash | 14 (20) | 25 (18) |
| Abdominal pain | 14 (20) | 16 (11) |
| Arthralgia | 13 (19) | 18 (13) |
| Anemia | 13 (19) | 16 (11) |
| Cough | 12 (17) | 24 (17) |
| Ascites | 12 (17) | 15 (11) |
| Nausea | 12 (17) | 14 (10) |
| Hypothyroidism | 11 (16) | 23 (16) |
| Weight decreased | 10 (14) | 22 (16) |
| Epistaxis | 10 (14) | 19 (13) |
| Edema peripheral | 10 (14) | 13 (9) |
| Nasopharyngitis | 10 (14) | 11 (8) |
| Back pain | 10 (14) | 11 (8) |
| Blood creatinine increased | 10 (14) | 6 (4) |
| Hypoalbuminemia | 9 (13) | 17 (12) |
| Dysphonia | 9 (13) | 16 (11) |
| Vomiting | 9 (13) | 12 (9) |
| Hyponatremia | 9 (13) | 8 (6) |
| Blood alkaline phosphatase increased | 8 (12) | 15 (11) |
| Thrombocytopenia | 8 (12) | 15 (11) |
| Upper respiratory tract infection | 8 (12) | 13 (9) |
| Dyspnea | 8 (12) | 12 (9) |
| Hyperglycemia | 8 (12) | 9 (6) |
| Headache | 7 (10) | 18 (13) |
| Musculoskeletal pain | 7 (10) | 16 (11) |
| Asthenia | 7 (10) | 12 (9) |
| Abdominal pain upper | 7 (10) | 8 (6) |
| Urinary tract infection | 7 (10) | 8 (6) |
| Infusion-related reaction | 6 (9) | 19 (13) |
| Insomnia | 4 (6) | 19 (13) |
Bevacizumab was withdrawn due to adverse events in 7 patients (10%) in group A-1 and 39 patients (28%) in group A-2. Adverse events leading to bevacizumab withdrawal in group A-1 were upper gastrointestinal hemorrhage (n = 2 [3%]), proteinuria (n = 2 [3%]), colitis (n = 1 [1%]), gastrointestinal hemorrhage (n = 1 [1%]), and venous embolism (n = 1 [1%]).
Discussion
Since its approval in 2020, atezolizumab + bevacizumab has emerged as the global standard of care in the 1L treatment of HCC. Results from this exploratory analysis of the phase III IMbrave150 study indicate that the efficacy and safety of atezolizumab + bevacizumab were similar between patients who skipped bevacizumab due to bevacizumab AESIs and patients who did not. OS and PFS were similar between the two groups (A-1 and A-2), with comparable percentages of patients receiving subsequent systemic and local therapies after atezolizumab + bevacizumab. These findings were consistent with a previous subgroup analysis of Taiwanese patients in the IMbrave150 and GO30140 studies, which showed that bevacizumab interruption did not significantly impact OS or PFS [13]. Grade 3/4 adverse events and any-grade proteinuria, hypertension, and fatigue occurred in more patients in group A-1 than in group A-2; these safety findings are comparable with a previous report of bevacizumab interruption [14].
The most common AESIs leading to bevacizumab interruption were proteinuria and hypertension. The majority of patients restarted bevacizumab treatment after interruption. Retrospective analyses of the relationship between specific risk factors and treatment outcomes may be confounded by immortal time bias due to the misclassification or exclusion of time intervals from the observation period [15]. In this exploratory analysis, landmark analyses of OS and PFS were conducted only in patients who received atezolizumab + bevacizumab for ≥6 months, and only patients who skipped bevacizumab due to bevacizumab AESIs were included in group A-1, in order to minimize immortal time bias. These landmark analyses were performed so that group A-2 excluded patients with early disease progression who did not have sufficient exposure to bevacizumab. The 6-month time point for the landmark analyses was determined based on the median time to onset (2-3 months) of the major AESIs leading to bevacizumab interruption in IMbrave150 (hypertension and proteinuria) [10]. This 6-month landmark was also used in a previous study of bevacizumab interruption in IMbrave150 [13].
To investigate the impact of immortal time bias in patients who received atezolizumab + bevacizumab for ≥3 months, separate analyses were performed to compare OS and PFS by whether bevacizumab was skipped due to AESIs. Compared with the main ≥6-month landmark analysis, the ≥3-month landmark analysis showed similar trends in OS in the group that skipped bevacizumab, whereas there appeared to be a trend toward worsened OS in the group that did not skip bevacizumab. These data potentially suggest that immortal time bias may have had a greater impact on the results of the ≥3-month analysis than on those of the ≥6-month analysis. Additionally, time-dependent analyses, in which the first occurrence of bevacizumab AESIs leading to bevacizumab interruption was included as the time-dependent variable, showed that HRs for OS and PFS were close to 1. These findings were consistent with the main ≥6-month landmark analysis. Overall, these separate analyses further supported the appropriateness and robustness of the ≥6-month landmark analysis to investigate the impact of bevacizumab interruption due to bevacizumab AESIs.
Previous studies evaluating the impact of bevacizumab interruption on OS and PFS with bevacizumab-containing treatment regimens have led to inconsistent conclusions. Two studies in colorectal cancer showed that bevacizumab interruption can potentially accelerate tumor regrowth and revascularization and increase tumor resistance [11, 12]. In a Japanese real-world study of patients with advanced HCC treated with atezolizumab + bevacizumab, early bevacizumab interruption (within the first 9 weeks of treatment) was associated with worse OS and PFS [14]. However, bevacizumab interruption did not have a meaningful impact on OS and PFS in an analysis of Taiwanese patients in IMbrave150 and GO30140 [13], as well as in this analysis. Discrepancies in study design between the previous Japanese real-world study and the current analysis include (1) the inclusion of only systemic treatment-naive patients in this analysis, (2) the focus on early interruption in the real-world analysis, and (3) the evaluation of bevacizumab interruption only due to bevacizumab AESIs in this analysis. Further, the high rate of bevacizumab reintroduction (78%) in this analysis may have contributed to the maintenance of survival outcomes in group A-1, suggesting the importance of reintroducing bevacizumab when safe to do so, after treatment interruption due to AESIs.
In IMbrave150, 58% of patients in the atezolizumab + bevacizumab arm experienced AESIs related to bevacizumab, with 29% of AESIs leading to bevacizumab interruption [8, 9]. In this analysis, the most common AESIs leading to bevacizumab interruption were proteinuria and hypertension. Hypertension and proteinuria are well known adverse drug reactions that are commonly seen with bevacizumab [10] and in phase III trials of bevacizumab combinations across cancer types [16–18]. Of note, hypertension and proteinuria have been shown to be associated with improved OS outcomes and have been proposed as clinical biomarkers of response to bevacizumab [19–21]. Results from this analysis also showed comparable OS and numerically longer PFS in patients who skipped bevacizumab due to bevacizumab AESIs than in those who did not.
Overall, the safety profiles of atezolizumab and bevacizumab were largely similar between patients who skipped bevacizumab due to bevacizumab AESIs and those who did not, but grade 3/4 AEs occurred at a higher incidence in group A-1. This higher incidence of grade 3/4 AEs in group A-1 is expected as these high-grade AEs were likely causes of bevacizumab interruption. Any-grade proteinuria, hypertension, and fatigue also occurred at a higher incidence in group A-1 than in group A-2. These results are consistent with those of a real-world study of early bevacizumab interruption in HCC, where proteinuria and fatigue occurred at a higher incidence in the bevacizumab interruption group [14].
In this analysis, bevacizumab interruption typically occurred after administration of bevacizumab for >6 months. The most common AESIs leading to bevacizumab interruption were hypertension and proteinuria in the first 6 months after treatment and proteinuria throughout the treatment period. In IMbrave150, hypertension events were managed with concomitant mediation, whereas proteinuria events were managed solely with bevacizumab interruption. Hence, the duration of bevacizumab interruption due to hypertension may be shorter than that due to proteinuria; this potentially explains the trends observed. Further management strategies for proteinuria are required in future studies.
Limitations of this study include its exploratory and nonrandomized nature, as well as the small sample size. Additionally, the primary analysis only included patients who skipped bevacizumab due to bevacizumab AESIs in group A-1; therefore, the generalizability of these findings to bevacizumab interruption due to other reasons remains unclear. Despite these limitations, results from this analysis suggest that patients can continue to derive benefit from atezolizumab + bevacizumab even if bevacizumab treatment is interrupted due to AESIs.
Conclusions
Results of this exploratory analysis of IMbrave150 suggest that skipping bevacizumab due to bevacizumab AESIs did not have a considerable impact on the efficacy and safety of atezolizumab + bevacizumab. Although limitations due to the nonrandomized and exploratory nature of this comparison should be acknowledged, the results indicate that patients who skip bevacizumab due to bevacizumab AESIs can continue to derive benefit from atezolizumab + bevacizumab and that bevacizumab was reintroduced in the majority of these patients. These results further support atezolizumab + bevacizumab as the standard-of-care treatment in patients with advanced, unresectable HCC.
Acknowledgments
The authors would like to thank the patients who participated in the trial, the patients’ families, and the investigators and staff at all clinical study sites. Third-party medical writing assistance for this manuscript was provided by Akshaya Srinivasan, PhD, of Nucleus Global, an Inizio Company.
Statement of Ethics
IMbrave150 was carried out in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients gave written informed consent to participate in the study. Protocol approval was obtained from the Institutional Review Board or Ethics Committee at each site. The first Institutional Review Board approval for IMbrave150 was granted on December 19, 2017, from the City of Hope National Medical Center, Duarte, CA, USA (IRB No. 20172734; Western Institutional Review Board, Inc, Puyallup, WA, USA), in addition to multiple other Ethics Committee/Institutional Review Board approvals obtained across all participating sites in the different countries of enrollment. An independent data monitoring committee reviewed unmasked safety and trial conduct data approximately every 6 months until study unblinding. The study sponsor supplied the study drugs and collaborated with academic authors on the study design, data collection, data analysis, and data interpretation.
Conflict of Interest Statement
Masatoshi Kudo reports the following conflicts of interest: honoraria payment to self from Bayer, Chugai Pharmaceutical Co. Ltd., Eli Lilly, Eisai, and Takeda; research funding to institution from AbbVie, Chugai Pharmaceutical Co. Ltd., EA Pharma, Eisai, F. Hoffmann-La Roche Ltd, GE HealthCare, Gilead Sciences, Otsuka, Sumitomo Dainippon Pharma, Taiho, and Takeda; and Editor in Chief of Liver Cancer. Kaoru Tsuchiya reports the following conflicts of interest: advisory/consultancy fees to self from Chugai Pharmaceutical Co. Ltd. and Eisai; speakers bureau participation for Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, and Takeda; and research funding to institution from F. Hoffmann-La Roche Ltd. Yu-Yun Shao reports the following conflicts of interest: honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd, Ipsen, Merck, and Ono. Richard S. Finn reports the following conflicts of interest: consulting fees to self from AstraZeneca, Bayer, CStone Pharmaceuticals, Eisai, Eli Lilly, Exelixis, F. Hoffmann-La Roche Ltd., Hengrui, Merck, and Pfizer; research funding to institution from Adaptimmune, Bristol Myers Squibb, Eisai, Eli Lilly, Merck, Pfizer, and F. Hoffmann-La Roche Ltd.; and Editorial Board Member of Liver Cancer. Peter R. Galle reports the following conflicts of interest: consulting fees to self from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp & Dohme, and Sirtex Medical; honoraria payment to self from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp & Dohme, and Sirtex Medical; advisory fees to self from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp & Dohme, and Sirtex Medical; and research funding to institution from Bayer and F. Hoffmann-La Roche Ltd. Michel Ducreux reports the following conflicts of interest: honoraria, consulting fees, or advisory fees to self from Amgen, AstraZeneca, Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, Merck Serono, Pierre Fabre, and Servier; travel support from Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, Merck Sharp & Dohme, and Servier; speakers bureau participation for Amgen, Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, and Merck Serono; and research funding to institution from Bayer and F. Hoffmann-La Roche Ltd. Ann-Lii Cheng reports the following conflicts of interest: research funding to institution from F. Hoffmann-La Roche Ltd. Tatsuya Yamashita reports the following conflicts of interest: speakers bureau participation for Bayer and Chugai Pharmaceutical Co. Ltd. and research funding to institution from F. Hoffmann-La Roche Ltd. Hironori Koga reports the following conflicts of interest: research funding to institution from AbbVie, Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo, and F. Hoffmann-La Roche Ltd. Ryosuke Take, Kyoko Yamada, Takashi Asakawa, and Yuki Nakagawa reports the following conflicts of interest: employment by Chugai Pharmaceutical Co. Ltd. Masafumi Ikeda reports the following conflicts of interest: honoraria to self from Bayer, Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, and Takeda; advisory/consulting fees to self from Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, Merck Sharp & Dohme, and Takeda; and research funding to institution from Bayer, Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Merck Sharp & Dohme, and Takeda.
Funding Sources
This study was sponsored by F. Hoffmann-La Roche Ltd. Third party medical writing assistance was sponsored by Chugai Pharmaceutical Co. Ltd.
Author Contributions
Masatoshi Kudo contributed to conceptualization, investigation, data curation, writing – review and editing, supervision, and project administration. Kaoru Tsuchiya, Richard S. Finn, and Ann-Lii Cheng contributed to investigation and writing – review and editing. Yu-Yun Shao contributed to conceptualization, methodology, and writing – review and editing. Peter R. Galle contributed to investigation, resources, and writing – review and editing. Michel Ducreux contributed to conceptualization, investigation, and writing – review and editing. Tatsuya Yamashita contributed to resources and writing – review and editing. Hironori Koga contributed to investigation, resources, and writing – review and editing. Ryosuke Take contributed to conceptualization, data curation, writing – original draft, and writing – review and editing. Kyoko Yamada contributed to conceptualization, writing – original draft, writing – review and editing, and supervision. Takashi Asakawa contributed to conceptualization, methodology, formal analysis, writing – original draft, writing – review and editing, and supervision. Yuki Nakagawa contributed to conceptualization, methodology, formal analysis, writing – original draft, and writing – review and editing. Masafumi Ikeda contributed to investigation, writing – review and editing, supervision, and project administration.
Funding Statement
This study was sponsored by F. Hoffmann-La Roche Ltd. Third party medical writing assistance was sponsored by Chugai Pharmaceutical Co. Ltd.
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 3. Tecentriq (atezolizumab) [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2017. [Google Scholar]
- 4. Pharmaceuticals and Medical Devices Agency . Review report (tecentriq) 2020. Available from:https://www.pmda.go.jp/drugs/2020/P20200925002/450045000_23000AMX00014_A100_1.pdf.
- 5. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee MS, Ryoo B-Y, Hsu C-H, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–20. [DOI] [PubMed] [Google Scholar]
- 7. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(pt 2):117–24. [DOI] [PubMed] [Google Scholar]
- 8. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. [DOI] [PubMed] [Google Scholar]
- 9. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. [DOI] [PubMed] [Google Scholar]
- 10. Kudo M, Ikeda M, Zhu AX, Qin S, Kim TY, Lim HY, et al. 169P IMbrave150: management of adverse events of special interest (AESIs) for atezolizumab (atezo) and bevacizumab (bev) in unresectable HCC. Ann Oncol. 2020;31:S1304–5. [Google Scholar]
- 11. Cacheux W, Boisserie T, Staudacher L, Vignaux O, Dousset B, Soubrane O, et al. Reversible tumor growth acceleration following bevacizumab interruption in metastatic colorectal cancer patients scheduled for surgery. Ann Oncol. 2008;19(9):1659–61. [DOI] [PubMed] [Google Scholar]
- 12. Becherirat S, Valamanesh F, Karimi M, Faussat AM, Launay JM, Pimpie C, et al. Discontinuous schedule of bevacizumab in colorectal cancer induces accelerated tumor growth and phenotypic changes. Transl Oncol. 2018;11(2):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shao Y-Y, Feng Y-H, Yen C-J, Yang T-S, Shen Y-C, Chao Y, et al. Bevacizumab and atezolizumab as first-line therapy for advanced hepatocellular carcinoma: a Taiwanese subgroup analysis on efficacy and safety. J Formos Med Assoc. 2022;121(12):2430–7. [DOI] [PubMed] [Google Scholar]
- 14. Hatanaka T, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, et al. Association of early bevacizumab interruption with efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma: a landmark analysis. Hepatol Res. 2022;52(5):462–70. [DOI] [PubMed] [Google Scholar]
- 15. Yadav K, Lewis RJ. Immortal time bias in observational studies. JAMA. 2021;325(7):686–7. [DOI] [PubMed] [Google Scholar]
- 16. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34. [DOI] [PubMed] [Google Scholar]
- 17. Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, et al. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–55. [DOI] [PubMed] [Google Scholar]
- 18. Niizeki T, Tokunaga T, Takami Y, Wada Y, Harada M, Shibata M, et al. Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a propensity score matching analysis. Target Oncol. 2022;17(6):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28(6):949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moisuc DC, Marinca MV, Gafton B, Alexa-Stratulat T, Pavel-Tanasa M, Cianga P. Antiangiogenic drug-induced proteinuria as a prognostic factor in metastatic colorectal cancer. Curr Oncol. 2022;29(6):3996–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimose S, Iwamoto H, Tanaka M, Niizeki T, Kajiwara M, Itano S, et al. Association between adverse events and prognosis in patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Cancers. 2022;14(17):4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification. Further inquiries can be directed to the corresponding author.