Abstract
Background:
Baricitinib, a Janus Kinase (JAK) inhibitor, has emerged as a potential therapeutic option for systemic lupus erythematosus (SLE). This systematic review aims to synthesize evidence from randomized controlled trials (RCTs) evaluating the potential of baricitinib in treating SLE.
Methods:
A systematic search was conducted across electronic databases to identify relevant RCTs assessing baricitinib in patients with SLE. Studies reporting outcomes such as the Systemic Lupus Erythematosus Responder Index-4 (SRI-4), adverse events, and safety profiles were included. Data extraction and quality assessment were performed following PRISMA guidelines.
Results:
A total of four studies were evaluated for efficacy and safety of baricitinib therapy. Three studies reported SRI-4, British Isles Lupus Assessment Group (BILAG), and Systemic Lupus Erythematosus Disease Activity Index-2000 (SLEDAI-2K), except for Dorner and colleagues Only Dorner and colleagues and Wallace and colleagues discuss the anti-dsDNA titres following treatment with baricitinib. The findings consistently demonstrated improved efficacy of baricitinib compared to placebo, particularly in terms of SRI-4 scores. Higher dosages of baricitinib showed significant improvement in disease activity and severity indices. Adverse events, including infections and gastrointestinal disturbances, were reported.
Conclusion:
Baricitinib holds promise for treating SLE, but caution is needed due to potential adverse events. Careful patient selection and monitoring are crucial. Future research should prioritize long-term safety and comparative effectiveness studies to better understand baricitinib’s role in managing SLE.
Keywords: baricitinib, systemic lupus erythematosus, Janus Kinase inhibitor, randomized controlled trial, efficacy, safety
Introduction
Highlights
A systematic search was conducted across electronic databases to identify relevant randomized controlled trials (RCTs) assessing baricitinib in patients with systemic lupus erythematosus (SLE).
Studies reporting outcomes such as the Systemic Lupus Erythematosus Responder Index-4 (SRI-4), adverse events, and safety profiles were included.
Four RCTs met the inclusion criteria and were included in the analysis. The findings consistently demonstrated improved efficacy of baricitinib compared to placebo, particularly in terms of SRI-4 scores. Higher dosages of baricitinib showed significant improvement in disease activity and severity indices.
Baricitinib shows promise as a therapeutic option for patients with SLE, with significant improvements in disease activity observed in the included studies.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease affecting multiple body systems. It is characterized by systemic inflammation and widespread immune dysregulation, producing autoantibodies1. The pathogenesis of SLE is believed to involve abnormalities in both the innate and adaptive arms of the immune system, which are interconnected by a positive feedback loop. Several cytokines, including IFNs, IL-6, IL-12, IL-17, IL-23, TNF, and B cell activating factors, have been implicated in developing SLE2,3.
One of the critical features of SLE is the presence of high serological activity, including antibodies against double-stranded DNA (anti-dsDNA). These antibodies have been linked to multiple end-organ injuries in SLE4. The Janus kinase (JAK) family of intracellular, non-receptor tyrosine kinases is associated with many critical cytokines that are implicated in the immune dysregulation seen in SLE5. Baricitinib is a selective and reversible JAK1/JAK2 inhibitor that is administered orally and has been approved for the treatment of moderate-to-severe active rheumatoid arthritis in adults in over 75 countries, including the USA, Japan, and countries in the European Union6.
Baricitinib inhibits JAK1/JAK2, which may impact the release of pro-inflammatory cytokines, such as type I IFNs, IFN-γ, IL-6, IL-12, and IL-235. This systematic review aims to summarize all clinical trials that have studied the efficacy and safety of baricitinib in the treatment of SLE.
Several randomized controlled trials have examined different treatments for SLE. However, none have directly compared the efficacy and safety of baricitinib doses. To address this gap, we conducted a systematic review by pooling data from previous RCTs. We aimed to compare the effectiveness and safety profiles of baricitinib at 2 and 4 mg doses, along with placebo, in the treatment of individuals with SLE.
Materials and methods
This systematic review and meta-analysis fully comply with the preferred reporting items for the systematic review and meta-analysis (PRISMA) 2020 statement, with its protocol registered in PROSPERO CRD42023443627. The work has been reported in line with the STROCSS criteria7. The work has been reported in line with AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines.
Database and literature search strategy
We performed a systematic review of the PubMed and Google Scholar, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials (The Cochrane Library) databases from inception to 1 January 2023, to find articles providing information on the efficacy and safety of baricitinib in patients with SLE. The following keywords were used: Systemic lupus erythematosus OR SLE, Janus kinase OR JAK Inhibitors OR Baricitinib, Randomized Controlled Trials OR RCT. The search results were not filtered or otherwise limited. Non-English language content was translated using Google’s translate tool. Two authors (F.A., Z.H.) independently screened the databases and the trial registries and extracted relevant data. The Quality assessment was done by two authors (Z.A., T.H.). Disagreements and Discrepancies about the relevance of the sources were resolved by mutual consensus from the author (R.W.).
Selection procedure and eligibility criteria
To be considered, studies had to meet the following criteria:
They needed to be either randomized controlled trials (RCTs) or observational studies.
The studies must have included adult patients over the age of 18 who had been diagnosed with systemic lupus erythematosus.
The effectiveness of baricitinib had to be evaluated.
The exclusion criteria were as follows:
Trials that included only children patients with SLE were excluded.
Reviews, letters, editorial comments, author responses, case reports, and reports that mainly focused on laboratory findings were also excluded.
Studies that had no usable or partial data reported were also excluded.
In case of any disagreement over the inclusion of a particular study, a third author was contacted to settle the issue.
Data extraction
Two reviewers independently examined and evaluated the studies. The following data were collected: study characteristics (e.g. author, year, study design, and treatment duration); patient characteristics (e.g. population, sex, age, and sample size); Outcomes and side effects.
Quality assessment
The quality of the included RCTs will be assessed independently by two authors using RevMan 5.4.1 software, in accordance with the Cochrane Handbook and Risk Of Bias (RoB) tool8. The risk of bias assessment will include five items: (1) adequate sequence generation; (2) allocation concealment, (3) incomplete outcome data; (4) free of selective reporting; and (5) free of other biases. The judgments will be categorized as “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (unclear risk of bias). All analyses will be based on previously published studies, and therefore no ethical approval or patient consent will be required.
Data synthesis
Employing meta-analytic methods, we aimed to quantify the combined effects of studies for key outcomes of interest. However, we opted to prioritize a systematic review approach over a meta-analysis due to significant variations in treatment durations and methodologies across the included studies. We analyzed treatment conditions, such as varied doses and treatment durations, to ascertain whether they led to an increase, reduction, or no change in the outcomes.
Results
Summary of literature search and screening process
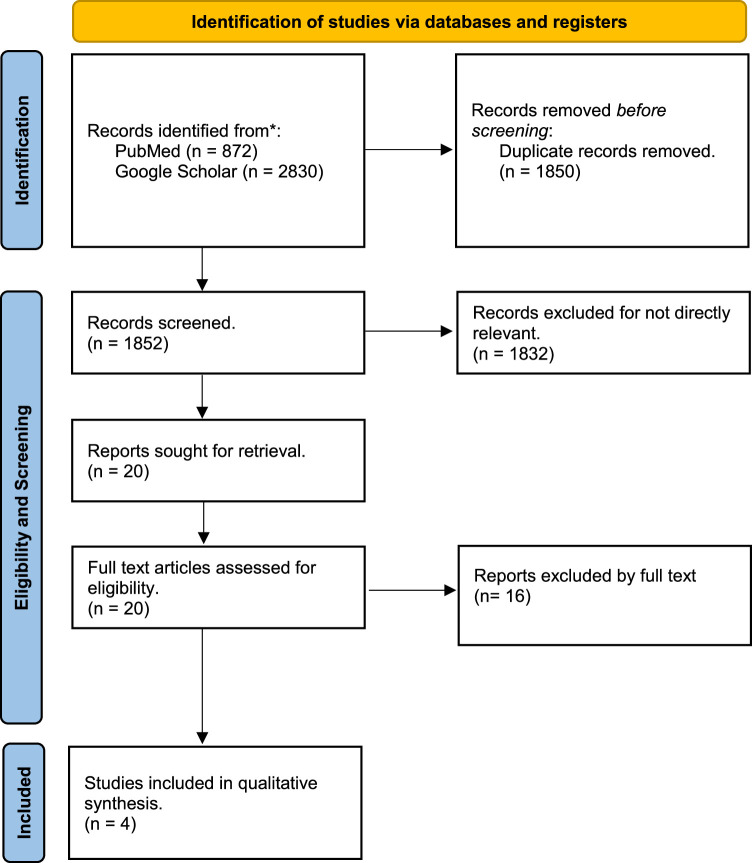
The PRISMA flow diagram in Fig. 1 illustrates the process of searching and screening literature. The initial and subsequent searches of all electronic databases yielded 3702 records, out of which 1850 duplicates were removed. After going through the eligibility and screening process, 1832 records that were not directly relevant were excluded. We obtained and reviewed the full-text versions of 20 papers, out of which only four satisfied the inclusion criteria. Therefore, only four publications were included in the systematic review9–12.
Figure 1.
Preferred reporting items for the systematic review and meta-analysis (PRISMA) flowchart of the systematic review process.
General characteristics of included studies
The study characteristics are succinctly reported in Table 1. All four included studies were randomized control trials, of which one was a phase II trial, and two were phase III trials. The trials had three comparators: patients receiving a placebo or 2 or 4 mg of baricitinib. All results mentioned henceforth will compare the outcomes between the two dosages of baricitinib and placebo by assessing the change from baseline data. Patients with active lupus nephritis and CNS lupus were excluded from all the studies. The duration of treatment was 24 weeks in two studies and 52 weeks in the remaining two. Included patients were aged 18 or above unanimously in all studies; hence, conclusions pertaining to a single age group could not be extrapolated from this data. All included patients tested positive for either antinuclear antibody (ANA), anti-dsDNA antibody, or anti-smith antibody.
Table 1.
Study characteristics of the included articles.
| References | Design | Patient characteristics | Inclusion criteria | Exclusion criteria | Treatment duration | Outcomes | Side effects |
|---|---|---|---|---|---|---|---|
| Wallace et al.9 | RCT | Total: 314 (105 Placebo, 105 Baricitinib 2 mg, 104 Baricitinib 4 mg) | 1. Age ≥18 years 2. SLE diagnosis ≥24 weeks prior 3. Meeting ACR or ≥4 SLICC criteria 4. Positive ANA or anti-dsDNA 5. Active arthritis/rash (SLEDAI-2K) 6. ANA (≥1:80) and/or anti-dsDNA (≥30 IU/ml) 7. SLEDAI-2K score ≥4 |
1. Severe lupus nephritis or CNS lupus 2. Recent serious infection 3. Severe systemic disorders 4. High-dose corticosteroids (>20 mg/day prednisone) or recent dose change 5. Recent NSAID dose adjustment 6. Recent use or dose change of anti-malarials, immunosuppressants, or cytotoxic agents |
24 weeks | Primary outcome: resolution of rash or arthritis (SLEDAI-2k) at 24 weeks. Secondary outcomes: changes in SLEDAI-2K score and PGA at week 24. |
One deep-vein thrombosis (baricitinib 4 mg), one serious infection, and one herpes zoster case (placebo and baricitinib 4 mg). No deaths, malignancies, or major cardiovascular events. |
| Dorner et al.10 | RCT | Total: 274 (90 Placebo, 92 Baricitinib 2 mg, 92 baricitinib 4 mg) | 1. Age ≥18 years, diagnosed with SLE 2. Positive ANA or anti-dsDNA 3. SLEDAI-2k score ≥4 4.Arthritis or rash per SLEDAI-2k |
1. Active severe lupus nephritis 2. Active CNS lupus |
24 weeks | Primary outcome: resolution of rash or arthritis (SLEDAI-2k) at 24 weeks. Secondary outcomes: changes in SRI-4 and others. |
Not Specified |
| Morand et al.11 | RCT | Total: 760 (253 Placebo, 255 Baricitinib 2mg, 252 Baricitinib 4 mg) | 1. Age ≥18 years 2. SLE diagnosis ≥24 weeks prior, meeting ≥4 of 11 ACR criteria 3. Positive ANA, anti-dsDNA, or anti-smith antibody 4. Active disease: SLEDAI-2k ≥6, with ≥1 BILAG A or 2 BILAG B scores at screening despite SOC medication |
1. Severe active lupus nephritis 2. Severe active CNS lupus 3. Treated or occurrence of any systemic inflammatory condition other than SLE |
52 weeks | Primary Outcome: Achieving an SLE Responder Index (SRI)-4 response at week 52, with criteria such as ≥4-point improvement in SLEDAI-2K score, no worsening in Physician Global Assessment (increase ≤3 points [10 mm]), and no new BILAG A or >1 new BILAG B. Secondary outcomes: SRI-4 response at weeks 24 and 52, reaching lupus low disease activity state (LLDAS) at week 52, maintaining prednisone ≤7.5 mg/day between weeks 40–52, time to first severe flare, changes in Worst Pain Numeric Rating Scale (NRS), and changes in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score at week 52. |
Most events were mild or moderate in severity. Serious adverse events were observed in 10% of participants in the baricitinib 4 mg group, 9% in the baricitinib 2 mg group, and 7% in the placebo group. More serious infections were reported in patients receiving baricitinib 4 mg (3% participants) and baricitinib 2 mg (4% participants) compared with placebo (1% participants). No deaths were reported in the baricitinib 4 mg group, one in the baricitinib 2 mg group (due to COVID-19), and one in the placebo group. |
| Petri et al.12 | RCT | Not specified | 1. Age ≥18 years 2. SLE diagnosis ≥24 weeks 3. Meeting ≥4 of 11 revised ACR criteria 4. Positive for ANA, anti-dsDNA, or anti-smith antibody 5. SLEDAI-2k score ≥6 at screening, ≥4 at baseline 6. ≥1 BILAG A or 2 BILAG B scores |
1. Severe active lupus nephritis 2. Severe active CNS lupus 3. Systemic active inflammatory disease other than SLE |
52 weeks | Primary outcome: Proportion of participants achieving SRI-4 response at 52 weeks with baricitinib 4 mg plus SOC compared to placebo plus SOC. Secondary outcome: Proportion of patients achieving SRI-4 response at 24 weeks, lupus low disease activity state response at 52 weeks, reduction in prednisone dose to ≤7.5 mg/day between weeks 40–52, time to first severe flare over 52 weeks, change in worst pain NRS at week 52, change in FACIT-Fatigue score at week 52, and proportion of patients achieving SRI-4 response at 52 weeks with baricitinib 2 mg plus SOC. |
Tuberculosis, herpes zoster, opportunistic infections, malignancies, hepatic events, MACE and VTE |
BILAG, British Isles Lupus Assessment Group; ACR, Urine albumin to creatinine ratio; CNS, central nervous system; MACE, Major adverse cardiovascular events; RCT, randomized controlled trial; SLICC, Systemic Lupus International Collaborating Clinics; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000; SRI-4, Systemic Lupus Erythematosus Responder Index-4; VTE, Venous thromboembolism.
Risk of bias of included studies
The summary of the risk of bias in studies included in our systematic review is shown in Fig. 2. Figure 3 shows the risk of bias summary of included studies.
Figure 2.

Risk of bias graph of included studies.
Figure 3.
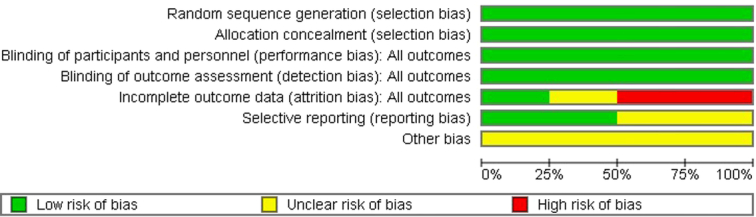
Risk of bias summary of included studies.
Summary of outcomes in included studies
Included studies reported the efficacy of baricitinib in treating patients with established SLE. The treatment duration does not differ in each study, facilitating the generalizability of the outcomes.
Systemic lupus erythematosus responder index-4 (SRI-4)
Three studies (Wallace and colleagues, Petri and colleagues, Moran and colleagues) reported this outcome directly, while Dorner and colleagues concluded anti-dsDNA titres post-treatment. Wallace and colleagues reported the outcome as an odds ratio for each dosage of baricitinib compared to placebo. An overall increase in response at 24 weeks was noted, with an odds ratio of 1.3 when 2 mg of baricitinib was administered compared to 2.0 with 4 mg; the response was significant with the higher dosage (p value of 0.0151 vs. 0.44). Morand and colleagues followed a similar trend at 52 weeks, with a hazard ratio of 1.14 with 2 mg and 1.57 with 4 mg of baricitinib. The response was significant for 4 mg of the drug (p value= 0.016 vs. 0.47). Treatment was administered for 52 weeks in the study conducted by Petri and colleagues; however, the response did not show significant variations between the two dosages (hazard ratio of 1.05 in 2 mg vs. 1.07 in 4 mg). Dorner and colleagues demonstrated an insignificant difference between the two dosages and placebo in lieu of the response on anti-dsDNA titres.
Systemic lupus erythematosus disease activity index-2000 (SLEDAI-2K)
This outcome was reported by three studies, with the exception being Dorner and colleagues, and was reported as the least square (LS) mean change from baseline. Wallace and colleagues reported a decrease in the outcome in all three groups, with the greatest decrease observed in the highest dosage group (−4.4, compared to −4.1 and −3.8 in the 2 mg and placebo groups, respectively). The differences were not, however, significant. Morand and colleagues followed a similar receding pattern at 52 weeks; nonetheless, the change observed in the 4 mg group was determined to be significant (p value=0.014). A slight discordance was observed in the results reported by Petri and colleagues, where a greater decrease (4.14) in the placebo group was noted in comparison to the 2 mg group (4.12). Nonetheless, these values were non-significant, with a p value of 0.45. Patients administered 4 mg of the drug showed the greatest response of −4.46; this value was also non-significant.
British isles lupus assessment group (BILAG)
The outcome under discussion was not reported by Dorner and colleagues. The BILAG score was assessed under the criteria that there is no new BILAG A disease activity and not more than one new disease activity falling under BILAG B (the disease status is not deteriorating). Wallace and colleagues and Morand and colleagues reported results favoring the intervention groups, with odds ratios of greater than 1 in each study. Petri and colleagues, however, gave conflicting results, with odds ratios of 0.92 in the 2 mg group and 0.82 in the 4 mg group. The results in all the studies were non-significant, with a p value greater than 0.05. The greatest response was evaluated by Morand and colleagues with odds ratios of 1.23 and 1.49 in the two drug dosages, respectively.
Cutaneous lupus erythematosus disease area and severity index (CLASI) activity score
CLASI activity score was reported as LS mean change from baseline by Wallace and colleagues, while odds ratios signifying greater than 50% reduction in the score by Morand and colleagues and Petri and colleagues. It was not assessed by Dorner and colleagues. Wallace et al. illustrated an overall reduction in all three groups; however, the greatest reduction at 24 weeks was observed in the placebo group (2.8 compared to 1.7 in the 2 mg and 2.3 in the 4 mg groups). This change was also significantly more compared to the 2 mg dose of Baricitinib (p=0.0371) while being non-significant compared to the greater drug dosage. The results reported by Petri and colleagues at 52 weeks were in symmetry with those by Wallace and colleagues, with the odds ratios being reported as 0.69 and 0.78 in the lower and higher drug dosages, respectively. The results were not significant. Morand and colleagues published results that were incongruent with those published by the other two studies. Odds ratios of 1.02 in patients receiving 2 mg of the drug and 1.22 in the patients receiving 4 mg of the drug were reported here. The results were, nonetheless, non-significant. A proper conclusion of the CLASI score activity could not be extrapolated from these results.
Anti-dsDNA titres
While all four included studies have reported baseline titres for this crucial antibody, only Dorner and colleagues and Wallace and colleagues discuss the altering dynamics following treatment with baricitinib. Dorner and colleagues reported the changing levels using LS mean change at 4, 12, and 24 weeks, with the greatest dip being observed at 12 weeks, followed by a paradoxical increase in the level of the titres. The change from baseline at 12 weeks was 17.1 IU/ml in the placebo group, −14.6 IU/ml in the 2 mg dose group, and -24.6 IU/ml in the 4 mg dose group. At 24 weeks, the change is 62.0 IU/ml in the placebo group, −6.9 IU/ml in the 2 mg group, and 93 IU/ml in the 4 mg group. According to Wallace and colleagues, the LS mean change in the placebo group was 55.4 IU/ml, 1.0 IU/ml in the 2 mg group, and 48.5 IU/ml in the 4 mg group. The comparison with placebo was non-significant at both dosages of baricitinib.
Discussion
Baricitinib, an inhibitor of JAK, has garnered attention as a potential therapeutic avenue for various autoimmune conditions, including SLE13. Our systematic review synthesized evidence from four RCTs assessing the efficacy of baricitinib in treating SLE. The included studies consistently demonstrated a trend towards improved efficacy of baricitinib compared to placebo, particularly regarding the Systemic Lupus Erythematosus Responder Index-4 (SRI-4). Higher dosages of baricitinib showed significant improvement compared to lower dosages and placebo, with reductions observed in disease activity and severity indices, although not always statistically significant.
While the efficacy of baricitinib appears promising, it is crucial to consider its safety profile. Adverse events reported in the included studies varied, with common adverse events including infections, gastrointestinal disturbances, and laboratory abnormalities such as neutropenia and liver function test elevations. Although the overall incidence of adverse events was manageable, clinicians should exercise caution when prescribing baricitinib, particularly in patients with underlying comorbidities or risk factors.
The safety profile of baricitinib also warrants attention regarding long-term use and potential risks of serious adverse events, including opportunistic infections and thromboembolic events14. While the included studies provided valuable insights into the short-term safety profile of baricitinib, further research is needed to assess its long-term safety and tolerability, especially in real-world clinical settings.
Despite the promising efficacy and manageable safety profile observed in the included studies, several limitations should be acknowledged. These include the limited duration of follow-up in some trials, heterogeneity in patient populations and study designs, and potential biases inherent in the reporting of adverse events. Furthermore, the generalizability of findings may be limited by the inclusion criteria and patient characteristics of the included studies.
Moving forward, future research efforts should focus on addressing these limitations and expanding our understanding of baricitinib’s efficacy and safety in managing SLE. Long-term observational studies and real-world evidence analyses are needed to assess the durability of treatment response and evaluate the risk-benefit profile of baricitinib in diverse patient populations15. Additionally, comparative effectiveness studies against other biological or targeted therapies may provide valuable insights into the positioning of baricitinib within the treatment landscape of SLE16.
These findings hold significant implications for clinical practice, suggesting that baricitinib, particularly at higher dosages, may offer a valuable therapeutic option for patients with SLE. The observed improvements in disease activity and severity indices indicate the potential for baricitinib to alleviate symptoms and enhance the quality of life for individuals living with SLE. Clinicians may consider incorporating baricitinib into treatment regimens, especially for patients with refractory disease or those intolerant to conventional therapies.
Conclusion
In summary, this systematic review highlights the potential efficacy of baricitinib in treating SLE, particularly in improving disease activity indices. While our findings suggest promising results, caution is warranted due to potential adverse events and limitations in study designs. Moving forward, further research is needed to confirm these findings, address safety concerns, and optimize treatment strategies for SLE patients. Despite these limitations, this review contributes valuable insights to the field and underscores the importance of continued investigation into baricitinib’s role in SLE management.
Ethical approval
This paper did not involve patients; therefore, no ethical approval was required.
Consent
This paper did not involve patients; therefore, no consent was required.
Source of funding
No funding was acquired for this paper.
Author contribution
H.H.S.: conceptualization; data curation; formal analysis; methodology; writing—original draft. F.A.: data curation; investigation; methodology; resources; software; writing—original draft. Z.H.: data curation; formal analysis; methodology; software; validation; writing—original draft. R.W.: methodology; investigation; resources; writing—review and editing. S.A.R.: methodology; investigation; resources; writing—review and editing. T.H.: methodology; investigation; resources; writing—review and editing. Z.A.: methodology; investigation; resources; writing—review and editing. S.A.Z.: methodology; investigation; resources; writing—review and editing. M.S.H.: methodology; investigation; resources; writing—review and editing. M.A.W.Z.: methodology; investigation; resources; writing—review and editing. M.A.H.: data curation; formal analysis; methodology; project administration; resources; visualization; writing—review and editing.
Conflicts of interest disclosure
The authors declare that there is no conflict of interest.
Research registration unique identifying number (UIN)
Name of the registry: Not applicable.
Unique Identifying number or registration ID: Not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
This systematic review and meta-analysis fully comply with the preferred reporting items for the systematic review and meta-analysis (PRISMA) 2020 statement [8], with its protocol registered in PROSPERO CRD42023443627.
Guarantor
Hussain Haider Shah.
Data availability statement
The data presented in this study are available within the article and its Supplementary Materials.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 21 June 2024
Contributor Information
Hussain Haider Shah, Email: hussainhydershah03@gmail.com.
Faiza Ashfaque, Email: Faizaaay75@gmail.com.
Zeenat Hadi, Email: zeenathadi99@gmail.com.
Radeyah Waseem, Email: radeyahwaseem@hotmail.com.
Sameer Abdul Rauf, Email: sameerrauf80@gmail.com.
Tooba Hussain, Email: toobahussain92@gmail.com.
Zahra Anas, Email: zahraanas179@gmail.com.
Syeda Alishah Zehra, Email: aiyakami123@gmail.com.
Muhammad Sheheryar Hussain, Email: baig2001@outlook.com.
Muhammad Abdul Wasay Zuberi, Email: abdulwasayasad@gmail.com.
Md Ariful Haque, Email: arifulhaque58@gmail.com.
References
- 1.Tsokos GC, Lo MS, Costa Reis P, et al. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 2016;12:716–730. [DOI] [PubMed] [Google Scholar]
- 2.Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013;382:819–831. [DOI] [PubMed] [Google Scholar]
- 3.Larosa M, Zen M, Gatto M, et al. IL-12 and IL-23/Th17 axis in systemic lupus erythematosus. Exp Biol Med (Maywood) 2019;244:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Xia Y. Anti-double stranded DNA antibodies: origin, pathogenicity, and targeted therapies. Front Immunol 2019;10:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017;16:843–862. Erratum in: Nat Rev Drug Discov. 2017;17:78. [DOI] [PubMed] [Google Scholar]
- 6.Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 2010;184:5298–5307. [DOI] [PubMed] [Google Scholar]
- 7.Mathew G, Agha R.for the STROCSS Group . STROCSS 2021: Strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:222–231; Erratum in: Lancet. 2018 Aug 11;392(10146):476. [DOI] [PubMed] [Google Scholar]
- 10.Dörner T, van Vollenhoven RF, Doria A, et al. Baricitinib decreases anti-dsDNA in patients with systemic lupus erythematosus: results from a phase II double-blind, randomized, placebo-controlled trial. Arthritis Res Ther 2022;24:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morand EF, Vital EM, Petri M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet 2023;401:1001–1010. [DOI] [PubMed] [Google Scholar]
- 12.Petri M, Bruce IN, Dörner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet 2023;401:1011–1019. [DOI] [PubMed] [Google Scholar]
- 13.Nikolopoulos D, Parodis I. Janus kinase inhibitors in systemic lupus erythematosus: implications for tyrosine kinase 2 inhibition. Front Med 2023;10:1217147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis 2022;81:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieber T, Feist E, Irvine AD, et al. A review of safety outcomes from clinical trials of baricitinib in rheumatology, dermatology and COVID-19. Adv Ther 2022;39:4910–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trøseid M, Arribas JR, Assoumou L, et al. EU SolidAct study group . Efficacy and safety of baricitinib in hospitalized adults with severe or critical COVID-19 (Bari-SolidAct): a randomised, double-blind, placebo-controlled phase 3 trial. Crit Care (London, England) 2023;27:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within the article and its Supplementary Materials.