Abstract
Background and objective:
Abdominal surgery stands as one of the most frequently conducted procedures across surgical specialties, accounting for up to half of surgery-related expenses. Hemodynamic instability emerges as a significant concern during anaesthesia and surgery, provoked by the stress of intubation, surgical incision, and anaesthetic agents. Following abdominal surgery, pain is an inevitable consequence, typically managed with opioid-based analgesia. However, the adverse effects associated with opioids often overshadow their analgesic benefits, particularly in the context of abdominal surgery. Consequently, there exists a necessity to explore and assess alternative non-opioid pain management options post-abdominal surgery as part of a broader strategy to reduce opioid usage. The primary aim of this investigation is to assess the effectiveness of varying doses of dexmedetomidine in regulating intraoperative hemodynamics and alleviating postoperative pain in patients undergoing abdominal surgery.
Methods:
Ethical clearance and institutional review board were obtained from the ethical clearance committee of Dilla University College of Medicine and Health Sciences with protocol unique number of duirb/008/22-01. Our trial has been prospectively registered on the Pan African Clinical Trial Registry with a unique identification number for the registry PACTR202208813896934. Statistical package and analysis were performed by using SPSS version 25. The distribution of data was checked by using Shapiro–Wilk test and the homogeneity of variance was checked by Levene’s test. Analysis of variance (ANOVA) and Kruskal–Wallis H test were used for normally distributed continuous data and non-normally distributed or non-parametric data, respectively. P value less than 0.05 with a power of 90% was considered statistically significant.
Result:
There was a statistically significant increase in mean SBP in the control group at the different critical time points (P<0.05), as compared to the baseline value, while there was no significant difference in mean systolic blood pressure (SBP) between the baseline and all other levels for group 2 and group 3. A statistically significant increase in mean arterial pressure (MAP) was detected in the control group at immediately after intubation (P=0.009) as compared to the baseline value, while a statistically significant reduction in mean heart rate (HR) was observed in group 3 at 15th min after infusion and at 30th 30 min after induction compared to baseline with a P value of 0.002 and 0.008, respectively.
Conclusion:
Perioperative low-dose infusion of dexmedetomidine at the rate of 0.4 mcg/kg/h is a useful anaesthesia adjuvant to control hemodynamic stress response to critical periods. It is wise to use this infusion dose as part of general anaesthesia to achieve better hemodynamic stability.
Keywords: Anaesthesia, dexmedetomidine, Ethiopia, haemodynamic, perioperative, surgery
Background
Highlights
There was a statistically significant increase in mean systolic blood pressure (SBP) in the control group at the different critical time points.
Significant increase in mean arterial pressure (MAP) was detected in the control group at immediately after intubation as compared to the baseline.
There was no statistically significant difference between the groups of the study in terms of postoperative adverse reactions.
Abdominal surgery ranks among the most prevalent procedures within surgical specialties, accounting for a substantial portion, up to 50%, of expenses related to surgery1. Open surgery, characterized by extensive tissue trauma and sympathetic activation induced by the surgical stress response, is typically associated with such procedures. Furthermore, postoperative pain emerges as the predominant concern following open surgery, affecting over 80% of patients with varying degrees of severity2,3. This pain often becomes the leading cause of delayed discharge for patients undergoing these procedures2.
Patients undergoing surgery under general anaesthesia commonly experience adverse effects such as coughing, agitation, pain, hypertension, bradycardia, or tachycardia. The occurrence of coughing can be as high as 82.5%4. Notably, coughing often peaks during extubation, posing discomfort to patients and potentially leading to complications such as hypertension, tachycardia, myocardial ischaemia, and laryngospasm. Various methods and medications have been employed to mitigate or diminish coughing episodes during general anaesthesia4,5. Ensuring patient comfort and safety during general anaesthesia necessitates the effective utilization of sedative-hypnotic and analgesic agents.
Hemodynamic instability poses a significant challenge during anaesthesia and surgery, primarily stemming from the stress induced by intubation, surgical incision, and the administration of anaesthetic medications. This complication is particularly prominent among patients with cardiovascular disorders and older individuals6. Research indicates that ~23.0% of patients experience hemodynamic fluctuations following the induction of general anaesthesia6. Furthermore, another study reveals that the prevalence of hypotension (systolic blood pressure<80 mmHg for more than 5 min) and hypertension (systolic blood pressure>160 mmHg for more than 5 min) stands at 26% and 20%, respectively6,7.
Effective management of perioperative and postoperative pain, maintenance of hemodynamic stability, proper sedation, and successful awakening constitute critical aspects of anaesthetic management. However, conventional sedative and analgesic medications come with potential adverse effects, such as respiratory depression, which can delay extubation. Additionally, they may contribute to significant tachyphylaxis and tolerance, impede gut motility, and prolong the need for intensive care and hospitalization8.
Pain is inevitable following abdominal surgery, and opioid-based postoperative analgesia is frequently employed to manage it. However, the adverse effects associated with opioids often overshadow their analgesic benefits, particularly in the context of abdominal surgery. Consequently, it has been advised to reserve opioid use for cases where non-opioid medications fail to provide adequate pain relief9,10. Therefore, there is a pressing need to explore and assess alternative non-opioid pain medications following abdominal surgery as part of a strategy aimed at reducing opioid usage.
Dexmedetomidine is a highly selective α2-adrenergic agonist known for its sedative, anxiolytic, hypnotic, analgesic, and sympatholytic properties11–15. It exhibits anxiolytic and moderate analgesic effects while causing minimal respiratory depression, even at higher doses of 2 µg/kg16,17. Continuous intravenous administration of dexmedetomidine during abdominal surgery has been shown to effectively reduce postoperative morphine requirements and provide analgesia without increasing side effects18,19. Additionally, its sympatholytic and antinociceptive effects contribute to maintaining hemodynamic stability during surgical stimulation20.
Studies have demonstrated that dexmedetomidine administration during or at the end of surgery can mitigate stress and cough responses, decrease postoperative pain, and reduce postoperative nausea and vomiting (PONV)21. However, higher doses or administration at the end of surgery have been associated with delayed awakening, bradycardia, and other complications22. Administering dexmedetomidine before induction can attenuate stress and cough responses, alleviate postoperative pain, and reduce PONV while minimizing impacts on recovery time and heart rates23,24.
Despite its benefits, there are dose-related controversies25,26; and some studies have shown that perioperative dexmedetomidine fails to improve postoperative analgesic consumption and recovery in specific patient populations27. Thus, this clinical trial aims to investigate the effects of different dexmedetomidine doses on anaesthesia quality in patients undergoing abdominal surgery. This research will help guide clinicians toward evidence-based care for surgical patients and may serve as a foundation for future studies in this area, particularly in Ethiopia where similar studies are lacking.
Objectives and hypothesis
The main objective of this study was to investigate the effectiveness of different doses of dexmedetomidine on intraoperative hemodynamic profiles and postoperative pain in patients undergoing abdominal surgery. In the null hypothesis (H0), we hypothesized that the change in mean hemodynamic profiles between the three groups is the same (µ1=µ2=µ3), while our alternative hypothesis (HA), was designed to show the change in mean hemodynamic profiles between the three groups are not the same (µ1≠µ2≠µ3).
Methodology
A double-blind randomized controlled trial was conducted at Dilla University Referral Hospital from 4 January 2022, to 3 January 2024. The hospital, situated 360 km south of Addis Ababa in Ethiopia, serves a population exceeding 4 million in the Gedeo Zone and neighbouring areas. It possesses around 500 hospital beds and is affiliated with Dilla University College of Medicine and Health Sciences. Ethical clearance was obtained from the university’s Ethical Clearance Committee, and the study was registered with the Pan African Clinical Trial Registry (PACTR202208813896934) and the Research Registry (researchregistry10074). The study adhered to the ethical principles outlined in the World Medical Association Declaration of Helsinki28. Patients aged 18-65 years with American Society of Anesthesiologists (ASA) class I and II undergoing elective abdominal operations were included, with exclusions for various medical conditions like history of PONV, motion sickness, bradycardia, atrioventricular block, and severe cardiac dysfunction, diabetes, hypertension, coronary heart disease, liver, and kidney function seriously damaged, chronic pain, chronic opioid users, upper respiratory tract infection, asthma, and smoking.
Sample size and sampling procedure
The largest sample size was calculated based on the previous study done in India that shows the change in hemodynamic profiles between groups26. With pooled SD of 7.07, A priori power analysis for a one-way ANOVA with 3 groups was conducted to determine sample size using an alpha=0.017 (adjusting for the probability of a Type I error using a Bonferroni correction) to be a sample of 30 subjects per group with 90% power to detect the difference in the mean arterial pressure (MAP) between the three groups. By adding a 10% attrition rate and assuming a balanced design the total sample size was 99. Based on situational analysis with exclusion criteria for the last two years at Dilla University Referral Hospital, a total of around 200 patients had undergone major abdominal surgery under general anaesthesia. A systemic random sampling method was applied with the probability of 50% being included in the study (k=½). Considering the operation schedule as a sample frame, a random start was used to select every eligible participant.
Blinding and randomization
Randomization was accomplished using a lottery method, wherein one of three sealed envelopes containing letters ‘A,’ ‘B,’ or ‘C’ was drawn. ‘A’ represented the NS group (control), ‘B’ indicated the Dex (0.4 µg/kg/hr.) group, and ‘C’ represented the Dex (0.6 µg/kg/h.) group. Both patients and data collectors remained blinded to the group allocation.
Data collection procedures
Data collection involved a questionnaire covering patients’ socio-demographic details, preoperative diagnosis, and starvation period before surgery, ASA status, weight, height, BMI, primary and secondary outcome variables, and other relevant information. The data collection tool was prepared in the English language and was collected by three postgraduate students who were already blinded to the group of study.
The conduct of anaesthesia and clinical outcome variables
All patients meeting the eligibility criteria were informed about the study’s benefits, risks, and objectives the night before surgery. On the morning of surgery, written informed consent was verified along with specific consent for participation in the research. Standard premedication including paracetamol 1 g PO, dexamethasone 4 mg, and metoclopramide 10 mg was administered to all patients following the hospital protocol. General anaesthesia with endotracheal intubation was uniformly administered to all groups, without any regional or neuraxial anaesthesia. Standard ASA monitors, comprising pulse oximetry, electrocardiography, non-invasive blood pressure, and temperature monitor, were applied to all participants. Patients were preloaded with 20 ml/kg of crystalloid solution, and baseline hemodynamics were recorded three times before the start of dexmedetomidine infusion. The infusion of the study drug commenced 15 min before anaesthesia induction by an anaesthetist not involved in the study.
After 3 min of pre-oxygenation, fentanyl 1–2 μg/kg was given over 30 seconds, and additional tramadol 50 mg for pain management was allowed as per the protocol. Patients underwent induction with intravenous propofol at a dosage of 2 mg/kg, followed by suxamethonium at a dosage of 1.5 mg/kg for intubation. Subsequently, vecuronium was administered at a dosage of 0.07-0.1 mg/kg to maintain muscle relaxation, and mechanical ventilation was initiated. Anaesthesia was sustained with a 1.2% end-tidal concentration of isoflurane in 100% O2 with a flow rate of 3 l/min, maintaining tidal volume and respiratory rate to achieve the target end-tidal CO2.
Once patients were categorized into three groups (referred to as ‘A,’ ‘B,’ or ‘C’ groups) through a randomized selection process, the principal investigator aseptically prepared the study drug based on weight from a standard concentration within one hour before administration. Group ‘A,’ designated as the control group, received a volume-matched infusion of normal saline (NS) prepared similarly. Groups ‘B’ and ‘C’ received infusions of dexmedetomidine at weight-based doses of 0.4 µg/kg/hr and 0.6 µg/kg/h, respectively, in accordance with prior research findings25,26. Infusions were prepared separately for each group in a designated operating room. To prepare the infusion, 0.5 ml of dexmedetomidine containing 50 µg of the drug was withdrawn and diluted up to 50 ml with normal saline, resulting in a final concentration of 1 mcg/ml. Dexmedetomidine or NS infusions were administered using an automated Mindray infusion pump throughout the surgical procedure, adjusted based on the patient’s weight to achieve the targeted infusion rate. After setting the infusion rate, the drug-containing bag was covered with a drape to conceal the grouping of the patient from the assessor. Consequently, the bags and volumes of prepared solution were identical across all groups, with only the infusion rate differing based on the patient’s weight and assigned group.
If deemed necessary, additional emergency medications were administered. Surgery commenced only after ensuring adequate depth of anaesthesia, thus ensuring that pain did not influence the primary endpoints. Participants experiencing complications were deemed lost to follow-up, and appropriate management protocols were continued. At the end of the surgery, infusion of the study drug ceased, anaesthesia was reversed, and patients were extubated before being transferred to the post-anaesthesia care unit (PACU). Discharge from the PACU occurred after two hours, provided the Aldrete score reached 10.
The primary endpoint was to evaluate the efficacy of various doses of dexmedetomidine infusion in mitigating hemodynamic responses to critical events such as laryngoscopy, intubation, incision, and extubation in patients undergoing open abdominal surgery. Changes in hemodynamic parameters (mean arterial pressure, systolic blood pressure, and heart rate) from baseline were compared with subsequent measurements. Secondary endpoints included assessing extubation time, postoperative sedation levels in the PACU, incidence of postoperative nausea and vomiting, pain scores, analgesic requirements, time to first analgesic request, and occurrence of adverse effects. Postoperative analgesia was administered based on patient-reported pain or a Visual Numerical Rating Scale (VNRS) score of greater than or equal to 4, following hospital protocol for analgesic drug administration.
The frequency and intensity of coughing during the recovery phase were evaluated using a grading system, where grade 0 denoted no coughing, grade 1 indicated mild, occasional coughing, grade 2 signified moderate, frequent coughing lasting less than 5 seconds with no impact on extubation, and grade 3 represented severe, continuous coughing lasting 5 seconds or more, affecting extubation29. Sedation levels were assessed at various intervals postoperatively (1, 15, 30, 60 to 120 min) using the Ramsay sedation scale, a recognized tool for measuring sedation or agitation30. The severity of PONV was gauged using an 11-point verbal numerical rating scale (VNRS), where 0 indicated no nausea, and 10 represented the most severe and intolerable nausea31. In the event of nausea or vomiting, intravenous metoclopramide 10 mg and dexamethasone 4 mg were administered.
Data quality assurance
The evaluation tool underwent pretesting with 5% of the sample size, distinct from the main study participants, before actual data collection. Throughout the data collection process, diligent supervision and follow-up were implemented. Supervisors reviewed each questionnaire daily, with additional scrutiny by the principal investigator to ensure data completeness and consistency.
Data processing and analysis procedure
Following data collection, a meticulous error check was performed, and the coded data were entered into the SPSS version 25 statistical package for analysis. Before analysis, the distribution of data was verified using the Shapiro–Wilk test for normality and Levene’s test for homogeneity of variance. Analysis of variance (ANOVA) and the Kruskal–Wallis H test were employed for normally distributed continuous data and non-normally distributed or non-parametric data, respectively. In cases where ANOVAs or the Kruskal–Wallis H test yielded significance, the Tukey post hoc test was utilized for between-group comparisons, while the Pearson χ2 test was applied for categorical variables. A two-way mixed design ANOVA was conducted to ascertain potential interactions between between-subjects and within-subjects factors. Normally distributed numerical data was presented as mean ± standard deviation, while non-normally distributed numerical data was expressed as ranked mean, and categorical data as proportions (%). A P value of less than 0.05, with a statistical power of 90%, was considered indicative of statistical significance.
Operational definitions of variables
Hemodynamic
The overall circulation of blood in our body and the forces involved in it.
Hemodynamic instability
one or more out-of-range vital sign measurements, such as low/high blood pressure and abnormal heart rate (arrhythmias)>20% increment or decrement from baseline.
Effectiveness
The ability of a drug not to bring significant hemodynamic change as compared to baseline.
Critical period
It is a perioperative period when there is maximum stimulation on the patient secondary to the application of a laryngoscope, tracheal intubation, surgical incision, and extubation that results in hemodynamic instability.
Baseline
The first set of vital signs measured on a patient before the study drugs were given.
Immediately after intubation
This is the immediate time in seconds/minute after a successful laryngoscope and tracheal intubation is performed.
Immediately after extubation
The immediate time in seconds/minutes after the successful removal of the tracheal tube is performed.
Result
A total of 99 patients were assessed for eligibility but only 96 participants (thirty-two in each three study groups) participated in this study. Three patients were excluded from the study, so they were not included in the randomization and analysis (Fig. 1). There was no statistically significant difference among the three study groups with regard to the age, height, and weight of the participants (P value> 0.05) as shown in (Table 1).
Figure 1.

Flow diagram of the study.
Table 1.
Sociodemographic characters, duration of surgery and anaesthesia.
| Parameters | Group 1 | Group 2 | Group 3 | P value |
|---|---|---|---|---|
| Age in years (mean±SD) | 41.28±12.652 | 41.19±12.820 | 39.97±10.793 | 0.890 |
| Sex of participants, n (%) | ||||
| Male | 19 (59.4) | 12 (37.5) | 21 (65.6) | 0.060 |
| Female | 13 (40.6) | 20 (62.5) | 11 (34.4) | |
| Height of participants in centimeters (mean±SD) | 165.38±5.830 | 165.91±4.947 | 166.28±5.431 | 0.798 |
| Weight of participants (mean±SD) | 65.97±6.029 | 65.63±5.824 | 66.78±6.469 | 0.740 |
| ASA physical status, n (%) | ||||
| ASA 1 | 21 (65.6) | 26 (81.3) | 27 (84.4) | 0.161 |
| ASA 2 | 11 (34.4) | 6 (18.8) | 5 (15.6) | |
| Type of incision, n (%) | ||||
| Midline incision | 10 (31.3) | 8 (25.0) | 5 (15.6) | 0.86 |
| Upper gastrointestinal | 10 (31.3) | 10 (31.3) | 10 (31.3) | |
| Lower gastrointestinal | 8 (25.0) | 9 (28.1) | 11 (34.4) | |
| Gynaecological abdominal incision | 4 (12.5) | 5 (15.6) | 6 (18.8) | |
| Duration of surgery in min (mean±SD) | 92.28±7.336 | 93.53±10.115 | 90.88±8.613 | 0.482 |
| Duration of anaesthesia in min (mean±SD) | 97.12±8.218 | 98.59±10.478 | 95.91±8.129 | 0.492 |
ASA, American Society of Anaesthesiologist.
A χ2 test for association was conducted, and there was no statistically significant difference between the three study groups in terms of gender χ2 (2)=5.622, P=0.06, ASA physical status χ2 (2)=3.656, P=0.161 and type of surgery, χ2 (6)=2.552, P=0.863.
A one-way Welch ANOVA was conducted to determine if there is any difference among study groups with regard to duration of surgery Welch’s F(2, 60.978)=0.646, P=0.527 and anaesthesia Welch’s F(2, 61.308)=0.656, P=0.522 shows insignificant difference. There is also no significant difference between the groups when comparing the total amount of fluid consumed F (2,93)=0.064, P=0.938, total amount vasopressors consumed Welch’s F (2, 61.95)=0.062, P=0.94 and operative blood loss Welch’s F (2, 58.037)=0.020, P=0.980.
Primary outcome variables
A significant difference was observed between the study groups on mean SBP @15th minute after infusion F (2,93)=16.790, P less than 0.0005, mean SBP @immediately after intubation F (2, 93)=18.052, P<0.0005, mean SBP @5th min after induction F (2, 93)=18.900, P less than 0.0005, mean SBP @10th min after induction F (2, 93)=10.412, P less than 0.0005, mean SBP @immediately after extubation F (2, 93)=16.704, P<0.0005 and mean SBP @5th min after extubation F (2, 93)=5.103, P=0.008. A Post hoc analysis shows a significantly reduced mean SBP in group 3 and group 2 as compared to the control group on these aforementioned time intervals with P less than 0.05. This reduction in mean SBP is also significant between group 2 and group 3 with a significant reduction in group 3 as compared to group 2 @5th min after induction (P=0.009).
The MAP was significantly reduced in group 2 and group 3 as compared to the control group @immediately after intubation with P less than 0.05, while significant reduction is observed only in group 3 as compared to group 1(the control group) @15th min after infusion, @5th min after induction and @immediately after extubation with P value of 0.05, 0.008 and 0.025, respectively.
A significant difference in mean HR between the groups was observed only @15th min after infusion F (2, 93)=9.226, P<0.0005. A Post hoc analysis shows a significant drop in mean HR in group 3 as compared to the control one P less than 0.0005 (Table 2).
Table 2.
Changes in Hemodynamic Profiles over time.
| Time | Variables | Group 1 (N=33) Mean ± SD | Group 2 (N=33) Mean ± SD | Group 3 (N=33) Mean ± SD | P |
|---|---|---|---|---|---|
| Baseline | SBP | 124.47±6.08 | 124.66±4.17 | 124.66±7.07 | 0.989 |
| MAP | 95.72±7.42 | 97.19±7.79 | 96.19±8.68 | 0.754 | |
| HR | 93.00±10.22 | 91.97±10.62 | 92.91±10.71 | 0.910 | |
| @15th min after infusion | SBP | 130.38±6.93 | 123.72±6.36* | 122.09±4.65* | <0.0005 |
| MAP | 97.50±8.89 | 92.47±8.14 | 91.88±11.20* | 0.038 | |
| HR | 98.03±13.68 | 91.47±14.98 | 84.22±9.220* | <0.0005 | |
| @immediately after intubation | SBP | 134.75±7.41 | 127.13±9.35* | 122.69±7.45* | <0.0005 |
| MAP | 104.97±11.56 | 95.31±12.77* | 93.53±9.18* | <0.0005 | |
| HR | 106.22±16.09 | 99.25±21.31 | 99.69±20.25 | 0.277 | |
| @5th min after induction | SBP | 131.06±6.63 | 125.50±8.33* | 120.09±6.29*,** | <0.0005 |
| MAP | 98.09±11.26 | 94.59±7.93 | 91.22±7.35* | 0.012 | |
| HR | 95.69±18.61 | 95.66±21.01 | 89.13±9.60 | 0.216 | |
| @10th min after induction | SBP | 128.63±9.72 | 122.25±8.34* | 120.31±3.23* | <0.0005 |
| MAP | 95.63±10.61 | 93.34±10.22 | 93.31±8.79 | 0.565 | |
| HR | 89.16±14.70 | 90.06±14.73 | 87.59±9.59 | 0.752 | |
| @15th min after induction | SBP | 124.78±10.80 | 119.69±9.32 | 120.72±5.15 | 0.053 |
| MAP | 94.69±10.51 | 93.09±11.42 | 93.22±11.62 | 0.819 | |
| HR | 88.81±14.38 | 87.84±12.71 | 87.00±11.27 | 0.853 | |
| @30th min after induction | SBP | 123.59±6.50 | 121.22±4.55 | 121.59±5.37 | 0.187 |
| MAP | 92.56±10.16 | 90.63±11.88 | 91.34±6.05 | 0.721 | |
| HR | 87.75±14.36 | 88.28±12.46 | 84.13±10.47 | 0.357 | |
| @immediately after extubation | SBP | 133.09±6.31 | 127.59±6.36* | 124.56±5.22* | <0.0005 |
| MAP | 102.63±13.45 | 99.22±11.82 | 95.41±5.76* | 0.033 | |
| HR | 95.00±15.60 | 97.97±14.02 | 94.47±10.45 | 0.539 | |
| @5th min after extubation | SBP | 129.63±6.54 | 125.88±7.35 | 123.31±9.63* | 0.008 |
| MAP | 96.28±12.39 | 96.59±14.07 | 95.28±9.38 | 0.902 | |
| HR | 91.91±13.89 | 93.75±14.70 | 93.75±14.03 | 0.836 | |
| @10th min after extubation | SBP | 124.34±11.12 | 121.47±7.70 | 122.16±7.73 | 0.413 |
| MAP | 91.28±9.99 | 90.50±9.45 | 90.19±5.75 | 0.872 | |
| HR | 86.38±9.68 | 84.13±8.96 | 88.56±10.86 | 0.204 |
HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure.
P<0.05 compared to Group 1 (Control group).
P<0.05 compared to Group 2.
Comparison of repeated measures between/within-group effect
There was a statistically significant interaction between the group and time on mean SBP F (12.634, 587.495)=3.395, P less than 0.0005, partial η2=0.068 ε=0.702. The main effect of the group showed that there was a statistically significant difference in mean SBP between groups F (2, 93)=22.425, P less than 0.0005, partial η2=0.325. The main effect of time showed a statistically significant difference in mean SBP at the different time points F (6.317, 587.495)=12.465, P less than 0.0005, partial η2=0.118 ε=0.702. A pairwise comparison indicates a statistically significant increase in mean SBP in the control group at 15th minutes after infusion (P=0.004), at immediately after intubation (P<0.0005), at the 5th minute after induction (P=0.007), and at immediately after extubation (P<0.0005) as compared to baseline value while there was no significant difference in mean SBP between the baseline and all other levels for group 2 and group 3.
There was a statistically significant interaction between the group and time on mean MAP F (12.071, 561.295)=1.993, P=0.023, partial η2=0.041 ε=0.671. The main effect of the group showed that there was a statistically significant difference in mean MAP between groups F (2, 93)=3.146, P=0.048, partial η2=0.063. The main effect of time also showed a statistically significant difference in mean MAP at the different time points F (6.035, 561.295)=9.480, P<0.0005, partial η2=0.093 ε=0.671. A pairwise comparison indicates a statistically significant increase in mean MAP in the control group at immediately after intubation (P=0.009) as compared to the baseline value (Fig. 2).
Figure 2.

Trends of mean arterial pressure (MAP). Hint:1- baseline, 2- at 15th min after infusion, 3- at immediately after intubation, 4- at 5th min after induction, 5- at 10th min after induction, 6- at 15th min after induction, 7- at 30th min after induction, 8- at immediately after extubation, 9- at 5th min after extubation, 10 at 10th min after extubation.
There was a statistically significant interaction between the group and time on mean HR F (8.288, 385.401)=2.323, P=0.018, partial η2=0.048 ε=0.460. The main effect of the group showed that there was no statistically significant difference in mean HR between groups F (2, 93)=0.802, P=0.451, partial η2=0.017. The main effect of time also showed a statistically significant difference in mean HR at the different time points F (4.144, 385.401)=19.550, P<0.0005, partial η2=0.174 ε=0.460. A pairwise comparison indicates a statistically significant increase in mean HR in the control group at immediately after intubation (P=0.003) as compared to the baseline value while there is a significant decrease of mean HR in group 2 at the 10th min after extubation as compared to the baseline value (P=0.040). A statistically significant reduction in mean HR is observed in group 3 at the 15th min after infusion and at the 30th min after induction as compared to baseline with a P value of 0.002 and 0.008, respectively (Fig. 3).
Figure 3.
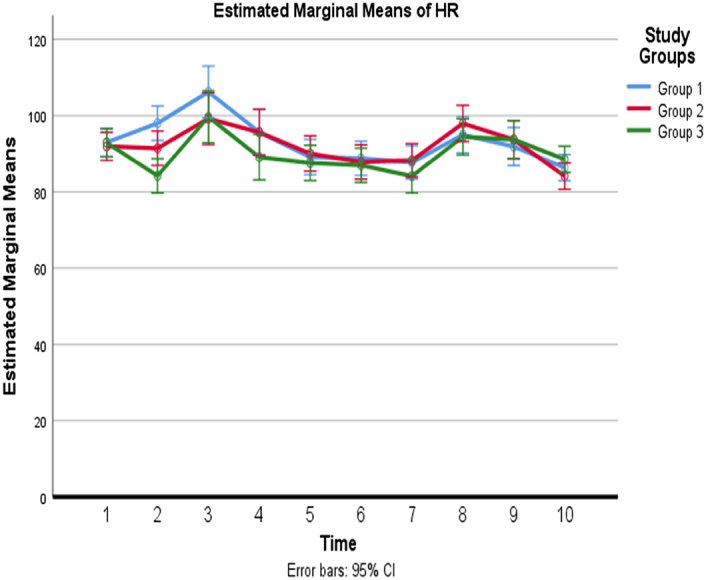
Trends of mean heart rate (HR). Hint:1- baseline, 2- at 15th min after infusion, 3- at immediately after intubation, 4- at 5th min after induction, 5- at 10th min after induction, 6- at 15th min after induction, 7- at 30th min after induction, 8- at immediately after extubation, 9- at 5th min after extubation, 10 at 10th min after extubation.
Secondary outcome variables
There was no statistically significant difference between the groups of the study in terms of postoperative adverse reactions [χ2 (10)=11.533, P=0.318] as assessed by χ2 test, while a statistically significant difference was observed concerning intraoperative complications among the groups [χ2 (10)=21.824, P=0. 016]. There was a strong association between the group of study and the presence of intraoperative complications, Cramer’s V=0.329, P=0.023. This finding is noted as a higher number of patients develop hypertension and tachycardia in the control group as compared to group 2 and group 3 while delayed awakening and hypotension were observed in a higher number of patients in group 3 as compared to the other two groups.
When comparing the grades of coughing for the study groups a significant difference was observed [χ2 (6)=17.509, P=0.008]. There was a strong association between the group of study and the grades of coughing, Cramer’s V=0.300, P=0.008. A higher number of patients develop grade 2 and 3 coughing in the control group.
A one-way ANOVA shows that there was no statistically significant difference between all three study groups when comparing the hemodynamic profiles (mean SBP, MAP HR) on discharge to PACU.
The Kruskal–Wallis test showed that the distribution of pain NRS scores was the same at 0, 6th, 12th, 18th, and 24th h across categories of the three study groups (P>0.05). There was a statistically significant difference in the mean rank of the NRS score at the 3rd h [χ2 (2)=7.426, P=0.024]. Post hoc analysis shows a significantly reduced NRS score in group 3 than the group 2 and the control group with a mean rank score of 41.2, 45.4, and 58.9, respectively. The Kruskal–Wallis test showed that the distribution of PONV VNRS score was the same at all-time points across categories of the three study groups (P>0.05), and there was no significant difference in anti-emetic consumption across all groups.
There was a statistically significant difference between the groups in terms of time to the first analgesic request F (2.93)=10.105, P<0.0005. Post hoc analysis shows the increased time for the first rescue analgesic requirement (in min) in group 2 and group 3 as compared to the control group with P values of 0.006 and less than 0.0005, respectively.
A statistically significant reduction in total analgesics consumption over 24 h (in mg) is observed in group 2 and 3 as compared to a control group with a P value of 0.020 and 0.014, respectively (Table 3).
Table 3.
Postoperative analgesic requirements.
| Group | Time for first rescue analgesic requirement (in min) | Total analgesics consumption over 24 h (in mg) |
|---|---|---|
| Group NS | 48.44±28.325 | 106.25±59.229 |
| Group Dex 0.4 | 92.34±66.308a | 73.44±47.493a |
| Group Dex 0.6 | 109.22±64.447a | 71.88±33.451a |
NS, normal saline.
Statistically significant as compared to baseline.
The mean sedation score between the groups was higher in group 3 when compared with group two and the control group at 1, 15, 30, and 60 min of PACU, but there were no statistically significant differences between groups in terms of postoperative sedation score in PACU at all levels F (2.93)=1.519, P=0.224, partial η2=0.032 (Table 4).
Table 4.
Changes in mean sedation score in PACU.
| Postoperative sedation score in PACU | |||||
|---|---|---|---|---|---|
| Group | 1 min | 15 min | 30 min | 60 min | 120 min |
| Group NS | 2.72±0.457 | 2.63±0.554 | 2.56±0.504 | 2.59±0.499 | 2.47±0.507 |
| Group Dex 0.4 | 2.81±0.644 | 2.62±0.492 | 2.56±0.504 | 2.50±0.508 | 2.50±0.508 |
| Group Dex 0.6 | 2.97±0.647 | 2.81±0.471 | 2.63±0.492 | 2.56±0.504 | 2.44±0.504 |
NS, normal saline; PACU, post-anaesthesia care unit
Discussion
In this current study, we found a profoundly decreased mean SBP in group 3 and group 2 as compared to the control group at critical time intervals with P less than 0.05. This reduction in mean SBP is also significant between group 2 and group 3 with a significant reduction in group 3 as compared to group 2 @5th min after induction (P=0.009). The Mean MAP was also significantly reduced in group 2 and group 3 as compared to the control group @immediately after intubation with P less than 0.05 while a significant drop in mean HR in group 3 as compared to the control one P less than 0.0005 was detected. Our finding means there were significantly attenuated hemodynamic profiles in group 2 and group 3 while exaggeratedly raised hemodynamic profiles in the control groups at critical time points like laryngoscopy, skin incision, and extubation.
The hemodynamic instability is an inevitable major complication of anaesthesia and surgery due to stress from intubation, surgical incision, and anaesthetic medications. Evidence supports that the prevalence of hemodynamic fluctuations after induction of general anaesthesia is about 23.0%6. The pre-induction infusion of dexmedetomidine supports its‘ analgesic and hemodynamic stabilization role for intubation and other critical time points that minimize the expected side effects as observed in our findings.
Our result is in line with a study done in India by Manne et al.26 where they found significant hemodynamic stress response following laryngoscopy, tracheal intubation, and extubation in the control group as compared to the dexmedetomidine group. A study done in China by Ye et al.25 indicated a decrement in HR at 5 min after intubation in D1 group, decreased at 1 min before intubation (T2), being intubated (T3), 5min after intubation (T4) and being extubated (T7) in D2 group and T2–3, T7–9 in D3 group (P<0.05). In our finding, a statistically significant reduction in mean HR is observed only in group 3 at the 15th min after infusion and the 30th min after induction as compared to baseline. This variability is believed to be related mainly to a difference in the way anaesthesia is conducted and the surgical approach.
Our study noted that a higher number of patients developed hypertension and tachycardia in the control group as compared to group 2 and group 3 which is in line with a study done in Ukraine by Bielka et al.32, while hypotension was observed in a higher number of patients in group 3 as compared to the other two groups. The presence of hypotension in more patients in group 3 is explained may be by a relatively higher dose of dexmedetomidine blunt the hemodynamic responses. Choi et al.24, a group of researchers from the Republic of Korea in their study elaborated that there is no significant difference between the study and control group concerning extubation time, which is contrary to our evidence where we found delayed awakening in a higher number of patients in group 3 as compared to the other two groups. This finding of our study is also against another study done in Turkey by Gurbet et al.18 which states the similarity between groups for mean times to extubation of the trachea. The difference in dose of dexmedetomidine 0.5 vs. 0.6 µg/kg and the variability in infusion time may bring this discrepancy.
In our study, a higher number of patients developed grade 2 and 3 coughing in the control group, which is a result comparable study done by Ye et al.25. PONV VNRS scores were similar between groups at all corresponding times throughout observation and this in line with study done by Gurbet et al.18. and Ye et al.25, where the incidence of PONV among groups at different time points was insignificant.
In this current study, a significantly reduced NRS score was encountered in group 3 than the group 2 and control group. There was also increased time for the first rescue analgesic requirement (in min) and a statistically significant reduction in total analgesics consumption over 24 h (in mg) in group 2 and 3 as compared to the control group. Our finding is comparable with a study done in Egypt by Bakri et al.33 which says early postoperatively, pain severity was significantly lower in the Dexmed group, but sedation scores were significantly higher. The first analgesic request was significantly delayed in the Dexmed group (P=0.02), while the total amounts of intraoperative and postoperative analgesic administered were significantly lower in the Dexmed group.
Contrary to our findings study done in China by Mao et al.27 found that perioperative dexmedetomidine did not decrease the number of analgesic requirements in the first postoperative 72 h (dexmedetomidine group: 12.14±4.76, saline group: 10.89±5.66; P=0.367). Likewise, they also stated groups did not differ concerning total postoperative analgesic requirements, postoperative pain, incidence of adverse events, surgical recovery (assessed at postoperative days 2 and 5 using the surgical recovery scale), length of hospital stay, hospital cost, incidence of chronic pain, or quality of life. The major reason for these contradictory findings is the variability in study participants and the timing and duration of infusion as well as the difference in follow-up period between our recent study and their study. In line with our study, these same researchers also found that dexmedetomidine had beneficial effects on decreasing intraoperative opioid consumption and improving postoperative sleep quality.
In our finding even though the mean sedation score between the groups was higher in group 3 when compared with group two and control group, that is not statistically significant. Sedation decreases gradually after stopping the infusion. We followed patients for 120 min as the elimination half‑life of dexmedetomidine is 2 h.
This study witnessed the fact that critical incidences like laryngoscopy, intubation, skin incision, and extubation do significantly increase the hemodynamic profiles in patients undergoing abdominal surgery as seen in group NS. Dexmedetomidine attenuates this sympathoadrenal response and provides hemodynamic stability34. The effective attenuation dose with minimum side effects noted in our study was 0.4 mcg/kg/h infusion.
Our study fails to demonstrate a significant difference in the incidence of PONV and Sedation score in the PACU between the groups. This result might be explained as all our study subjects took premedication for PONV, and the patients’ unawareness of their condition after surgery due to the residual anaesthetic agent effect may have kept the participants in all groups in a comparably sedated state.
Strengths of the study
Being double blinding randomized controlled trial (RCT) and homogeneous population in both groups concerning socio-demographic aspects
Limitations
Our study has some limitations such as lack of control over confounding factors like incision size and type of surgery.
Conclusion
Perioperative low-dose infusion of dexmedetomidine at the rate of 0.4 mcg/kg/h is a useful anaesthesia adjuvant to control hemodynamic stress response to laryngoscopy, intubation, skin incision, and extubation in patients undergoing abdominal surgery. It also provides lighter sedation and reduces the postoperative analgesic requirements without any significant adverse effects.
Recommendations
We suggest that clinicians use dexmedetomidine at the rate of 0.4 mcg/kg/h as part of general anaesthesia to achieve better hemodynamic stability. We also recommend that researchers perform further studies with a large sample size, with invasive BP measurement and multicenter RCT.
Ethical approval
Ethical clearance was obtained from the College of Medicine and health sciences of Dilla University institutional review board with Protocol Unique No: duirb/008/22-01 before the start of the study. The purposes and the importance of the study were clearly explained & written informed consent was obtained from each study participant.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
There is no funding from any institution to conduct this study.
Author contribution
All authors have made substantial contributions to the conception, design, analysis, and interpretation of data and participated in the critical review and editing of the manuscript drafts for scientific merit and depth.
Conflicts of interest disclosure
Nothing to declare.
Research registration unique identifying number (UIN)
It has been registered with the unique identifying number of researchregistry10074 https://www.researchregistry.com/browse-the-registry#home/
Guarantor
Seyoum Hailu.
Data availability statement
All datasets used and analyzed during this study are available from the first author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgment
The author would like to thank Dilla University for giving me an opportunity to conduct this study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 29 April 2024
Contributor Information
Seyoum Hailu, Email: seyoumhailu44@gmail.com.
Shimelis Abbabu, Email: yshimelis.1@gmail.com.
Ashenafi Seifu, Email: seifuashenafi@gmail.com.
Naol Gorde, Email: naolpeace@yahoo.com.
Aschalew Besha, Email: aschalewbesha5@gmail.com.
References
- 1.Pfuntner A, Wier LM, Stocks C. Most Frequent Procedures Performed in U.S. Hospitals, 2011. 2013 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [PubMed]
- 2.Ung JWLJ. Postoperative pain management: Study of patients’ level of pain and satisfaction with health care providers’ responsiveness to their reports of pain. Nurs Health Sci 2003;5:13–21. [DOI] [PubMed] [Google Scholar]
- 3.and ASoAACoS. Parameters P. Practice guidelines for acute pain management in the perioperative setting. An update report by the American Society of Anesthesiologists task force on acute pain management. Anesth Essays Res 2010;116:248–273. [DOI] [PubMed] [Google Scholar]
- 4.Safavi M, Honarmand A, Khazaei M. The effects of propofol, ketamine and combination of them in prevention of coughing and laryngospasm in patients awakening from general anesthesia: A randomized, placebo-controlled, double blind clinical trial.. Adv Biomed Res 2016;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HY, Kim JY, Ahn SH, et al. Predicting effective remifentanil concentration in 95% of patients to prevent emergence cough after laryngomicroscopic surgery. Medicine (Baltimore) 2018;97:e11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawasaki S, Kiyohara C, Tokunaga S, et al. Prediction of hemodynamic fluctuations after induction of general anesthesia using propofol in non-cardiac surgery: a retrospective cohort study. BMC Anesthesiol 2018;18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair BG, Horibe M, Newman S-F, et al. Anesthesia information management system-based near real-time decision support to manage intraoperative hypotension and hypertension. Anesth Analg 2014;118:206–214. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MHAT, Shannon E. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med 1999;27:1714–1720. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet HDJ. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921–1928. [DOI] [PubMed] [Google Scholar]
- 10.Estebe JP, Morel M, Daouphars T, et al. Lessons from the analysis of a retrospective cohort of patients who underwent large open abdominal surgery under total intravenous opioid-free anesthesia. Drugs 2021;8:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Revista Brasil Anestesiol 2012;62:118–133. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, He J, Yu N, et al. Mechanisms of dexmedetomidine in neuropathic pain. Front Neurosci 2020;14:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weerink MAS, Struys M, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet 2017;56:893–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villela NR, Nascimento P, Júnior. Dexmedetomidine in anesthesiology. Rev Bras Anestesiol 2003;53:97–113. [DOI] [PubMed] [Google Scholar]
- 15.Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia, and beyond.. Expert Opin Drug Metab Toxicol 2008;4:619–627. [DOI] [PubMed] [Google Scholar]
- 16.Belleville, WD JP, Bloor BC, et al. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology 1992;77:1125–1133. [DOI] [PubMed] [Google Scholar]
- 17.Ebert TJHJ, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000;93:382–394. [DOI] [PubMed] [Google Scholar]
- 18.Gurbet A, Basagan-Mogol E, Turker G, et al. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Canadian J Anesth J CanadienAnesthesie 2006;53:646–652. [DOI] [PubMed] [Google Scholar]
- 19.Kim NY, Kwon TD, Bai SJ, et al. Effects of dexmedetomidine in combination with fentanyl-based intravenous patient-controlled analgesia on pain attenuation after open gastrectomy in comparison with conventional thoracic epidural and fentanyl-based intravenous patient-controlled analgesia. Int J Med Sci 2017;14:951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arcangeli A, D’Alò C, Gaspari R. Dexmedetomidine use in general anesthesia. Curr Drug Targets 2009;10:687–695. [DOI] [PubMed] [Google Scholar]
- 21.Dong CS, Zhang J, Lu Q, et al. Effect of Dexmedetomidine combined with sufentanil for post-thoracotomy intravenous analgesia: a randomized, controlled clinical study. BMC Anesthesiol 2017;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu SLY, Wang S, Xu S, et al. Effects of intravenous infusion of lidocaine and dexmedetomidine on inhibiting cough during the tracheal extubation period after thyroid surgery. BMC Anesthesiol 2019;19:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Wang W, Shan Z, et al. Dexmedetomidine as an adjuvant for patients undergoing breast cancer surgery: a meta-analysis. Medicine 2020;99:e23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JJ, Kim K, Park HY, et al. CONSORT the effect of a bolus dose of dexmedetomidine on postoperative pain, agitation, and quality of recovery after laparoscopic cholecystectomy. Medicine 2021;100:e24353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Q, Wang F, Xu H, et al. Effects of dexmedetomidine on intraoperative hemodynamics, recovery profile, and postoperative pain in patients undergoing laparoscopic cholecystectomy: a randomized controlled trial.. BMC Anesthesiol 2021;21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manne GR, Upadhyay MR, Swadia V. Effects of low dose dexmedetomidine infusion on the hemodynamic stress response, sedation and post-operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian J Anesth 2014;58:726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao Y, Sun X, Si L, et al. Perioperative dexmedetomidine fails to improve postoperative analgesic consumption and postoperative recovery in patients undergoing lateral thoracotomy for thoracic esophageal cancer: a randomized, double-blind, placebo-controlled trial. Pain Res Manag 2020;2020:4145893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Association WM. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 29.Minogue SCRJ, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Amesth Analg 2004;99:1253–1257. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974;2:656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meek R, Egerton‐Warburton D, Mee MJ, et al. Measurement and monitoring of nausea severity in emergency department patients: a comparison of scales and exploration of treatment efficacy outcome measures. Acad Emerg Med 2015;22:685–693. [DOI] [PubMed] [Google Scholar]
- 32.Bielka K, Kuchyn I, Babych V, et al. Dexmedetomidine infusion as an analgesic adjuvant during laparoscopic сholecystectomy: a randomized controlled study. BMC Anesthesiol 2018;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamed H, Bakri EAI, Ibrahim A. Comparison of dexmedetomidine and dexamethasone for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean J Anesthesiol 2015;68:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panchgar VS, Sunitha AN, Dhulkhed HB, et al. The effectiveness of intravenous dexmedetomidine on perioperative hemodynamics, analgesic requirement, and side effects profile in patients undergoing laparoscopic surgery under general anesthesia. Anesth Essay Res 2017;11:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used and analyzed during this study are available from the first author upon reasonable request.


