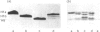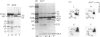Abstract
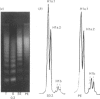
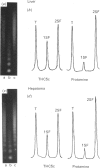
The H1 histones serve as general repressors of gene expression by inducing the formation of a compact chromatin structure, whereas the high-mobility-group (HMG) non-histone chromosomal proteins have roles in maintaining the structure and function of transcriptionally active chromatin. The distribution of the H1 histone subtypes and HMG proteins among various trout tissues (liver, hepatocellular carcinoma, testis and erythrocyte) was determined. Histone H1b was present in the chromatin of liver, but not in the chromatin of hepatocellular carcinoma, testis or erythrocyte. Nuclease-resistant regions of liver chromatin had elevated levels of histone H1b. Histone H1b was isolated, and the N-terminal amino acid sequence of histone H1b was found to be highly similar to that of mammalian histone H1(0) and duck H5. HMG proteins T1, T2, T3, H6, C, D and F were associated with liver and hepatocellular-carcinoma chromatin, with hepatocellular carcinoma containing higher levels of HMG T1 and F. Testis and erythrocyte had HMG T2 and H6 as their predominant HMG proteins. Most of the HMG H6 of hepatocellular carcinoma, but not of liver, was located in a chromatin fraction that was soluble at physiological ionic strength and enriched in transcriptionally active DNA. These alterations in the chromatin distribution and content of hepatocyte HMG proteins and H1 histone subtypes may contribute to aberrant hepatocyte gene expression in the hepatocellular carcinoma.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Breuer B., Bouterfa H., Doenecke D. Early increase in histone H1(0) mRNA during differentiation of F9 cells to parietal endoderm. EMBO J. 1988 Oct;7(10):3003–3008. doi: 10.1002/j.1460-2075.1988.tb03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
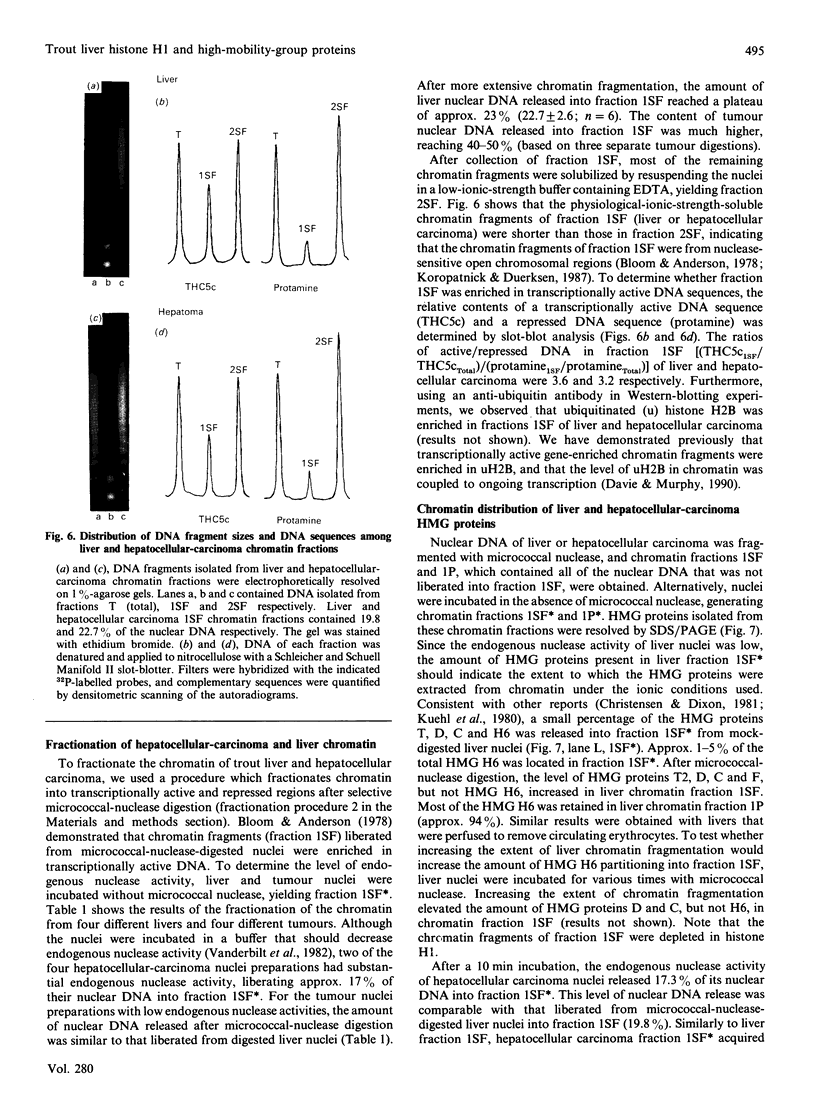
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991 Apr 4;350(6317):429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- Brown E., Goodwin G. H. Comparison of the high-mobility-group chromosomal proteins in rainbow-trout (Salmo gairdnerii) liver and testis. Biochem J. 1983 Dec 1;215(3):531–538. doi: 10.1042/bj2150531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Goodwin G. H., Mayes E. L., Hastings J. R., Johns E. W. Heterogeneity of proteins resembling high-mobility-group protein HMG-T in trout testes nuclei. Biochem J. 1980 Nov 1;191(2):661–664. doi: 10.1042/bj1910661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Crippa M. P., Pash J. M. Immunochemical analysis of the exposure of high mobility group protein 14 and 17 surfaces in chromatin. J Biol Chem. 1990 Nov 25;265(33):20077–20080. [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Comparison of the high mobility group proteins and their chromatin distribution in trout testis and liver. J Biol Chem. 1981 Jul 25;256(14):7549–7556. [PubMed] [Google Scholar]
- Cole R. D. Microheterogeneity in H1 histones and its consequences. Int J Pept Protein Res. 1987 Oct;30(4):433–449. doi: 10.1111/j.1399-3011.1987.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Croston G. E., Kerrigan L. A., Lira L. M., Marshak D. R., Kadonaga J. T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991 Feb 8;251(4994):643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Delcuve G. P., Nickel B. E., Moirier R., Bailey G. Reduced levels of histones H1o and H1b, and unaltered content of methylated DNA in rainbow trout hepatocellular carcinoma chromatin. Cancer Res. 1987 Oct 15;47(20):5407–5410. [PubMed] [Google Scholar]
- Davie J. R., Murphy L. C. Level of ubiquitinated histone H2B in chromatin is coupled to ongoing transcription. Biochemistry. 1990 May 22;29(20):4752–4757. doi: 10.1021/bi00472a002. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Numerow L., Delcuve G. P. The nonhistone chromosomal protein, H2A-specific protease, is selectively associated with nucleosomes containing histone H1. J Biol Chem. 1986 Aug 5;261(22):10410–10416. [PubMed] [Google Scholar]
- Davie J. R. Peptide mapping of basic proteins by proteolysis in acetic acid/urea-minislab polyacrylamide gels. Anal Biochem. 1985 Feb 1;144(2):522–526. doi: 10.1016/0003-2697(85)90149-6. [DOI] [PubMed] [Google Scholar]
- Delcuve G. P., Davie J. R. DNA methylation pattern and restriction endonuclease accessibility in chromatin of a germ-line specific gene, the rainbow trout protamine gene. Nucleic Acids Res. 1987 Apr 24;15(8):3385–3396. doi: 10.1093/nar/15.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorbic T., Wittig B. Chromatin from transcribed genes contains HMG17 only downstream from the starting point of transcription. EMBO J. 1987 Aug;6(8):2393–2399. doi: 10.1002/j.1460-2075.1987.tb02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorbic T., Wittig B. Isolation of oligonucleosomes from active chromatin using HMG17-specific monoclonal antibodies. Nucleic Acids Res. 1986 Apr 25;14(8):3363–3376. doi: 10.1093/nar/14.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Mendelson E., Landsman D., Bustin M. Immunofractionation of DNA sequences associated with HMG-17 in chromatin. Exp Cell Res. 1986 Oct;166(2):486–496. doi: 10.1016/0014-4827(86)90493-3. [DOI] [PubMed] [Google Scholar]
- Gabrielli F., Tsugita A. H1(0) histones of normal and cancer human cells. Amino acid composition of H1 purified by polyacrylamide gel electrophoresis. Mol Cell Biochem. 1986 Aug;71(2):129–134. doi: 10.1007/BF00214771. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Cole R. D. The distribution of H1 histone is nonuniform in chromatin and correlates with different degrees of condensation. J Biol Chem. 1984 Nov 25;259(22):14237–14242. [PubMed] [Google Scholar]
- Jin Y. J., Cole R. D. H1 histone exchange is limited to particular regions of chromatin that differ in aggregation properties. J Biol Chem. 1986 Mar 5;261(7):3420–3427. [PubMed] [Google Scholar]
- Koropatnick J., Duerksen J. D. Nuclease sensitivity of alpha-fetoprotein, metallothionein-1, and immunoglobulin gene sequences in mouse during development. Dev Biol. 1987 Jul;122(1):1–10. doi: 10.1016/0012-1606(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Kuehl L., Lyness T., Dixon G. H., Levy-Wilson B. Distribution of high mobility group proteins among domains of trout testis chromatin differing in their susceptibility to micrococcal nuclease. J Biol Chem. 1980 Feb 10;255(3):1090–1095. [PubMed] [Google Scholar]
- Lennox R. W., Cohen L. H. The production of tissue-specific histone complements during development. Biochem Cell Biol. 1988 Jun;66(6):636–649. doi: 10.1139/o88-073. [DOI] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Diversity of sequences of polyadenylated cytoplasmic RNA from rainbow trout (Salmo gairdnerii) testis and liver. Biochemistry. 1977 Mar 8;16(5):958–964. doi: 10.1021/bi00624a023. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Macleod A. R., Wong N. C., Dixon G. H. The amino-acid sequence of trout-testis histone H1. Eur J Biochem. 1977 Aug 15;78(1):281–291. doi: 10.1111/j.1432-1033.1977.tb11739.x. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Davie J. R. The protamine gene chromatin in developing trout testis exists in an altered state. Biochim Biophys Acta. 1989 Jan 23;1007(1):23–29. doi: 10.1016/0167-4781(89)90125-5. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Roth S. Y., Cook R. G., Allis C. D., Davie J. R. Changes in the histone H2A variant H2A.Z and polyubiquitinated histone species in developing trout testis. Biochemistry. 1987 Jul 14;26(14):4417–4421. doi: 10.1021/bi00388a034. [DOI] [PubMed] [Google Scholar]
- Rabbani A., Goodwin G. H., Walker J. M., Brown E., Johns E. W. Trout liver high mobility group non-histone chromosomal proteins. FEBS Lett. 1980 Jan 14;109(2):294–298. doi: 10.1016/0014-5793(80)81108-2. [DOI] [PubMed] [Google Scholar]
- Roche J., Girardet J. L., Gorka C., Lawrence J. J. The involvement of histone H1[0] in chromatin structure. Nucleic Acids Res. 1985 Apr 25;13(8):2843–2853. doi: 10.1093/nar/13.8.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin S. M., Cole R. D. H1 histones of trout. J Biol Chem. 1981 Jan 10;256(1):442–444. [PubMed] [Google Scholar]
- Singh J., Dixon G. H. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry. 1990 Jul 3;29(26):6295–6302. doi: 10.1021/bi00478a026. [DOI] [PubMed] [Google Scholar]
- Sinnhuber R. O., Hendricks J. D., Wales J. H., Putnam G. B. Neoplasms in rainbow trout, a sensitive animal model for environmental carcinogenesis. Ann N Y Acad Sci. 1978 Sep 29;298:389–408. doi: 10.1111/j.1749-6632.1977.tb19280.x. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Harris M. R., Sigournay C. M., Mayes E. L., Bustin M. A survey of H1o-and H5-like protein structure and distribution in higher and lower eukaryotes. Eur J Biochem. 1984 Jan 16;138(2):309–317. doi: 10.1111/j.1432-1033.1984.tb07916.x. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Borun T. W., Charpentier R., Cristofalo V. J., Croce C. M. Normal and neoplastic human cells have different histone H1 compositions. J Biol Chem. 1982 May 25;257(10):5337–5338. [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]