Abstract
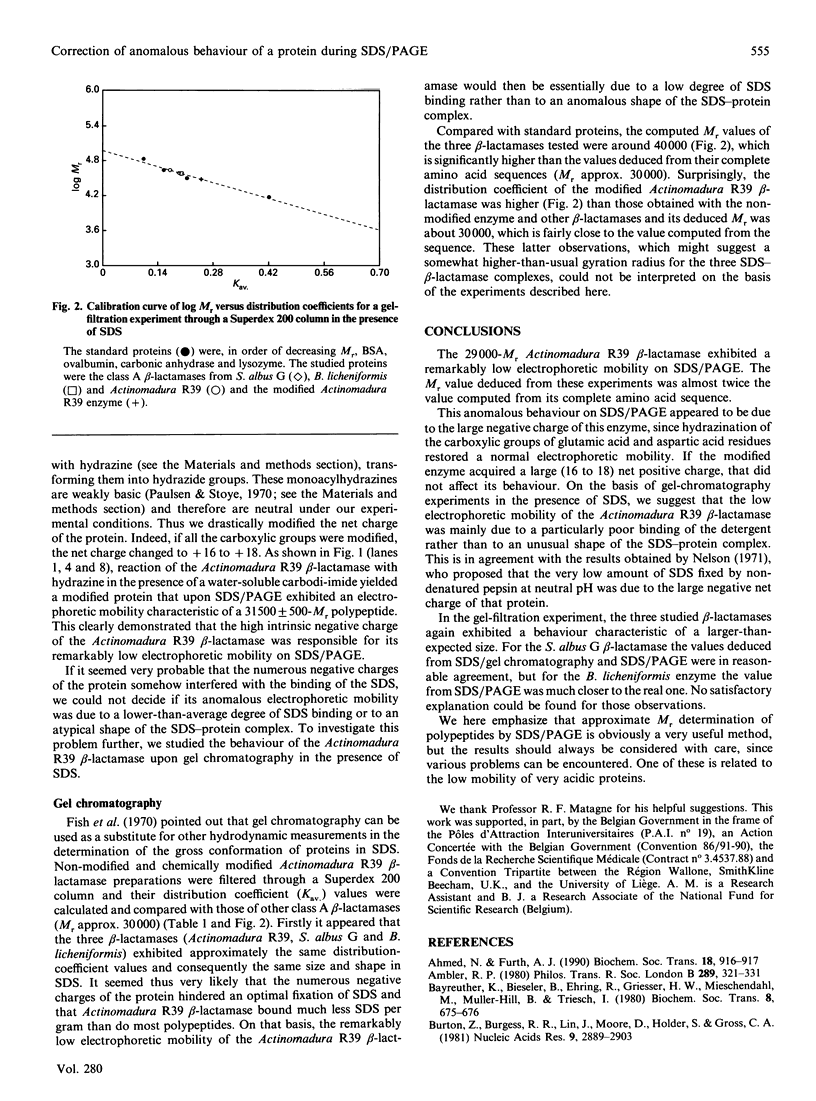
The 29,000-Mr Actinomadura R39 beta-lactamase exhibited a remarkably low electrophoretic mobility on SDS/PAGE, yielding an Mr value almost twice that computed from the corresponding gene sequence. We showed that chemical modification of the carboxylic groups of glutamic acid and aspartic acid residues restored a normal electrophoretic mobility and that the anomalous behaviour of that protein on SDS/PAGE was due to its very large negative charge at neutral pH. We also compared the behaviour of the same enzyme on gel filtration in the presence of SDS with those of other class A beta-lactamases (Mr approx. 30,000). These experiments suggested that the very low electrophoretic mobility of the Actinomadura R39 beta-lactamase upon SDS/PAGE was more probably due to a low degree of SDS binding rather than to an unusual shape of the SDS-protein complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed N., Furth A. J. Cross-linking, fragmentation and anomalous behaviour on SDS gels, of proteins glycated in vitro. Biochem Soc Trans. 1990 Oct;18(5):916–917. doi: 10.1042/bst0180916. [DOI] [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehottay P., Dusart J., De Meester F., Joris B., Van Beeumen J., Erpicum T., Frère J. M., Ghuysen J. M. Nucleotide sequence of the gene encoding the Streptomyces albus G beta-lactamase precursor. Eur J Biochem. 1987 Jul 15;166(2):345–350. doi: 10.1111/j.1432-1033.1987.tb13521.x. [DOI] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Ghuysen J. M., Van Beeumen J., Delcambe L., Dierickx L. Purification and properties of the exocellular beta-lactamase of Actinomadura strain R39. Biochim Biophys Acta. 1982 Jan 4;700(1):24–32. doi: 10.1016/0167-4838(82)90287-4. [DOI] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Klein D., Noël M., Ghuysen J. M., Delcambe L., Dierickx L. The exocellular beta-lactamase of Streptomyces albus G. Purification, properties and comparison with the exocellular DD-carboxypeptidase. Biochem J. 1981 Jan 1;193(1):75–82. doi: 10.1042/bj1930075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- Georges E., Mushynski W. E. Chemical modification of charged amino acid moieties alters the electrophoretic mobilities of neurofilament subunits on SDS/polyacrylamide gels. Eur J Biochem. 1987 Jun 1;165(2):281–287. doi: 10.1111/j.1432-1033.1987.tb11439.x. [DOI] [PubMed] [Google Scholar]
- Houba S., Willem S., Duez C., Molitor C., Dusart J., Frère J. M., Ghuysen J. M. Nucleotide sequence of the gene encoding the active-site serine beta-lactamase from Actinomadura R39. FEMS Microbiol Lett. 1989 Dec;53(3):241–246. doi: 10.1016/0378-1097(89)90224-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann E., Geisler N., Weber K. SDS-PAGE strongly overestimates the molecular masses of the neurofilament proteins. FEBS Lett. 1984 May 7;170(1):81–84. doi: 10.1016/0014-5793(84)81373-3. [DOI] [PubMed] [Google Scholar]
- Matagne A., Joris B., Van Beeumen J., Frère J. M. Ragged N-termini and other variants of class A beta-lactamases analysed by chromatofocusing. Biochem J. 1991 Feb 1;273(Pt 3):503–510. doi: 10.1042/bj2730503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A., Misselyn-Bauduin A. M., Joris B., Erpicum T., Granier B., Frère J. M. The diversity of the catalytic properties of class A beta-lactamases. Biochem J. 1990 Jan 1;265(1):131–146. doi: 10.1042/bj2650131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattice W. L., Riser J. M., Clark D. S. Conformational properties of the complexes formed by proteins and sodium dodecyl sulfate. Biochemistry. 1976 Sep 21;15(19):4264–4272. doi: 10.1021/bi00664a020. [DOI] [PubMed] [Google Scholar]
- Nelson C. A. The binding of detergents to proteins. I. The maximum amount of dodecyl sulfate bound to proteins and the resistance to binding of several proteins. J Biol Chem. 1971 Jun 25;246(12):3895–3901. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The molecular weights of vertebrate histones exploiting a modified sodium dodecyl sulfate electrophoretic method. J Biol Chem. 1971 Dec 25;246(24):7557–7560. [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]