Abstract
Background
Safe and effective management of venous vascular access is a key component of electrophysiology (EP) procedures. Recently, the Z-stitch method has been developed for effective venous hemostasis. However, the standard postprocedure protocol often includes prolonged bed rest, which may affect patient satisfaction. The ZEBRA (Z stitch Early Bed Rest Assessment) study aims to systematically investigate and quantify patient satisfaction metrics and safety parameters associated with the early mobilization after Z-stitch placement.
Objective
This study primarily investigates whether early mobilization following Z-stitch placement in venous vascular access management during EP procedures enhances patient satisfaction without compromising safety.
Methods
In this prospective, multicenter, randomized clinical trial, approximately 200 patients undergoing various EP procedures at Oregon Health and Science University and Veterans Affairs Portland Health Care System will be randomly assigned to either a 1- or 4-hour bed rest regimen post–Z stitch. Patient satisfaction will be assessed through survey, alongside monitoring for hematomas, bleeding complications, and other safety endpoints. The study includes stratification based on heparin administration and sheath size to ensure robust and nuanced data analysis.
Results
We anticipate that early mobilization will lead to higher patient satisfaction scores. We also expect to closely monitor and report the incidence of hematomas, pain medication use, healthcare costs, patient outcomes at 30 days, time to ambulation, and hospital readmissions or emergency visits related to groin complications.
Conclusion
The ZEBRA study is poised to fill a critical knowledge gap in postprocedure care in EP labs. By rigorously evaluating the impact of early mobilization on patient satisfaction and safety, this study could significantly influence future guidelines and improve patient experiences in EP procedures.
Keywords: Z stitch, Venous vascular hemostasis, Electrophysiology procedures, Postprocedure management, Randomized clinical trial
Key Findings.
-
▪
This is a randomized trial of groin management and mobilization strategies after electrophysiology procedures.
-
▪
All patients receive Z stitch(es) for hemostasis after the procedure and are randomized to mobilization at 1 hour vs 4 hours.
-
▪
All Z stitches are removed at 4 hours.
-
▪
Primary outcome is patient satisfaction, with groin complications as a secondary outcome.
Introduction
Venous vascular access management is a cornerstone in electrophysiology (EP) procedures.1 Traditionally, direct manual pressure was the primary method for achieving hemostasis after sheath removal. This approach has several drawbacks including patient discomfort, prolonged immobilization, and the need for reversal of anticoagulation. Hemostasis by manual pressure also has variable efficacy based on technique and experience. Recently, the Z-stitch method for hemostasis has been developed (Figure 1). This involves placing a stitch with a deeper bite where the sheath enters the skin and re-enters more cranial and superficial relative to the sheath in a “Z” pattern. This bunches up the soft tissue directly adjacent to the venotomy site and effectively applies continuous pressure to the venotomy tract to achieve hemostasis.2,3 The Z-stitch method reduces reliance on manual pressure and allows for safe removal of sheaths without reversal of anticoagulation.4, 5, 6 In a recent randomized trial comparing the Z-stitch method and Perclose ProGlide vascular closure device (Abbott Vascular), there was no significant difference in access site complications but a substantial difference in cost.7 Despite its efficacy, common practice is still to commit patients with Z stitch(es) to several hours of bed rest.
Figure 1.
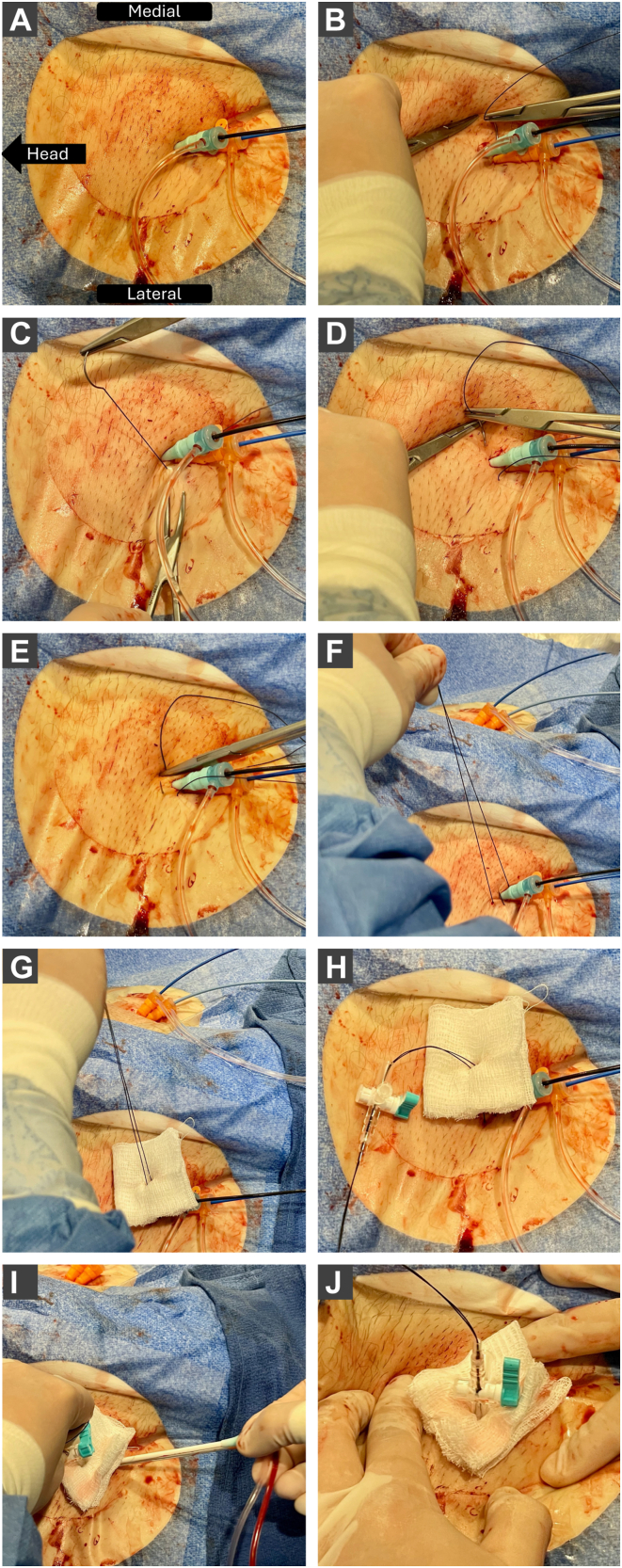
Z-stitch closure technique for femoral venous sheath removal. The sequential steps of a Z-stitch closure for femoral venous sheath removal. A: Initial view with indwelling venous sheaths in the right femoral vein. B, C: Placement of the first suture bite in a mediolateral direction beneath the venous sheaths, ensuring that it remains superficial to the femoral vessels. D, E: Positioning of the second suture bite cranial to the first, maintaining a superficial trajectory above the sheaths. F–H: Approximation of the suture, application of a protective gauze pad, and attachment of a stopcock. I: Removal of the venous sheaths with sustained downward pressure applied to the stopcock. J: Final appearance of the completed Z-stitch closure.
Patient satisfaction is becoming a core measure in healthcare interventions.8 The VASCADE device trial investigated a vascular closure device and highlighted the correlation between reduced bed rest duration and enhanced patient satisfaction. Early mobilization can minimize indwelling foley catheter use and has been demonstrated to mitigate the need for opioid analgesics and their associated risks.9,10 Additionally, early mobilization is recommended to reduce the risk of deep vein thrombosis.11 Implementation of the readily available and simple Z-stitch technique may help evolve postprocedure care protocols for patients undergoing invasive EP procedures. Efficacy and safety of the Z-stitch technique has been repeatedly demonstrated and it is plausible that with it in place patients should be able to mobilize earlier to decrease bedrest associated complications and facilitate earlier discharge.
However, currently there are no data regarding the effects of early mobilization on patient satisfaction and safety outcomes post–Z-stitch placement. The proposed ZEBRA (Z stitch Early Bed Rest Assessment) study aims to fill this gap by examining patient satisfaction scores and safety outcomes following early mobilization after Z-stitch placement. The study hypothesizes that early mobilization after Z-stitch placement can significantly improve patient satisfaction without compromising safety.
The ZEBRA study places a strong emphasis on monitoring for potential complications. The safety of patients, particularly in the context of a new technique like the Z stitch, is paramount. Therefore, the study is designed to meticulously track outcomes such as hematomas, bleeding complications, and other safety metrics.
Methods
Study design
The ZEBRA study is a prospective, multicenter, randomized clinical trial that aims to evaluate the efficacy and safety of the Z-stitch method in venous vascular access management during EP procedures. The primary objective is to assess whether early mobilization post–Z-stitch placement leads to enhanced patient satisfaction without compromising safety. This study was approved by the institutional review board at the Oregon Health and Science University (OHSU) (ID: STUDY00025985). This research adheres to the principles set forth in the Declaration of Helsinki.
Study population
The study aims to enroll approximately 200 patients at OHSU and the Veterans Affairs Portland Health Care System with approximately 100 randomized to each arm of the study. All subjects meeting the inclusion criteria from the date of the start of the study will be screened. Patients will be excluded based on the exclusion criteria listed subsequently. This research study is a multicenter study, and all procedures will take place at OHSU or the Veterans Affairs Portland Health Care System.
Recruitment
Appropriate patients will be screened for eligibility and approached for the study either in the outpatient clinic ahead of time or at the time of consenting for the procedure in the preprocedural area. Recruitment for this study will be conducted via verbal explanation of the study's objectives. No additional recruitment materials, advertisements, phone contact, or participant payments will be utilized.
Inclusion and exclusion criteria
The inclusion criteria for the study are patients between 10 and 99 years of age presenting for procedure in the OHSU EP lab for atrial fibrillation, atrial flutter, supraventricular tachycardia, diagnostic EP study, atrioventricular node ablation, left atrial appendage closure device placement, ventricular tachycardia, or premature ventricular contractions. The exclusion criteria for the study are those who are unable to consent, cases involving femoral arterial access, cases involving access with a >16F sheath (eg, patients needing a leadless pacemaker, patients with a body mass index >40 kg/m2, patients undergoing cardiovascular implantable electronic device lead extraction, thrombocytopenia, and bleeding diathesis.
Screen failures will be documented, and data collected during screening will be destroyed at the end of the study. Patients receiving large bore catheters (>16F) were excluded due to a higher perceived risk of complications in the group and lack of comprehensive data on the use of the Z stitch in this group. Furthermore, limited data exist on patients with a body mass index >40 kg/m2 and their risk of complications; in clinical practice, this group is also perceived to have a higher risk of bleeding complications.
Vulnerable populations
We plan to include children over 10 years of age in the current research project. Children undergoing cardiac EP procedures can benefit from the results of the study. The need for more controlled studies in children has been highlighted by various professional organizations, including the World Health Organization.12 Consent will be obtained from the research subject’s parent and assent from the research subject. This study does not include any other vulnerable populations such as pregnant women, neonates, decisionally impaired adults, or prisoners.
Consent process
The informed consent process for the study will adhere to standard procedures and will be executed in a face-to-face discussion. The process will be continuous, encouraging open communication with participants, and reminding them of their right to withdraw at any point, without repercussions. To minimize coercion and undue influence, the process will take place in a private, neutral setting with an emphasis on voluntary participation and ample time for participant queries and clarifications. Efforts will be made to ensure participant understanding by using comprehensible language, addressing any concerns, and providing contact information for future communications. Participants will have the assurance that their participation decision will not affect their medical care. The consent process for this study will remain unmodified, ensuring full informed consent from all participants. Non-English-speaking subjects will be accommodated by engaging a qualified interpreter, either over the phone or in person, to facilitate the consent discussion and a Short Form in the appropriate language will be used. In the case of children between 10 and 18 years of age included in the research study, we will seek parental permission and child assent. The study will not involve adults who are decisionally impaired or incapable of providing consent.
Randomization
Randomization in the ZEBRA study will be conducted at a 1:1 ratio using a computer-generated system. This process will occur while the subject is in the EP laboratory undergoing the procedure. Randomization will be executed by research personnel not involved in the Z-stitch procedure, ensuring an unbiased and objective assignment of participants to the study arms. This method ensures scientific rigor and minimizes selection bias.
Procedures
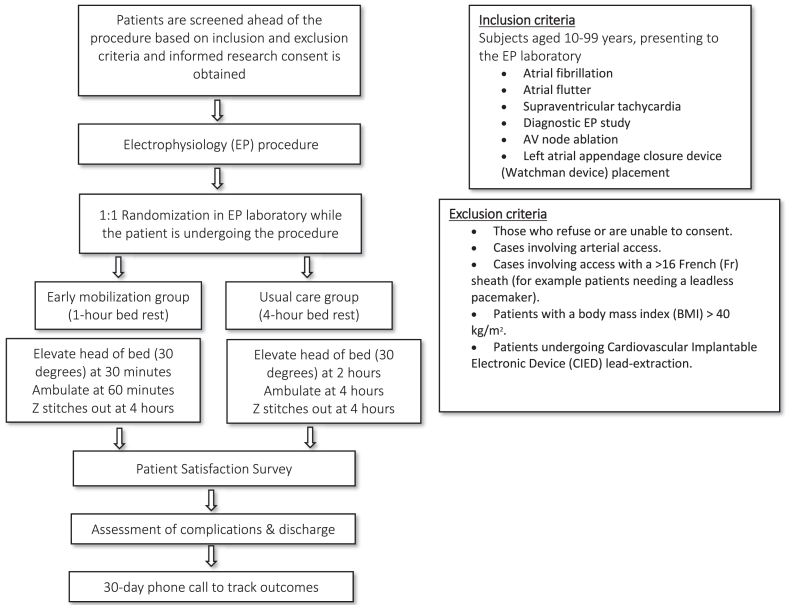
The duration of an individual subject's participation in the study is up to 30 days, with an initial procedure and bed rest period followed by a 30-day phone call follow-up for outcome tracking. The research procedures will be performed as follows (Figure 2):
-
•
Patients will be approached for participation in the study either in the outpatient clinic prior to the procedure or at the time of consenting for the procedure in the preprocedure area. Informed, written consent will be obtained at this time.
-
•
Patients will undergo their procedure in the EP laboratory, during which all venous access sites will be closed with Z stitches. We typically apply 1 Z stitch per side, but when access sites are spaced apart, multiple stitches may be placed to ensure hemostasis. Anticoagulation will not be reversed. There are no activated clotting time limitations on Z stitch placed and sheath removal.
-
•Following the procedure, patients will be randomized to 1 of 2 bed rest and Z stitch removal schemes:
-
▪Scheme 1: 1 hour of bedrest, head of bed elevated to 30° at 30 minutes, ambulation at 60 minutes, Z stitch(es) out at 4 hours, eligibility for discharge at 4.5 hours.
-
▪Scheme 2: 4 hours of bedrest, head of bed elevated to 30° at 2 hours, Z stitch(es) out and ambulation at 4 hours, eligibility for discharge at 4.5 hours.
-
▪
-
•
Prior to discharge, patients will be assessed for groin hematoma and other complications, and they will fill out a patient satisfaction survey.
-
•
A 30-day phone call follow-up will be performed to track outcomes.
-
•
Data about subjects will be collected from source records including patient medical charts, EP procedure records, and the patient satisfaction survey administered prior to discharge.
-
•Subjects may be withdrawn from the study without their consent under the following circumstances:
-
○Unplanned need for femoral arterial access during the procedure.
-
○Groin complications recognized during the procedure prior to randomization.
-
▪Development of a medical condition or complications that would make continued participation unsafe.
-
▪Failure to comply with the study procedures.
-
○
Figure 2.
ZEBRA (Z stitch Early Bed Rest Assessment) design overview with key steps. BMI = body mass index; CIED = cardiovascular implantable electronic device; EP = electrophysiology.
Data and privacy
We will collect data encompassing patients' medical records, procedure details, satisfaction scores, postprocedure complications, and 30-day outcomes. Throughout the study, we will safeguard participant privacy. Conversations during recruitment and consent will occur privately, and the information shared by patients will be exclusively used for this study. The research team will maintain confidentiality, excluding any personal identifiers from reports or publications. The data will be securely stored on an encrypted drive within the OHSU network system, accessible only to the authorized research team, for about 1 year following completion of the study. The principal investigator and a designated data manager will handle data receipt and transmission, which will be executed via encrypted channels for security. Any physical data will be securely stored in locked cabinets.
Data analysis
We will summarize key aspects such as demographic information of the participants, anticoagulant use, EP procedure performed, incidence of hematomas and other complications, utilization of analgesics, and outcomes at the 30-day mark using descriptive statistical methods, including the calculation of frequencies, percentages, means, and standard deviations. The primary endpoint of this study is aggregated patient satisfaction scores. The patient satisfaction survey used in this study has been attached as a supplement 1 with this manuscript. Satisfaction score will be calculated as an aggregate of scores for questions 1 to 3 in PART A and sum of scores for questions 1 to 3 in PART B or PART C depending on the randomization arm (Supplement 1). We will utilize a 2-tailed t-test under the intention-to-treat (ITT) principle to compare scores between the early (1 hour) and standard (4 hours) mobilization groups. ITT analysis, maintaining original group assignments irrespective of protocol adherence, mitigates bias from nonrandom participant loss, thereby upholding randomization benefits. To evaluate the disparities in the aggregated patient satisfaction scores between 2 groups, a 2-sample t test will be employed. Assuming an anticipated mean difference of 10, with an SD of 25 for both groups, and setting the significance level at .05 with a power of 80%, the required sample size was calculated to be 99 subjects per group. R version 4.1.2 (R Foundation for Statistical Computing) was used for power analysis. Secondary endpoints, such as hematoma rates and other complications, will be compared using chi-square or Fisher's exact tests, adhering to the ITT principle. Additionally, a multivariate logistic regression model will adjust for potential confounders like oral/systemic anticoagulation and sheath sizes, providing an independent estimate of early mobilization's impact on patient satisfaction and complication rates.
Risks to subjects
The potential risks associated with this study are negligible and predominantly pertain to the possibility of developing a hematoma or encountering other hemorrhagic complications. There are sparse data regarding hemorrhagic complications in the literature related to the Z-stitch procedure.2 In a single-center randomized study, the reported incidence of bleeding requiring action from a nurse or physician was 11.7%.4 The incidence of these complications is projected to be comparable across both study groups. Additionally, a slight risk pertaining to data privacy breaches is present, notwithstanding the stringent measures that will be instituted to minimize this exposure. It is imperative to acknowledge that all participants will undergo our current standard of care (ie, the Z-stitch procedure), consequently rendering the incremental risk attributable to the research as minimal.
Potential benefits to subjects
Potential benefits of participation may include shorter postprocedure bed rest, faster mobilization, and earlier discharge, potentially enhancing recovery satisfaction. However, these benefits, dependent on individual recovery, are not guaranteed for every participant.
Discussion
The ZEBRA study plays a crucial role in advancing the field of postprocedural care after EP interventions by focusing on the optimal management of the groin puncture site used for venous access. It critically re-examines traditional care protocols, with a keen emphasis on optimizing patient satisfaction while maintaining rigorous safety standards.8 The Z stitch is a significant advancement over conventional manual pressure techniques to achieve hemostasis.5 The study highlights a significant knowledge gap: the optimal duration of bed rest required following the application of the Z stitch is yet to be determined. Addressing the length of bed rest in a safe and effective manner not only stands to improve patient satisfaction, but also has a profound impact on the flow of patients in postprocedural areas, offering potential cost and time savings.13
Historically, the management of the groin after venous access has relied heavily on direct manual pressure to achieve hemostasis. A method that, while effective, has several downsides including patient discomfort and the requirement for prolonged immobilization.5 Vascular closure devices offer an alternative to manual compression, showing promise in reducing bed rest duration, enhancing patient satisfaction, and enabling more efficient utilization of healthcare resources.10,13,14 However, these devices come with their own set of limitations, including added costs and a learning curve for optimal utilization.
The ZEBRA study uses the Z-stitch technique as a cost-effective and efficient alternative to both manual compression and vascular closure devices. Unlike closure devices, the Z stitch does not introduce significant additional material costs to the procedure, as it utilizes suture material as its only consumable product, helping with potential cost savings. While the current study did not formally assess cost implications, the inherent cost-efficiency of the Z stitch suggests potential for significant savings, especially when considering the cumulative effect across numerous EP procedures performed over time.
Furthermore, the study's findings have the potential to inform future guidelines and practices in postprocedural care. By demonstrating that early mobilization does not compromise patient safety and may indeed enhance satisfaction, the ZEBRA study challenges the status quo and opens the door for more patient-centric care protocols.
Looking ahead, the implications of the ZEBRA study extend beyond the initial findings. The call for a larger, multicenter trial underscores the necessity to validate these results across a broader spectrum of healthcare settings and patient populations. Such studies could incorporate a formal cost-benefit analysis, comparing the Z stitch directly with various vascular closure devices and traditional manual pressure methods.
Conclusion
In summary, the ZEBRA study will contribute significantly to our understanding of post-procedural care of vascular access following EP procedures. We hope that the study will validate the safety and patient satisfaction associated with the Z stitch and early mobilization. As healthcare continues to evolve toward more efficient, patient-centered models, the insights from the ZEBRA study will undoubtedly play a crucial role in shaping future protocols and practices.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Consent will be obtained from the research subject’s parent and assent from the research subject.
Ethics Statement
This study was approved by the institutional review board at the Oregon Health and Science University (ID: STUDY00025985). This research adheres to the principles set forth in the Declaration of Helsinki.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2024.06.002.
Appendix. Supplementary Data
References
- 1.Ding W.Y., Khanra D., Kozhuharov N., et al. Incidence of vascular complications for electrophysiology procedures in the ultrasound era: a single-centre experience over 10,000 procedures in the long term. J Interv Card Electrophysiol. 2023;66:693–700. doi: 10.1007/s10840-022-01386-8. [DOI] [PubMed] [Google Scholar]
- 2.Sanghai S., Sandhu U., Verdick C., et al. Figure-of-eight sutures in fully anticoagulated patients after left atrial appendage occlusion may obviate need for anticoagulation reversal: vascular management after LAAO. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.121.010268. [DOI] [PubMed] [Google Scholar]
- 3.Payne J., Bickel T., Gautam S. Figure-of-eight sutures for hemostasis result in shorter lab recovery time after ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2018;41:1017–1021. doi: 10.1111/pace.13405. [DOI] [PubMed] [Google Scholar]
- 4.Pracon R., Bangalore S., Henzel J., et al. A randomized comparison of modified subcutaneous “Z”-stitch versus manual compression to achieve hemostasis after large caliber femoral venous sheath removal. Catheter Cardiovasc Interv. 2018;91:105–112. doi: 10.1002/ccd.27003. [DOI] [PubMed] [Google Scholar]
- 5.Mujer M.T., Al-Abcha A., Flores J., Saleh Y., Robinson P. A comparison of figure-of-8-suture versus manual compression for venous access closure after cardiac procedures: An updated meta-analysis. Pacing Clin Electrophysiol. 2020;43:856–865. doi: 10.1111/pace.14008. [DOI] [PubMed] [Google Scholar]
- 6.Yasar S.J., Bickel T., Zhang S., et al. Heparin reversal with protamine sulfate is not required in atrial fibrillation ablation with suture hemostasis. J Cardiovasc Electrophysiol. 2019;30:2811–2817. doi: 10.1111/jce.14253. [DOI] [PubMed] [Google Scholar]
- 7.Lodhi H., Shaukat S., Mathews A., Maini B., Khalili H. Comparison of figure-of-eight suture and Perclose ProGlide suture-mediated closure in large bore venous access hemostasis: a randomized controlled trial. Am J Cardiol. 2023;209:181–183. doi: 10.1016/j.amjcard.2023.09.105. [DOI] [PubMed] [Google Scholar]
- 8.Okunrintemi V., Nasir K. Optimizing patient-reported experiences for cardiovascular disease: current landscape and future opportunities. Methodist Debakey Cardiovasc J. 2020;16:220–224. doi: 10.14797/mdcj-16-3-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nollen J.-M., Pijnappel L., Schoones J.W., Peul W.C., Van Furth W.R., Brunsveld-Reinders A.H. Impact of early postoperative indwelling urinary catheter removal: a systematic review. J Clin Nursing. 2023;32:2155–2177. doi: 10.1111/jocn.16393. [DOI] [PubMed] [Google Scholar]
- 10.Natale A., Mohanty S., Liu P.Y., et al. Venous vascular closure system versus manual compression following multiple access electrophysiology procedures: the AMBULATE Trial. J Am Coll Cardiol EP. 2020;6:111–124. doi: 10.1016/j.jacep.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Gould M.K., Garcia D.A., Wren S.M., et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141 doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Clinical trials in children. https://www.who.int/clinical-trials-registry-platform/clinical-trials-in-children Available at:
- 13.Eldadah Z.A., Al-Ahmad A., Bunch T.J., et al. Same-day discharge following catheter ablation and venous closure with VASCADE MVP: a postmarket registry. J Cardiovasc Electrophysiol. 2023;34:348–355. doi: 10.1111/jce.15763. [DOI] [PubMed] [Google Scholar]
- 14.Dou E., Winokur R.S., Sista A.K. Venous access site closures using the VAS12CADE vascular closure system. J Vasc Interv Radiol. 2016;27:1885–1888. doi: 10.1016/j.jvir.2016.07.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.