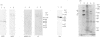Abstract
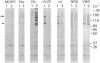
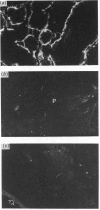
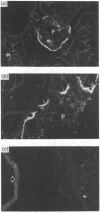
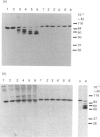
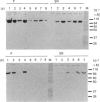
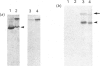
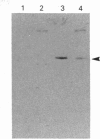
The heterodimeric vitronectin receptor (VNR) and platelet glycoprotein IIb/IIIa (GPIIb/IIIa) are two members of the integrin family of cell adhesion receptors that share the same beta subunit (GPIIIa). These proteins are involved in binding to vitronectin, fibrinogen and fibronectin and in cytoskeleton-membrane interactions. The present study shows that the human placental syncytiotrophoblast brush border membrane contains a heterodimer of subunit Mr values of 140,000 and 90,000 (non-reduced) or 125,000 and 100,000 (reduced). This protein was recognized by a monoclonal antibody to GPIIIa, rabbit antisera to the VNR and a human alloantiserum to GPIIIa. Brush border VNR-related protein bound to an immobilized peptide containing the Arg-Gly-Asp sequence and, less avidly, to immobilized fibrinogen. Only a small fraction of brush border VNR was associated with a cytoskeleton fraction. Membrane-bound brush border GPIIIa was distinct from that of platelets in its resistance to digestion by trypsin and Staphylococcus aureus V8 protease, and had a slightly lower mobility on SDS/PAGE. In addition, lectin-binding studies indicate glycosylation differences between microvillar and platelet GPIIIa heterodimers. Thus, although placental syncytiotrophoblast expresses a beta 3 integrin in its apical brush border, differences in protease sensitivity and carbohydrate content suggest that it may lack or mask certain antigenic determinants. This may be beneficial in avoiding harmful maternal alloantibody responses during pregnancy. Immunohistology showed that the VNR was present in syncytiotrophoblast apical but not basal plasma membranes, and was absent from other forms of trophoblast. The brush border VNR could function in localizing Arg-Gly-Asp-sequence-containing plasma proteins to the materno-trophoblastic interface.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Nagata K., Yamada K. M. Cell surface receptors for extracellular matrix components. Biochim Biophys Acta. 1990 Feb 28;1031(1):91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Beer J., Coller B. S. Evidence that platelet glycoprotein IIIa has a large disulfide-bonded loop that is susceptible to proteolytic cleavage. J Biol Chem. 1989 Oct 15;264(29):17564–17573. [PubMed] [Google Scholar]
- Biesecker G. The complement SC5b-9 complex mediates cell adhesion through a vitronectin receptor. J Immunol. 1990 Jul 1;145(1):209–214. [PubMed] [Google Scholar]
- Blanchette V. S., Chen L., de Friedberg Z. S., Hogan V. A., Trudel E., Décary F. Alloimmunization to the PlA1 platelet antigen: results of a prospective study. Br J Haematol. 1990 Feb;74(2):209–215. doi: 10.1111/j.1365-2141.1990.tb02567.x. [DOI] [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Olaniyan R. O., Vanderpuye O. A. An improved method for the preparation of human placental syncytiotrophoblast microvilli. Placenta. 1980 Oct-Dec;1(4):327–336. doi: 10.1016/s0143-4004(80)80034-8. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Bekeart L. S., Phillips D. R. Platelet glycoprotein IIb-IIIa-like proteins mediate endothelial cell attachment to adhesive proteins and the extracellular matrix. J Biol Chem. 1987 Jul 25;262(21):9935–9938. [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck P. J., De Mol M., Alessi M. C., Baudner S., Pâques E. P., Preissner K. T., Müller-Berghaus G., Collen D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin). J Biol Chem. 1988 Oct 25;263(30):15454–15461. [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H. C., Booth A. G. Calcium-sensitive, lipid-binding cytoskeletal proteins of the human placental microvillar region. J Cell Biol. 1987 Jul;105(1):303–311. doi: 10.1083/jcb.105.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. J., Gebbink R. K., Keijer J., Linders M., Preissner K. T., Pannekoek H. Alteration of serpin specificity by a protein cofactor. Vitronectin endows plasminogen activator inhibitor 1 with thrombin inhibitory properties. J Biol Chem. 1990 Aug 5;265(22):13029–13035. [PubMed] [Google Scholar]
- Faulk W. P., McIntyre J. A. Immunological studies of human trophoblast: markers, subsets and functions. Immunol Rev. 1983;75:139–175. doi: 10.1111/j.1600-065x.1983.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Favaloro E. J., Moraitis N., Bradstock K., Koutts J. Co-expression of haemopoietic antigens on vascular endothelial cells: a detailed phenotypic analysis. Br J Haematol. 1990 Apr;74(4):385–394. doi: 10.1111/j.1365-2141.1990.tb06324.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Poncz M., Steiner B., Rall S. C., Jr, Bennett J. S., Phillips D. R. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987 Dec 15;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Role of phosphorylation in mediating the association of myosin with the cytoskeletal structures of human platelets. J Biol Chem. 1982 Apr 25;257(8):4120–4126. [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J., Ryckwaert J. J., Pierschbacher M., Pytela R., Ruoslahti E., Plow E. F. Immunochemical and amino-terminal sequence comparison of two cytoadhesins indicates they contain similar or identical beta subunits and distinct alpha subunits. J Biol Chem. 1987 Apr 25;262(12):5437–5440. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kelley L. K., Smith C. H., King B. F. Isolation and partial characterization of the basal cell membrane of human placental trophoblast. Biochim Biophys Acta. 1983 Sep 21;734(1):91–98. doi: 10.1016/0005-2736(83)90079-2. [DOI] [PubMed] [Google Scholar]
- Krissansen G. W., Elliott M. J., Lucas C. M., Stomski F. C., Berndt M. C., Cheresh D. A., Lopez A. F., Burns G. F. Identification of a novel integrin beta subunit expressed on cultured monocytes (macrophages). Evidence that one alpha subunit can associate with multiple beta subunits. J Biol Chem. 1990 Jan 15;265(2):823–830. [PubMed] [Google Scholar]
- Kunicki T. J., Aster R. H. Isolation and immunologic characterization of the human platelet alloantigen, P1A1. Mol Immunol. 1979 Jun;16(6):353–360. doi: 10.1016/0161-5890(79)90100-7. [DOI] [PubMed] [Google Scholar]
- Labarrere C. A., McIntyre J. A., Faulk W. P. Immunohistologic evidence that villitis in human normal term placentas is an immunologic lesion. Am J Obstet Gynecol. 1990 Feb;162(2):515–522. doi: 10.1016/0002-9378(90)90421-3. [DOI] [PubMed] [Google Scholar]
- Lam S. C., Plow E. F., D'Souza S. E., Cheresh D. A., Frelinger A. L., 3rd, Ginsberg M. H. Isolation and characterization of a platelet membrane protein related to the vitronectin receptor. J Biol Chem. 1989 Mar 5;264(7):3742–3749. [PubMed] [Google Scholar]
- Lee M. C., Damjanov I. Lectin histochemistry of human placenta. Differentiation. 1984;28(2):123–128. doi: 10.1111/j.1432-0436.1984.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Argraves S., Suzuki S., Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Ramaswamy H., Hemler M. E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990 May;9(5):1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry M. V., Surolia A. Intrinsic fluorescence studies on saccharide binding to Artocarpus integrifolia lectin. Biosci Rep. 1986 Oct;6(10):853–860. doi: 10.1007/BF01116238. [DOI] [PubMed] [Google Scholar]
- Sheng Z., Vanderpuye O. A., Hull S. R., Carraway C. A., Carraway K. L. Topography and microfilament core association of a cell surface glycoprotein of ascites tumor cell microvilli. J Cell Biochem. 1989 Aug;40(4):453–466. doi: 10.1002/jcb.240400406. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Szulman A. E. The A, B and H blood-group antigens in human placenta. N Engl J Med. 1972 May 11;286(19):1028–1031. doi: 10.1056/NEJM197205112861904. [DOI] [PubMed] [Google Scholar]
- Truman P., Ford H. C. The brush border of the human term placenta. Biochim Biophys Acta. 1984 Jun 25;779(2):139–160. doi: 10.1016/0304-4157(84)90006-6. [DOI] [PubMed] [Google Scholar]
- Valentin N., Vergracht A., Bignon J. D., Cheneau M. L., Blanchard D., Kaplan C., Reznikoff-Etievant M. F., Muller J. Y. HLA-DRw52a is involved in alloimmunization against PL-A1 antigen. Hum Immunol. 1990 Feb;27(2):73–79. doi: 10.1016/0198-8859(90)90104-w. [DOI] [PubMed] [Google Scholar]
- Vanderpuye O. A., Carraway C. A., Carraway K. L. Microfilament association of ASGP-2, the concanavalin A-binding glycoprotein of the cell-surface sialomucin complex of 13,762 rat mammary ascites tumor cells. Exp Cell Res. 1988 Oct;178(2):211–223. doi: 10.1016/0014-4827(88)90392-8. [DOI] [PubMed] [Google Scholar]
- Vanderpuye O. A., Edwards H. C., Booth A. G. Proteins of the human placental microvillar cytoskeleton. alpha-Actinin. Biochem J. 1986 Jan 15;233(2):351–356. doi: 10.1042/bj2330351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen M., Uhlenbruck G., Ortmann M., Dufhues G., Fischer R. Occurrence and distribution of glycoconjugates in human tissues as detected by the Erythrina cristagalli lectin. J Histochem Cytochem. 1988 Apr;36(4):367–376. doi: 10.1177/36.4.3346539. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- van Kuppevelt T. H., Languino L. R., Gailit J. O., Suzuki S., Ruoslahti E. An alternative cytoplasmic domain of the integrin beta 3 subunit. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5415–5418. doi: 10.1073/pnas.86.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]