Abstract
Background:
Biologic therapies inhibiting the interleukin (IL) 4 or 5 pathways are very effective in the treatment of asthma, and other related conditions. However, the IL-4 and IL-5 cytokines also play a role in the generation of adaptive immune responses. Although these biologics do not cause overt immunosuppression, their effect in primary SARS-CoV-2 immunization has not been completely studied.
Objective:
To evaluate the antibody and cellular immunity after SARS-CoV-2 mRNA vaccination in patients on biologics (PoB).
Methods:
We enrolled patients with severe asthma or atopic dermatitis on benralizumab, dupilumab or mepolizumab who received the initial 2-dose SARS-CoV-2 mRNA vaccination adults in a prospective, observational study. As our control group we used a cohort of immunologically healthy subjects (no significant immunosuppression) who were not on biologics (NB). We used a multiplexed immunoassay to measure antibody levels, neutralization assays to assess antibody function, as well as flow cytometry to quantitate Spike-specific lymphocytes.
Results:
We analyzed blood from 57 PoB and 46 control subjects from NB group. PoB had lower levels of SARS-CoV-2 antibodies, pseudovirus neutralization, live virus neutralization, and frequencies of Spike-specific B and CD8 T cells at 6 months post vaccination. In subgroup analyses, patients with asthma on biologics had significantly lower pseudovirus neutralization compared to subjects with asthma who were not on biologics.
Conclusion:
PoB have reduced SARS-CoV-2-specific antibodies, neutralizing activity, as well as virus-specific B and CD8 T cells. These results have implications in considering developing a more individualized immunization strategy in patients who receive biologic medications blocking IL-4 or IL-5 pathways.
Keywords: Asthma biologics, SARS-CoV-2, COVID-19, mRNA vaccines, benralizumab, dupilumab, mepolizumab, antibody neutralization, memory B cells, memory T cells
Capsule Summary
In a prospective observational study, patients with asthma or atopic dermatitis treated with benralizumab, dupilumab, or mepolizumab exhibited lower post-vaccination SARS-CoV-2 antibody levels, neutralization activity, and Spike-specific lymphocyte memory responses than subjects not on biologics.
Introduction:
Within the first twelve months of the COVID-19 pandemic, two novel mRNA vaccines were developed and approved by the United States Food and Drug Administration (FDA) for emergency use: Pfizer-BioNTech’s Comirnaty and Moderna’s Spikevax. Upon two-dose administration, both vaccines conferred approximately 95% protection from infection with the wild type (WT) SARS-CoV-2 (1, 2), but the antibody response waned substantially over time (3, 4) and the breadth of neutralization against the emerging variants was low (5, 6). Furthermore, individual responses were highly variable, and some vulnerable populations did not mount adequate immunity (3, 7).
Some patients with asthma can have frequent symptoms and exacerbations, leading to poor quality of life and unscheduled healthcare visits. Many often benefit from biologic therapies that reduce symptoms, prevent exacerbations, and improve lung function (8). These biologic immunomodulators target various cytokines critical for disease pathogenesis, including interleukins (IL)-4, −5, and −13. However, these cytokines also play important roles in coordinating normal immunity. Although treatments with biologics are not generally associated with major risks of viral or bacterial infection, the extent of impairment of immune responses following primary SARS-CoV-2 vaccination remains unknown.
The IL-4 pathway, targeted by dupilumab, is essential for B cell development (9) as well as antibody class switching to immunoglobulins (Ig) E and G4 (10). Studies with IL-4 knock-out mice have shown lower levels of IgG1 (equivalent to human IgG4) and IgE (11). Additionally, CD4 T follicular helper cells (TFH) in germinal centers (GC) secrete IL-4 to promote the development of plasma cells (PC) and facilitate affinity maturation (12). Studies have also shown that IL-4 is necessary for the development of memory CD8 T cells (13). Interestingly, the IL-13 signaling pathway, also inhibited by dupilumab, has been implicated in B cell proliferation (14) and its administration to immunized mice increases antibody levels (15).
IL-5, directly targeted by mepolizumab, or indirectly through its receptor by benralizumab, plays an essential role in maintaining humoral immunity. In mice, IL-5 is important for differentiation and survival of PC, which are responsible for long-term antibody production (16–18). Bone marrow (BM) PC depend on eosinophil-secreted survival factors (19), and anti-IL-5 treatments result in decreased eosinophils and plasma cells (20). Moreover, these animal models of eosinophil depletion and IL-5 deficiency have shown reduced antibody levels after vaccination or infection (19, 21). Interestingly, mucosal antibody-secreting cells (22) and BM long-lived PC (unpublished data from our laboratory) have surface expression of the IL-5 receptor α suggesting that these cells which are responsible for durable humoral immunity may be bystander targets of benralizumab.
IL-4, IL-5 and IL-13 not only are implicated in atopic pathogenesis but are also essential cytokines of the normal immune response. Thus, blocking these cytokine pathways may have some unintended consequences. Prior studies assessing short-term vaccine response to a secondary vaccination found similar responses with severe asthma or atopic dermatitis treated with or without biologic therapies (23, 24). However, neither study evaluated a primary vaccination or response beyond four weeks post-immunization.
To address this gap in knowledge, we conducted a prospective observational study evaluating the immunity generated by a primary SARS-CoV-2 mRNA 2-dose vaccination regimen in patients treated with benralizumab, dupilumab or mepolizumab. We previously reported that patients on biologics (PoB) have reduced SARS-CoV-2-specific antibody responses at 3 months, compared to subjects not on biologics (NB) (25). Here, we perform a comprehensive assessment of the post-vaccination humoral and cellular immune responses and expand the follow-up period to six months after immunization. We show that patients receiving benralizumab, mepolizumab, or dupilumab have lower SARS-CoV-2 antibody levels, decreased neutralization ability, and reduced percentages of virus-specific B and CD8 T cells in comparison to controls with NB. In a subgroup analysis we found that PoB with asthma had significantly lower pseudovirus neutralization than subjects with asthma who were not on biologics (NB).
Methods:
Subject enrollment.
We conducted a prospective, observational clinical study of patients being treated with anti-IL-4 or anti-IL-5 biologics (benralizumab, dupilumab, or mepolizumab) for asthma or atopic dermatitis. We called this group “patients on biologics (PoB)”. Patients were screened and recruited from February 2021 to February 2022 from the dermatology and asthma clinics at Emory University (Atlanta, Georgia), and University of Colorado (Denver, Colorado). Subjects with chronic corticosteroid use and those with a prior history of COVID-19 infection were excluded (complete criteria in online supplement). As a control group we used samples from subjects who were not being treated with biologics. These subjects were concomitantly enrolled as immunologically healthy controls (IHC) that excluded conditions with immunosuppression (see online supplement for complete inclusion/exclusion criteria). We called this group “no biologics (NB)”. All research subjects from both study groups (PoB and NB) received a 2-dose SARS-CoV-2 mRNA vaccination regimen with Pfizer-BioNTech’s Comirnaty or Moderna’s Spikevax. Studies were approved by Institutional Review Boards at Emory University and University of Colorado. Peripheral blood samples were collected at days 30, 60, 90, and 180 after the second vaccination (Figure E1).
Flow cytometry.
We used spectral flow cytometry (5L UV Cytek Aurora) to characterize B and T cell populations. The online supplement contains complete antibody panel in tables E1 and E2, and gating strategies in figures E2 and E3. For the quantification of antigen-specific B cells, we used a tetramer-based detection of SARS-CoV-2 Spike and RBD proteins. To quantify antigen-specific T cells, we performed an in vitro AIM (Activation Induced Markers) assay previously described (29). We incubated peripheral blood mononuclear cells (PBMC) for 24 hours with commercially available SARS-CoV-2 Spike 15-mer sequences with 11 amino acid overlap peptide pool (Miltenyi Biotec PepTivator Prot_S Complete) (29). Separately, we incubated cells with Staphylococcal enterotoxin B (positive control) and a third well with no antigens (negative control). After the 24-hour incubation, cells were washed and stained with a mix of antibodies to assess the frequencies of AIM+ T cells using markers of activation specific for CD8 (CD69 and 4–1BB), CD4 (CD40L and OX40) and circulating TFH (CXCR5 of AIM+ CD4 T cells). Values were calculated after background (negative control) subtraction and negative values plotted at zero.
Antibody binding through multiplex immunoassay.
Three S1-Receptor Binding Domain (RBD) proteins (GenScript) for three variant strains of SARS-CoV-2: WT (Z03483), Delta (B.1.617.2; Z03613) and Omicron (B.1.1.529; Z03728) were tested. In addition, WT Spike S1 protein (ACROBiosystems S1N-C52H2) was obtained. Each protein was expressed with an N-terminal His6-Tag to facilitate purification, at least 90% pure and appeared as a predominant single band on SDS-PAGE analysis. Proteins were coupled to MagPlex Microspheres of spectrally distinct regions (Luminex; Austin, TX, USA) via carbodiimide coupling and tested against patient samples as previously described (26). Serum and plasma samples were tested at 1:500 dilution in duplicates. Results were analyzed on a Luminex FLEXMAP 3D instrument running xPONENT 4.3 software. Median fluorescent intensity (MFI) using individual PE-conjugated anti-IgG or combined (anti-IgA/anti-IgG/anti-IgM) detection antibodies on Enhanced PMT setting. The background value of the assay buffer was subtracted from serum or plasma results to obtain MFI minus background (Net MFI).
Pseudovirus neutralization.
We used pseudotyped lentiviruses against SARS-CoV-2 WT (Wuh-1), Delta (B.1.617.2) and Omicron (B.1.1.529) BA.1 variants in a single-round-of-infection assay, as previously described (7). To produce SARS-CoV-2 WT, Delta and Omicron pseudoviruses, an expression plasmid bearing codon optimized SARS-CoV-2 full-length S plasmid (parental sequence Wuhan-1, Genbank #: MN908947.3) was co-transfected into HEK293T cells (ATCC#CRL-11268) with plasmids encoding non-surface proteins for lentivirus production and a lentiviral backbone plasmid expressing a Luciferase-IRES-ZsGreen reporter, HIV-1 Tat and Rev packing plasmids (BEI Resources NR-53818). Pseudoviruses were harvested after 48 hours post transfection. Pseudoviruses were mixed with serial dilutions (1:50 to 1:328,050) of serum or plasma and were incubated for one hour at 37 °C in a 5% CO2 incubator, and thereafter added to monolayers of ACE-2-overexpressing 293T cells (BEI Resources NR-52511), in duplicates. 48 hours after infection, cells were lysed, luciferase was activated with the Luciferase Assay System (Bright-Glo Promega), and relative light units were measured on a synergy Biotek reader. Statistical analysis was performed using the software Graphpad Prism 9.0 for determination of ID50 (50% inhibitory dilution) through a dose-response curve fit with non-linear regression. Data are shown in a logarithmic scale and negative values are plotted at 1.99 log (lower limit of assay detection).
Live virus neutralization.
Four SARS-CoV-2 variants were tested: D614G, Delta (B.1.617.2), Omicron (B.1.1.529) BA.1 and BA.2. D614G (hCoV-19/USA/GA-EHC-083E/2020, EPI_ISL_454690) and BA.1 (hCoV19/EHC_C19_2811C, EPI_ISL_7171744) were isolated from residual nasopharyngeal swabs from patients in Atlanta, GA. B.1.617.2 (SARS-CoV-2/USA/MD-HP05647/2021) and BA.2 (SARS-CoV-2/USA/MD-HP24556/2022, EPI_ISL_8818457) variants were kindly provided by Andy Pekosz. All viruses were isolated by plaque purification on Vero-TMPRSS2 cells and passaged once prior to the generation of stocks. Stocks were deep sequenced to confirm identity with original nasal swabs or stocks as previously described (27). Live virus focus reduction neutralization tests (FRNT) were performed as previously described (27, 28).
Data processing and statistical analysis.
For flow cytometry data analysis, we used FlowJo version 10.8.1. Statistical analyses were performed using the software Graphpad Prism 9.5.0. To compare two groups, a non-parametric Mann-Whitney U test was applied to assess for differences in medians. To compare more than two groups, a Welch ANOVA test was performed. To analyze samples with multiple log10 data points on the x-axis (days post-vaccination analyses), a simple linear regression analysis with 95% confidence interval was performed. To assess correlation, a Pearson r correlation test was performed assuming Gaussian distribution of the data. A covariate analysis was used to assess for potential confounders. We considered all p-values equal to or lower than 0.05 as statistically significant.
Results:
Clinical characteristics of study groups.
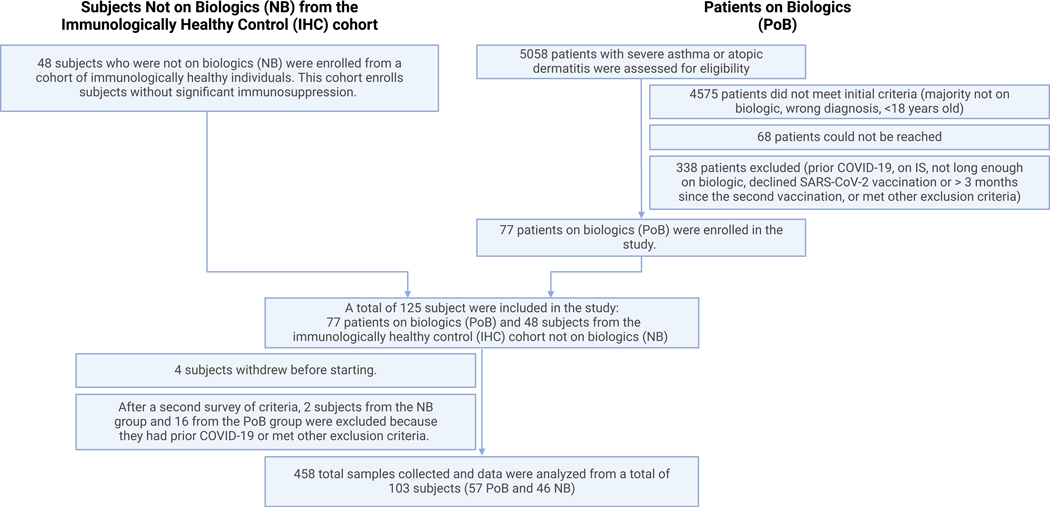
Between February and December 2021, we screened a total of 5,058 subjects. Among all screened subjects, 4575 did not meet all inclusion criteria, 68 could not be reached, and 338 met exclusion criteria or did not consent to participate (Figure 1). A total of 125 subjects participated in the study, 48 subjects enrolled as immunologically healthy controls (IHC) not on biologics (NB) and 77 patients on biologics (PoB) with severe asthma or atopic dermatitis. A total of 458 blood samples were collected. For those subjects who acquired SARS-CoV-2 infection during the study or had serological evidence of prior COVID-19, we removed their subsequent data points from the analysis. After excluding subjects with prior COVID-19 or those meeting other exclusion criteria, we analyzed a total of 103 subjects (57 PoB and 46 NB). Table 1 shows the subjects’ demographic and clinical characteristics. We found that 9 (20%) of the subjects enrolled in NB group as IHC had a diagnosis of asthma. In the PoB we had 47 subjects with asthma, 19 (33%) had atopic dermatitis (AD), and 9 (16%) had both clinical indications for biologic use. The rate of asthma exacerbations per subject with asthma in the previous year was 0.67 for the NB group vs 0.47 for the PoB group. The use of inhaled corticosteroids (ICS) in patients with asthma was 66% for NB group and 74% for PoB group.
Figure 1. Diagram of the enrollment of patients on biologics (PoB) and subjects not on biologics (NB) from the immunologically healthy control (IHC) cohort in our prospective, observational study.
COVID-19, Coronavirus disease 2019; IS, immunosuppression; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Clinical and demographic characteristics of research subjects.
| Subjects Not on Biologics (NB) from Immunologically Healthy Control (IHC) cohort | Patients on Biologics (PoB) | |
|---|---|---|
| Number of subjects | 46 | 57 |
| Average months on biologic prior to enrolment | n/a | 22 |
| Demographics | ||
| Average Age | 38 | 54 |
| Male | 14 (30%) | 24 (42%) |
| Female | 32 (70%) | 33 (58%) |
| White | 27 (60%) | 26 (46%) |
| Black | 7 (16%) | 20 (35%) |
| Asian | 10 (22%) | 7 (12%) |
| Other | 1 (2%) | 4 (7%) |
| Average body mass index | 26 | 31 |
| Current smokers | 0 (0%) | 2 (4%) |
| Former smokers | 6 (13%) | 9 (16%) |
| Never smokers | 39 (87%) | 46 (81%) |
| Type of vaccine received | ||
| Pfizer-BioNTech | 30 (67%) | 36 (63%) |
| Moderna | 15 (33%) | 21 (37%) |
| Underlying clinical condition | ||
| Subjects with asthma | 9 (20%) | 47 (82%) |
| Subjects without asthma | 37 (80%) | 10 (18%) |
| Subjects with atopic dermatitis | 0 (0%) | 19 (33%) |
| Subjects with both asthma and atopic dermatitis | 0 (0%) | 9 (16%) |
| Clinical characteristics of subjects with asthma | ||
| Asthma exacerbations in the previous year | 6 | 22 |
| Asthma exacerbations per subject with asthma in the previous year | 0.67 | 0.47 |
| Subjects with asthma on low dose ICS | 1 of 9 (11%) | 2 of 47 (4%) |
| Subjects with asthma on medium dose ICS | 2 of 9(22%) | 17 of 47 (36%) |
| Subjects with asthma on high dose ICS | 3 of 9 (33%) | 16 of 47(34%) |
| Subjects with asthma not on ICS | 3 of 9 (33%) | 12 of 47 (26%) |
| Subjects on ≥5 mg oral prednisone daily | 1 of 9 (11%) | 3 of 47 (9%) |
Vaccination-induced SARS-CoV-2-specific antibody levels are lower in PoB.
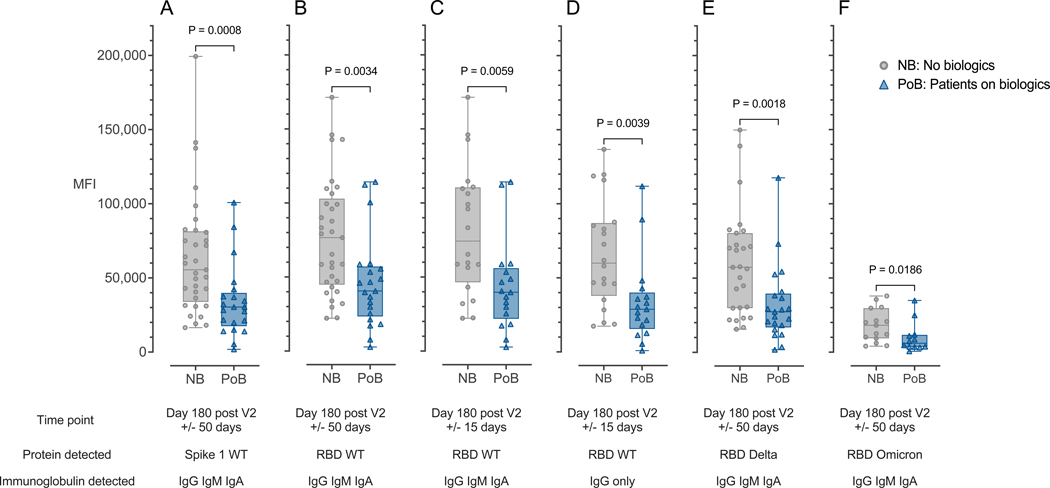
We used a multiplexed bead-based fluorescence antibody detection assay to assess lower levels of SARS-CoV-2 antibodies in samples from PoB and NB groups. Six months (day 180, +/− 50 days) after the second vaccination, we found that PoB had lower levels of immunoglobulins (combined IgG, IgM, IgA) against the wild-type (WT) SARS-CoV-2 Spike S1 protein (p=0.0008; Figure 2A) and receptor binding domain (RBD) (p=0.0034; Figure 2B). We also found differences between the groups when we analyzed a narrower 6-month time point (day 180, +/− 15 days) (p=0.0059; Figure 2C) and for RBD-specific IgG alone (p=0.0039; Figure 2D). PoB also had a lower response against RBD Delta (p=0.0018; Figure 2E) and Omicron variants (p=0.0186; Figure 2F).
Figure 2. Antibody binding is correlated with antibody levels.
(A) Combined IgA, IgG, and IgM immunoglobulins binding to WT SARS-CoV-2 Spike 1 protein measured at day 180 after V2 +/− 50 days. (B) Combined IgA, IgG, and IgM immunoglobulins binding to WT SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 50 days. (C) Combined IgA, IgG, and IgM immunoglobulins binding to WT SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 15 days. (D) Immunoglobulin IgG only binding to WT SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 15 days. (E) Combined IgA, IgG, and IgM immunoglobulins binding to the Delta-variant SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 50 days. (F) Combined IgA, IgG, and IgM immunoglobulins binding to the Omicron-variant SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 50 days. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab or mepolizumab; IgA, Immunoglobulin A; IgM, Immunoglobulin M; IgG, Immunoglobulin G; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger ribonucleic acid; RBD, receptor-binding domain; MFI, median fluorescence intensity; V2, second mRNA SARS-CoV-2 vaccination dose.
PoB exhibit lower neutralization activity, breadth, and potency against SARS-CoV-2 viruses.
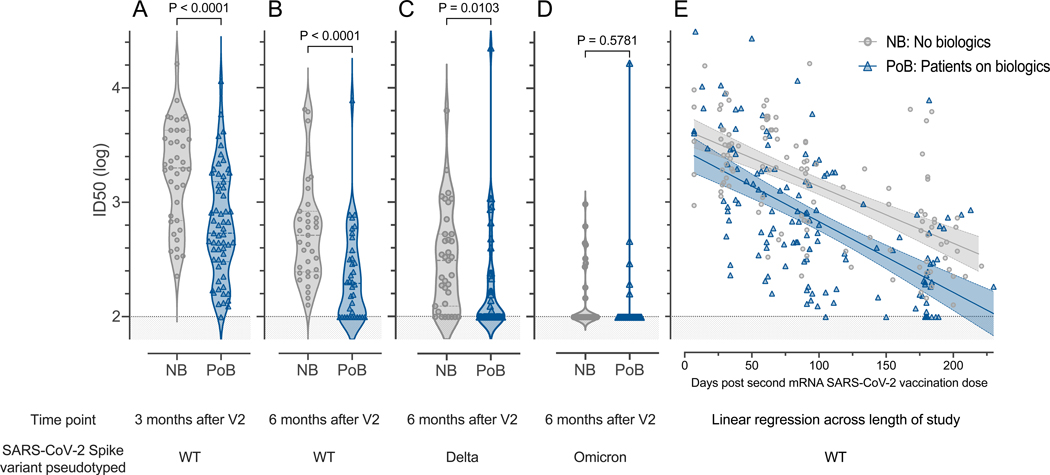
To further assess the function of subjects’ antibodies and their ability to prevent binding of SARS-CoV-2 to ACE-2 receptors, we tested their neutralization capacities in a luciferase-based pseudotyped neutralization assay. Consistent with their levels of SARS-CoV-2 antibodies, we found that PoB had lower neutralizing activity against SARS-CoV-2 WT than NB at three (p<0.0001; Figure 3A) and six months (p<0.0001; Figure 3B). We also observed lower neutralization activity against the Delta-variant in PoB compared to NB (p=0.0103; Figure 3C).
Figure 3. Pseudovirus neutralization.
All comparisons were performed between subjects not on biologics (NB) and patients on biologics (PoB) (A) Pseudovirus neutralization of WT SARS-CoV-2, comparison between three months after V2. (B) Pseudovirus neutralization of WT SARS-CoV-2 six months after V2. (C) Pseudovirus neutralization of Delta-variant SARS-CoV-2 six months after V2. (D) Pseudovirus neutralization of Omicron-variant BA.1 SARS-CoV-2 six months after V2. (E) Linear regression of data points from each group (NB and PoB) across the entire study follow-up period. Their 95% confidence intervals are represented by the shaded area. For all subfigures: the shaded area under 2 represents the lower limit of detection of the assay. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab and mepolizumab; V2, dose two of the SARS-CoV-2 mRNA vaccine; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger ribonucleic acid; ID50, 50% inhibitory dilution.
Very few of our subjects had neutralizing activity above the assay’s limit of detection against Omicron BA.1 and there were no significant differences between the two study groups (p=0.5781; Figure 3D). By longitudinally plotting all samples analyzed during the study follow-up period, we found that the difference in pseudovirus neutralizing activity against WT SARS-CoV-2 between groups was maintained across time (Figure 3E and Table E3 online supplement). Linear regression analysis showing the mean and 95% confidence interval of all data points shown in Figure 3E revealed that the groups separate at day 30 after the second vaccination dose and maintain their differences throughout the length of the study.
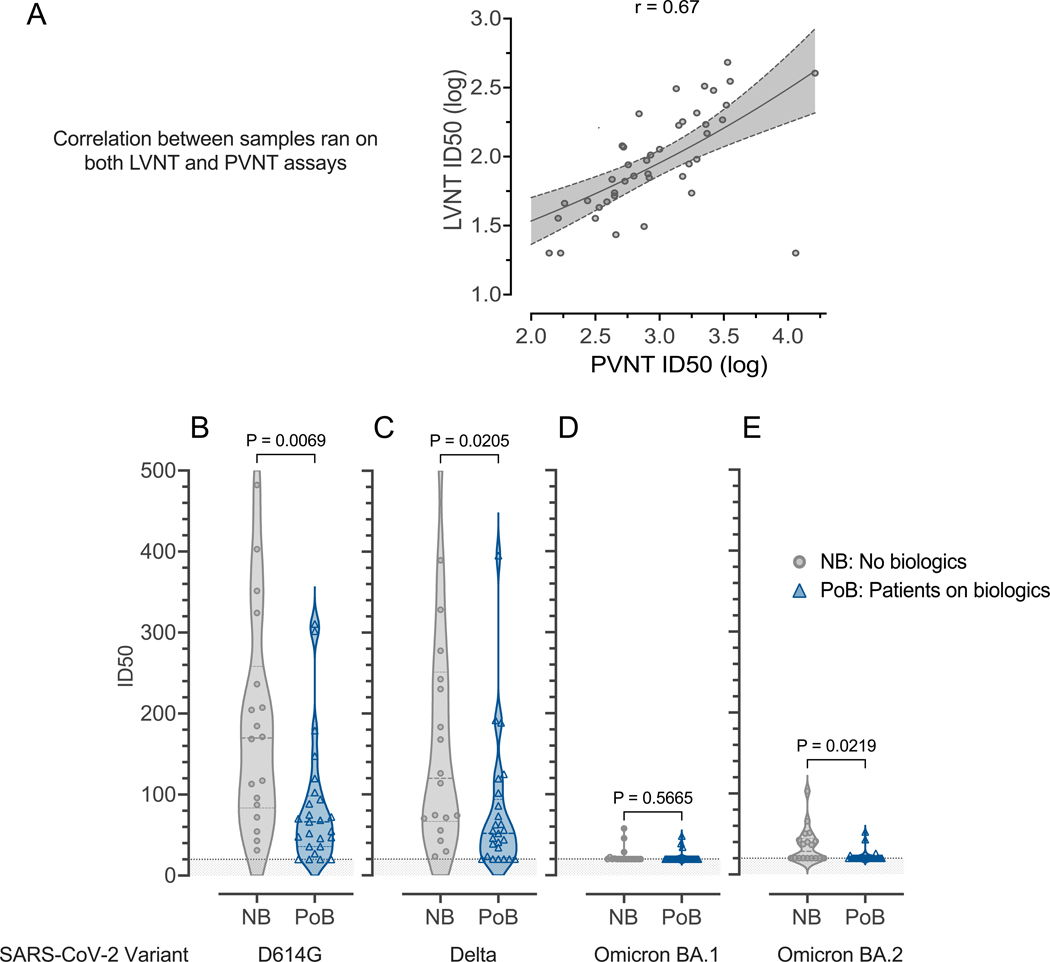
To validate results from the pseudotyped neutralization assay in a more biologically relevant experimental system, we characterized a subset of samples (between days 69 and 105 after the second vaccination) using live virus neutralization. Consistent with the lower RBD-specific antibody levels (Figure 2) and pseudovirus neutralization (Figure 3), we found that the samples from PoB had reduced ability to block live SARS-CoV-2 viruses from infecting ACE-2 expressing cells (Figure 4). We found a strong correlation among samples that were tested in both live- and pseudovirus neutralization assays (Pearson correlation coefficient 0.67, 95% CI 0.46–0.81, Figure 4A). We observed statistically significant differences between groups when analyzing the variants D614G (viruses that circulated in the first pandemic wave in 2020) (p=0.0069; Figure 4B), Delta (p=0.0205; Figure 4C), and Omicron BA.2 (p=0.0219; Figure 4E). However, there was low overall live-virus neutralization against the Omicron BA.1 variant and no significant difference between our study groups (p=0.5665; Figure 4D).
Figure 4. Live virus neutralization.
Samples collected between days 69 and 105 after the second mRNA SARS-CoV-2 vaccination (A) Correlation between live-virus neutralization and pseudovirus neutralization for samples run on both assays. (B) Live-virus neutralization of D614G-variant SARS-CoV-2. (C) Live-virus neutralization of Delta-variant SARS-CoV-2. (D) Live virus neutralization of Omicron-variant BA.1 SARS-CoV-2. (E) Live virus neutralization of Omicron-variant BA.2 SARS-CoV-2. For all subfigures: the shaded area under 20 represents the lower limit of detection of the LVNT assay. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab, and mepolizumab; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild-type; mRNA, messenger ribonucleic acid; ID50, 50% inhibitory dilution; PVNT, pseudovirus neutralization; LVNT, live virus neutralization.
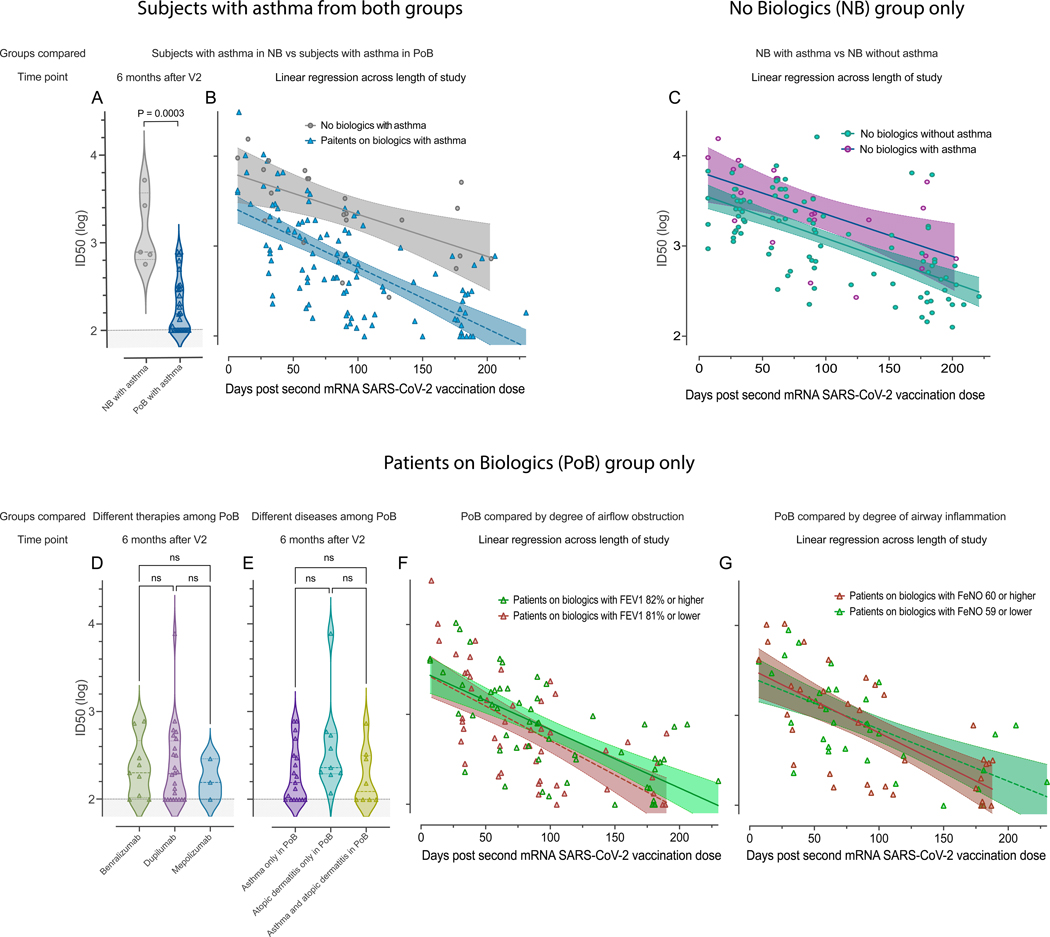
Subgroup analyses: Biologic use, and not asthma, is the driving variable of the decreased immunity observed.
We found the PoB with asthma had significantly lower pseudovirus neutralization than subjects with asthma from the IHC who were not on biologics (NB). This was true at the six-month time point post-vaccination and across the entire duration of the study (p=0.0003; Figure 5A and 5B. These results are consistent with the biologics being the variable driving the decreased pseudovirus neutralization in subjects with asthma.
Figure 5. Subgroup analyses.
Pseudovirus neutralization of WT SARS-CoV-2, comparison between subjects with asthma in the NB group and subjects with asthma in the PoB group (A) six months after V2 and (B) across the entire study follow-up period. (C) Pseudovirus neutralization of WT SARS-CoV-2, comparison between subjects with and without asthma in the no biologics control group across the entire study follow-up period. (D) Pseudovirus neutralization of WT SARS-CoV-2, comparison between different therapies (benralizumab, dupilumab, or mepolizumab) among all PoB six months after V2. (E) Pseudovirus neutralization of WT SARS-CoV-2, comparison between diseases (asthma, atopic dermatitis, or both) among all PoB six months after V2. (F) Pseudovirus neutralization of WT SARS-CoV-2 of patients with asthma on biologics stratified by the degree of airway obstruction across the entire study follow-up period. In green: patients with asthma on biologics who have an FEV1 of 82% of predicted or higher. In red: patients with asthma on biologics who have an FEV1 of 81% of predicted or lower. (G) Pseudovirus neutralization of WT SARS-CoV-2 of patients with asthma on biologics stratified by degree of airway inflammation across the entire study follow-up period. In red: patient with asthma on biologics who have a FeNO of 60ppb or higher. In green: patients with asthma on biologics who have a FeNO of 59 or lower. For subfigures A, D, and E: the shaded area under 2 represents the lower limit of detection of the assay. For subfigures B, C, F, and G: the diagonal lines represent the linear regression of data points from each group and their 95% confidence intervals are represented by the shaded area. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab and mepolizumab; V2, dose two of the SARS-CoV-2 mRNA vaccine; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger ribonucleic acid; ID50, 50% inhibitory dilution; ns, non-significant p>0.05; FEV1, forced expiratory volume in the first second; FeNO, fractional exhaled nitric oxide.
Within the NB group, subjects with asthma and those without asthma had similar pseudovirus neutralization across the length of the study (Figure 5C). This finding shows that asthma is not associated with lower pseudovirus neutralization levels in subjects without biologics.
Among PoB, there were comparable levels of neutralization between the patients treated with benralizumab, dupilumab or mepolizumab (p>0.05; Figure 5D). Similarly, among PoB, those with asthma had similar responses compared to patients with atopic dermatitis or those with both diseases (p>0.05; Figure 5E). Furthermore, no differences were notable even when stratifying by the degree of airflow obstruction (Figure 5F) or airway inflammation (Figure 5G). These findings show that PoB on biologics have low pseudovirus neutralization levels regardless of their underlying clinical condition, degree of airflow obstruction, or airway inflammation.
To further assess for confounding variables, we performed a co-variate biostatistical analysis. After adjusting for differences in the baseline characteristics of age, race, gender, and asthma status, we found that PoB had significantly lower values in the outcome variables of antibody binding to Spike and RBD, pseudovirus neutralization, and live virus neutralization than NB (p=< 0.05) (Table 2, first row). The adjusted covariates of age, race, gender, and asthma status were not significant in predicting the outcomes (P > 0.05) in all except for the live virus neutralization where subjects with asthma actually had a significantly higher neutralization than subjects without asthma. When analyzing the longitudinal results across the duration of the study of all research subjects we found that those on biologics had lower pseudovirus neutralization than those who were not on biologics (Table E1 first row, online supplement). However, when we compared all subjects with asthma to those without asthma there was not a statistically significant difference between groups (Table E1 second row, online supplement).
Table 2.
Covariate analysis
| Antibody binding IgG IgM, IgA Spike 1 WT | Antibody binding IgG IgM, IgA RBD WT | Antibody binding IgG only RBD WT | Pseudovirus neutralization WT | Pseudovirus neutralization WT Asthmatics in each group only | Live Virus Neutralization D614G | |
|---|---|---|---|---|---|---|
| PoB vs. NB | −34,778 (0.01) | −33,776 (0.02) | −36,253 (0.009) | −0.27 (0.05) | −0.95 (<.0001) | −130.66 (0.008) |
| Age | −28.12 (0.94) | −191.85 (0.60) | 23.85 (0.95) | −0.004 (0.25) | −0.006 (0.14) | −0.74 (0.56) |
| Race | ||||||
| Black | −17,474 (0.28) | −18,762 (0.26) | −8,153.52 (0.62) | −0.25 (0.07) | 0.03 (0.82) | −6.77 (0.86) |
| Asian | −32,959 (0.09) | −31,658 (0.11) | −17,623 (0.36) | −0.26 (0.25) | −0.12 (0.62) | 46.41 (0.39) |
| White (ref) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Female vs male | −4,144.48 (0.70) | 6,249.52 (0.58) | −4,282.71 (0.70) | 0.05 (0.61) | 0.16 (0.19) | −9.97 (0.76) |
| Asthma yes vs no | −2,582.92 (0.85) | 1,627.50 (0.91) | 2,715.83 (0.84) | −0.09 (0.48) | 97.93 (0.03) |
Table 2 Legend: After adjusting for differences in the baseline characteristics of age, race, gender, and asthma status we found that PoB had significantly lower values in the outcome variables of antibody binding and pseudovirus neutralization than NB (p =< 0.05, bold). The adjusted covariates of age, race, gender, and asthma status were not significant in predicting the outcomes (P > 0.05) in all except for the live virus neutralization where subjects with asthma actually had a significantly higher neutralization than subjects without asthma. The top number in each box represents the difference in means between the groups compared. For age that is a continuous variable, it represents the change in the outcome variable for every 1-year increase of age. The bottom number in parenthesis represents the p-value of each comparison. All comparisons in this table were done at the 6-month timepoint after the second vaccination dose.
In conjunction, these subgroup analyses and biostatistical adjustments for potential confounders confirmed that the decreased immunity observed in the patients on biologics is due to the use of biologics and not due to the underlying asthma condition.
PoB exhibit lower frequencies of post-vaccination SARS-CoV-2-specific B and CD8 T cells in peripheral blood.
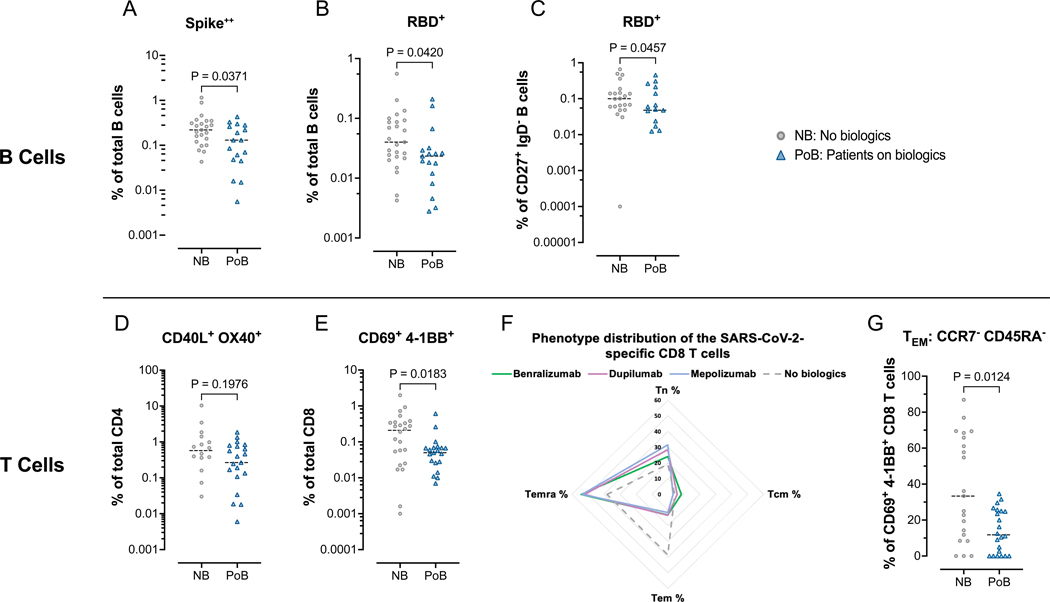
Using multi-color flow cytometry to assess the magnitude of virus-specific B cells, we constructed two-color fluorescently-conjugated tetramers of SARS-CoV-2 Spike and RBD proteins with a panel of 28-color to immunophenotype the peripheral B cells. PoB exhibited lower frequencies of Spike-specific (p=0.0371; Figure 6A) and RBD-specific B cells (p=0.0420; Figure 6B) than NB at the six-month time point (day 130–250) after the second vaccine dose. RBD-specific conventional memory B cells (IgD-, CD27+) were also significantly lower in the PoB than in NB (p=0.0457; Figure 6C). We did not find significant differences in the percentages of Spike or RBD-specific in other B cell subsets such as total double negative B cells (DN; IgD– CD27–) or their subpopulations DN1(CD21+CD11c–) and DN2 (CD21–CD11c+) (Figure E4 online supplement).
Figure 6. Flow cytometry assessment of SARS-CoV-2-specific B and T lymphocytes.
All data points in this figure are from days 130–250 after V2 (A-C) Fluorescently-conjugated Spike and RBD proteins in a tetramer conformation were used to identify ex-vivo SARS-CoV-2-specific B cells. All Spike-specific cell populations were identified using double-labeling of fluorochromes. (A) Percentages of Spike positive cells of total B cells. (B) Percentages of RBD positive cells of total B cells. (C) Percentages of RBD positive cells of switched memory B cells (CD27+, IgD–). (D-G) Ex-vivo PBMCs from NB and PoB were incubated for 24-hours with Spike peptides, with Staphylococcal enterotoxin B as a positive control, or with no antigens as a negative control. SARS-CoV-2 specific T cells were quantified using markers of activation (CD40L and OX40 for CD4 T cells, and CD69 and 4–1BB for CD8 T cells). SARS-CoV-2 specific values are calculated and plotted as the experimental condition with Spike peptides minus the experimental condition without peptides (negative control). (D) Percentages of CD40L+ OX40+ cells of total CD4 T cells. (E) Percentages of CD69+ 4–1BB+ cells of total CD8 T cells. (F) Radar plot showing the phenotypic distribution of SARS-CoV-2-specific CD8 T cells. (G) Comparisons of TEM percentages of the SARS-CoV-2 specific CD8 T cells. PBMC, peripheral blood mononuclear cells; NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab and mepolizumab; V2, dose two of the SARS-CoV-2 mRNA vaccine; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain; mRNA, messenger ribonucleic acid; TEM, effector memory CCR7– CD45RA–; TCM, central memory CCR7+ CD45RA–; TEMRA, terminally differentiated effector CCR7– CD45RA+, TN, naïve CCR7+ CD45RA+.
We performed an in vitro AIM assay (29) to characterize Spike-reactive T cells measuring the expression of surface activations markers (CD40L and OX40 for CD4; CD69 and 4–1BB for CD8 T cells) after a 24h PBMC stimulation with Spike overlapping peptides. We found no significant differences in the magnitude of Spike-reactive CD4 T cells between the groups (p=0.1976; Figure 6D). We did not observe significant differences in the percentages of antigen-specific TFH cells (Figure E5 online supplement). However, Spike-reactive CD8 T cells were lower in PoB than in NB (p=0.0183; Figure 6E) and we detected a significant reduction in the percentage of effector memory (TEM) CCR7– CD45RA– Spike-specific CD8 T cells (Figures 6F and 6G; p<0.0001).
Discussion:
Our results show that patients with severe asthma or atopic dermatitis, treated with benralizumab, dupilumab or mepolizumab have reduced antibody levels after SARS-CoV-2 mRNA vaccination, compared to subjects not on biologics. This study is a robust and comprehensive immunological analysis with a longitudinal follow-up after the primary SARS-CoV-2 mRNA vaccination in PoB. We found reduced levels of antibodies as early as one month that persisted to at least six months after the second vaccine dose.
We also found that sera from PoB had lower neutralizing ability against SARS-CoV-2 WT and Delta variant. Since all subjects in our study received the initial vaccine targeting WT SARS-CoV-2, it was not surprising that few individuals from both groups had detectable neutralizing antibodies against the highly mutated Omicron-variant. PoB also had reduced SARS-CoV-2 Spike- and RBD-specific B cells. This is consistent with the observed reduced neutralization, given that most of the ascribed neutralizing antibodies target RBD (30). As reduced neutralizing antibodies have been shown to afford less protection against COVID-19 infection (31), PoB could therefore be at a higher risk of becoming infected with SARS-CoV-2 than the general population. This study was not powered to investigate whether a reduction of immunity translates into higher risk of infection or disease severity. However, others have shown that dupilumab reduces the risk of severe COVID-19 (32). This complex balance of viral immunity in protection and pathogenesis is not necessarily mutually exclusive. So, it is possible that biologics decrease post-vaccination immunity but also provide protection by reducing inflammation during acute COVID-19. We could make an analogy of how systemic corticosteroids can reduce vaccine responses but, at the same time, decrease inflammation with severe COVID-19 disease (33).
Studies that have investigated the effect of asthma in post-vaccine immunity yield conflicting results. Some showed reduced responses in asthmatics compared to healthy controls after pneumococcal (34, 35) and influenza vaccination (36), while other studies showed similar responses between the groups (37–39). Similarly, the impact of asthma on COVID-19 severity is inconclusive (40–44). While one group found that COVID-19 vaccination was less effective at preventing COVID-19 hospitalization among asthmatics with a recent hospitalization (43), the remaining studies did not specifically assess the response after SARS-CoV-2 vaccination, and none of them independently assessed the risk of biologic use. Despite mixed results from previous publications, we were not able to explain the differences seen in our study by asthma as a disease.
Although there was not a perfectly paired control group of patients with severe asthma not on biologics, we evaluated 9 subjects with asthma in the NB group (Table 1). Despite the small number of subjects with asthma not on biologics, it was enough to consistently demonstrate across several subgroup comparisons and biostatistical adjustments for potential confounders that biologics and not the asthma condition is the variable responsible for the diminished immunity seen in the PoB group (Figure 5, and tables 2 and E1).
Several patient characteristics can negatively impact vaccine response, such as age (45), BMI (46, 47), and use of high-dose inhaled corticosteroids (48). Although many of these characteristics have not been studied in the setting of COVID-19 vaccines, these factors could have affected our results. For example, PoB in our study were not perfectly matched to the NB group (Table 1). The PoB were on average older and more obese. To address the issue of potential confounders, we performed statistical analyses adjusting for age, race, gender, and asthma status. When adjusting for these covariates, the differences found between PoB and NB groups still persisted (Table 2). Also, PoB had lower pseudovirus neutralization than the NB group across different age and BMI ranges (Figures E6 online supplement). The use of oral corticosteroid around the time of vaccination or sample collection as well as the use of inhaled corticosteroids did not explain the differences in pseudovirus neutralization seen between our groups (Figures E7, E8, and E9 online supplement). Thus, collectively, after adjusting for baseline differences and performing subgroup analyses, these potential confounders are unlikely to explain the differences between NB and PoB. Interestingly, we found lower responses in the patients on biologics who received the Pfizer-BioNTech versus Moderna vaccine at 6 months post-vaccination (Figure E10 and E11 online supplement). These differences may be due to the higher antigen dose and the longer interval between the doses in the Moderna vaccine strategy.
Our study shows that inhibition of the IL-4 or IL-5 pathways is associated with decreased post-vaccination SARS-CoV-2 specific immunity. We did not find significant post-vaccination differences among the patients on the individual biologics with different mechanisms of action (Figures 5D), but our study was not powered to detect differences between subgroups. Thus, further studies are needed. Since each biologic has a different mechanism of action targeting different molecules and receptors, the mechanism of impairing post-vaccination immunity for each biologic will likely be different. For example, the role of IL-4R inhibition may be important in the generation of germinal center antibody secreting cells (ASC) whereas the IL-5/IL-5R antagonist may play a role in the survival and maintenance of long-lived plasma cells (LLPC). We hypothesize that dupilumab targeting IL-4 would impair activation of B cells in the germinal centers (GC) (10–12) and potentially memory T cell responses (15). Since TFH IL-21 and IL-4 responses in the lymph node (LN) GC are important in primary immune responses, blocking IL-4 responses may have a role in generating ASC after primary vaccine responses with neoantigens such as in COVID-19 vaccines. Similarly, patients on Dupilumab compared to healthy adults may have no differences after secondary short-lived influenza vaccines since secondary vaccine responses may not require GC responses and arise much faster at day 5–7. As for biologics targeting the IL-5/IL-5R pathway, the inability to maintain durable antibody levels could be by depleting ASC which express the IL-5RA on its surface such as mucosal ASC and bone marrow LLPC (22). Thus, ASC within the tissue and BM microniches may be the targets of benralizumab. The effects of IL-4, IL-5, and IL-13 in Th2 polarization are well established but their role in the activation and effector function of T cells is less well documented (49). Even though no quantitative differences were found in Spike-reactive CD4 T cells between thegroups (Figure 6D), Spike-reactive CD8 T cells from PoB were decreased and exhibited a lower effector memory polarization than NB (Figure 6E, 6F and 6G). These results are consistent with a mouse study showing that IL-4 is necessary for the development of memory CD8 T cells (13). We appreciate that to evaluate each biologic mechanism of immune impairment would require much larger cohort, but so we hope to dissect these immune mechanisms in future studies and use the current study to build on these findings.
In conclusion, this study offers important immunological insights of primary immunization responses in PoB with identification of reduced antibody levels, neutralization activity, and virus-specific B and CD8 T cells after 6-month. In all, PoB have lower post-vaccination immunity and likely less protection against COVID-19. Due to the reduced post-vaccination responses observed in PoB, we recommend following the Centers for Disease Control recommendations regarding vaccination and infection prevention (50). Since uncontrolled asthma has been associated with worsening outcomes in COVID-19 disease (51), we encourage patients with severe asthma on biologics to continue their asthma-specific therapies. Future studies will be needed to design better vaccine strategies for patients on anti-IL4 or IL-5 therapies.
Supplementary Material
Clinical Implications.
Patients on benralizumab, dupilumab, or mepolizumab, had lower post-vaccination SARS-CoV-2 immunity than subjects not on biologics which could place them at increased risk of COVID-19 infection.
Acknowledgments:
We thank the trial participants, healthcare providers and research staff for their essential contributions. We also thank Dr. Robert Swerlick for help with patient recruitment.
Grant support:
NIH/National Institute of Allergy and Infectious Diseases: P01AI125180, U54CA260563, R01AI121252, R01AI172254, U01AI141993, and
NIH/National Heart, Lung, and Blood Institute: T32HL116271
NIH P51OD011132, 1U54CA260563, and NIH/NIAID CEIRR under contract 75N93021C00017 to Emory University
MCR and PAL are supported by: NIH/NHLBI T32HL116271.
NS is supported by NIH/NHLBI T32HL116271 and National Center for Advancing Translational Sciences of the NIH: UL1TR002378
NH is employed by MicroB-plex, Inc, No payments were received for this manuscript.
CS receives compensation and consulting fees from Insmed, Inc that are unrelated to this manuscript.
FH is in an Adjudication Committee Member, ASPEN Trial. Insmed Inc.
JR received funding from an NIH grant in the past 36 months.
IS receives funding from NIH/NIAID P01AI125180 and U54CA260563, receives royalties from BLI INC for Plasma cell survival media, consulting fees from GSK, Pfizer, Kayverna, Johnson & Johnson, Celgene, Bristol Myer Squibb, Visterra. Receives honoraria for presentations from Yale and Harvard Universities. Has a patent on plasma cell survival media.
FEL is supported by NIH/NIAID grants P01AI125180, U54CA260563, R01AI121252, R01AI172254, U01AI141993, research grants from Genentech and Gates Foundation. Receives royalties from BLI INC for Plasma cell survival media. Receives consulting fees from Be Bio Pharma. Received honoraria for presentations at University of Pennsylvania, University of Cincinnati, and Gerontological Advanced Practice Nurses Association. Has patents on plasma cell survival media and MENSA (media of elaborated newly synthesized antibodies). Is the founder and owner of MicroB-plex, Inc.
List of abbreviations
- IL
Interleukin
- PoB
Patients on biologics
- NB
Not on biologics
- FDA
Food and Drug Administration
- WT
Wild type
- Ig
Immunoglobulin
- PC
Plasma cell
- Tfh
T Follicular helper cells
- GC
Germinal Center
- BM
Bone Marrow
- IHC
Immunologically healthy controls
- RBD
Receptor-binding domain
- MFI
Median fluorescent intensity
- FRNT
Focus reduction neutralization tests
- PBMC
Peripheral blood mononuclear cells
- AIM
Activation induced markers
- AD
Atopic dermatitis
- ICS
Inhaled corticosteroids
- DN
Double negative B cells
- ASC
Antibody secreting cells
- LLPB
Long-lived plasma cell
- LN
Lymph node
- V2
second vaccination dose
- ID50
50% inhibitory dilution
- FEV1
Forced expiratory volume in the first second
- FeNO
fractional exhaled nitric oxide
Footnotes
Disclaimer: This work does not necessarily represent the views of the US Government or Department of Veterans Affairs.
Conflicts of interest
CEF, NC, AM, RN, MR, AMP, HQ, RPR, MW, FA, CP, WN, RP, CK, IH, SK, SU, TN, ZG, SS, SC have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2021;384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. New England Journal of Medicine. 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen DC, Lamothe PA, Woodruff MC, Saini AS, Faliti CE, Sanz I, et al. COVID-19 and plasma cells: Is there long-lived protection? Immunol Rev. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen JE, Addetia A, Dang HV, Stewart C, Brown JT, Sharkey WK, et al. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science. 2022;377(6608):890–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nooka AK, Shanmugasundaram U, Cheedarla N, Verkerke H, Edara VV, Valanparambil R, et al. Determinants of Neutralizing Antibody Response After SARS CoV-2 Vaccination in Patients With Myeloma. Journal of Clinical Oncology. 2022;40(26):3057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcgregor MC, Krings JG, Nair P, Castro M. Role of Biologics in Asthma. American Journal of Respiratory and Critical Care Medicine. 2019;199(4):433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofman F, Brock M, Taylor C, and Lyons B. IL-4 regulates differentiation and proliferation of human precursor B cells. The Journal of Immunology. 1998;141(4). [PubMed] [Google Scholar]
- 10.Moens LT, Stuart G. Cytokine-Mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Frontiers in Immunology. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254(5032):707–10. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202(4):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, et al. Interleukin 13 is a B cell stimulating factor. Journal of Experimental Medicine. 1994;179(1):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai YH, Mosmann TR. Mouse IL-13 enhances antibody production in vivo and acts directly on B cells in vitro to increase survival and hence antibody production. J Immunol. 1999;162(1):78–87. [PubMed] [Google Scholar]
- 16.Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Advances in immunology. 2009;101:191–236. [DOI] [PubMed] [Google Scholar]
- 17.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma Cell Survival Is Mediated by Synergistic Effects of Cytokines and Adhesion-Dependent Signals. The Journal of Immunology. 2003;171(4):1684–90. [DOI] [PubMed] [Google Scholar]
- 18.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral Immunity Due to Long-Lived Plasma Cells. Immunity. 1998;8(3):363–72. [DOI] [PubMed] [Google Scholar]
- 19.Chu VT, Fröhlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nature Immunology. 2011;12(2):151–9. [DOI] [PubMed] [Google Scholar]
- 20.Redfield RR, Rodriguez E, Luo Y, Rostami S, Parsons RF, Noorchashm H, et al. Interleukin 5 immunotherapy depletes alloreactive plasma cells. journal of surgical research. 2014;187(1):310–5. [DOI] [PubMed] [Google Scholar]
- 21.Strestik BD, Olbrich ARM, Hasenkrug KJ, Dittmer U. The role of IL-5, IL-6 and IL-10 in primary and vaccine-primed immune responses to infection with Friend retrovirus (Murine leukaemia virus). Journal of General Virology. 2001;82(6):1349–54. [DOI] [PubMed] [Google Scholar]
- 22.Corrado A, Ramonell RP, Woodruff MC, Tipton C, Wise S, Levy J, et al. Extrafollicular IgD+ B cells generate IgE antibody secreting cells in the nasal mucosa. Mucosal Immunology. 2021;14(5):1144–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blauvelt A, Simpson EL, Tyring SK, Purcell LA, Shumel B, Petro CD, et al. Dupilumab does not affect correlates of vaccine-induced immunity: A randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. Journal of the American Academy of Dermatology. 2019;80(1):158–67.e1. [DOI] [PubMed] [Google Scholar]
- 24.Zeitlin PL, Leong M, Cole J, Mallory RM, Shih VH, Olsson RF, et al. Benralizumab does not impair antibody response to seasonal influenza vaccination in adolescent and young adult patients with moderate to severe asthma: results from the Phase IIIb ALIZE trial. Journal of Asthma and Allergy. 2018;Volume 11:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Runnstrom MC, Morrison-Porter A, Ravindran M, Quehl H, Ramonell RP, Woodruff M, et al. Reduced COVID-19 Vaccine Response in Patients Treated with Biologic Therapies for Asthma. American Journal of Respiratory and Critical Care Medicine. 2022;205(10):1243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad NS, Nguyen DC, Kuruvilla ME, Morrison-Porter A, Anam F, Cashman KS, et al. One-Stop Serum Assay Identifies COVID-19 Disease Severity and Vaccination Responses. ImmunoHorizons. 2021;5(5):322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edara VV, Pinsky BA, Suthar MS, Lai L, Davis-Gardner ME, Floyd K, et al. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N Engl J Med. 2021;385(7):664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderheiden A, Edara VV, Floyd K, Kauffman RC, Mantus G, Anderson E, et al. Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies. Curr Protoc Immunol. 2020;131(1):e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020;183(4):1024–42 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nature Medicine. 2021;27(7):1205–11. [DOI] [PubMed] [Google Scholar]
- 32.Donlan AN, Mallawaarachchi I, Sasson JM, Preissner R, Loomba JJ, Petri WA. Dupilumab Use Is Associated With Protection From Coronavirus Disease 2019 Mortality: A Retrospective Analysis. Clin Infect Dis. 2023;76(1):148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheen YH, Kizilbash S, Ryoo E, Wi CI, Park M, Abraham RS, et al. Relationship between asthma status and antibody response pattern to 23-valent pneumococcal vaccination. J Asthma. 2020;57(4):381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung JA, Kita H, Dhillon R, Jacobson RM, Nahm MH, Park M, et al. Influence of asthma status on serotype-specific pneumococcal antibody levels. Postgrad Med. 2010;122(5):116–24. [DOI] [PubMed] [Google Scholar]
- 36.Velasco-Medina AA, García-León ML, Velázquez-Sámano G, Wong-Chew RM. The cellular and humoral immune response to influenza vaccination is comparable in asthmatic and healthy subjects. Human Vaccines & Immunotherapeutics. 2021;17(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juhn YJ, Kita H, Lee LA, Swanson RJ, Smith R, Bagniewski SM, et al. Childhood asthma and measles vaccine response. Ann Allergy Asthma Immunol. 2006;97(4):469–76. [DOI] [PubMed] [Google Scholar]
- 38.Yoo KH, Agarwal K, Butterfield M, Jacobson RM, Poland GA, Juhn YJ. Assessment of humoral and cell-mediated immune response to measles-mumps-rubella vaccine viruses among patients with asthma. Allergy Asthma Proc. 2010;31(6):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velasco-Medina AA, Garcia-Leon ML, Velazquez-Samano G, Wong-Chew RM. The cellular and humoral immune response to influenza vaccination is comparable in asthmatic and healthy subjects. Hum Vaccin Immunother. 2021;17(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunjaya AP, Allida SM, Di Tanna GL, Jenkins CR. Asthma and COVID-19 risk: a systematic review and meta-analysis. European Respiratory Journal. 2022;59(3):2101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terry PD, Heidel RE, Dhand R. Asthma in Adult Patients with COVID-19. Prevalence and Risk of Severe Disease. Am J Respir Crit Care Med. 2021;203(7):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang BZ, Chen Z, Sidell MA, Eckel SP, Martinez MP, Lurmann F, et al. Asthma Disease Status, COPD, and COVID-19 Severity in a Large Multiethnic Population. The Journal of Allergy and Clinical Immunology: In Practice. 2021;9(10):3621–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi T, Pan J, Vasileiou E, Robertson C, Sheikh A. Risk of serious COVID-19 outcomes among adults with asthma in Scotland: a national incident cohort study. The Lancet Respiratory Medicine. 2022;10(4):347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloom CI, Cullinan P, Wedzicha JA. Asthma Phenotypes and COVID-19 Risk: A Population-based Observational Study. American Journal of Respiratory and Critical Care Medicine. 2022;205(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clinical Infectious Diseases. 2021;73(11):2065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. International Journal of Obesity. 2012;36(8):1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Der Klaauw AA, Horner EC, Pereyra-Gerber P, Agrawal U, Foster WS, Spencer S, et al. Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanania NA, Sockrider M, Castro M, Holbrook JT, Tonascia J, Wise R, et al. Immune response to influenza vaccination in children and adults with asthma: effect of corticosteroid therapy. Journal of Allergy and Clinical Immunology. 2004;113(4):717–24. [DOI] [PubMed] [Google Scholar]
- 49.Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. IL-4: an important cytokine in determining the fate of T cells. Biophys Rev. 2014;6(1):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prevention CfDCa. COVID-19 prevention recommendations [Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
- 51.Karlsson Sundbaum J, Konradsen JR, Vanfleteren L, Axelsson Fisk S, Pedroletti C, Sjoo Y, et al. Uncontrolled asthma predicts severe COVID-19: a report from the Swedish National Airway Register. Ther Adv Respir Dis. 2022;16:17534666221091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.