Abstract
Background/Aims:
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, 103.4 million cases and 1.1 million deaths have occurred nationally as of November 2023. Despite the benefit of mitigating measures, the pandemic’s effect on participant safety is rarely documented.
Methods:
This study assessed noncompliance occurring from July 2019 to August 2021 that were stratified by the date of noncompliance (before or after restrictions). Events were described by size, site, noncompliance type, primary category, subcategory, and cause. In addition, noncompliance associated with COVID-19 was analyzed to determine characteristics.
Results:
In total, 323 noncompliance events occurred across 21,146 participants at risk in 35 protocols. The overall rate of noncompliance increased from 0.008 events per participant to 0.022 events per participant after the COVID-19 restrictions (p<0.001). For onsite protocols, the median within protocol change in rates was 0.001 (interquartile range 0.141) after the onset of COVID-19 restrictions (p=0.54). For large-sized protocols (n≥100), the median within protocol change in rates was also 0.001 (interquartile range 0.017) after COVID-19 restrictions (p=0.15). For events related to COVID-19 restrictions, 160/162 (99%) were minor deviations, 161/162 (99%) were procedural noncompliance, and 124/162 (77%) were an incomplete study visit.
Conclusion:
These noncompliance events have implications for clinical trial methodology because nonadherence to trial design can lead to participant safety concerns and loss of trial data validity. Protocols should be written to better facilitate the capture of all safety and efficacy data. This recommendation should be considered when changes occur to the protocol environment that are outside of the study team’s control.
Keywords: COVID-19, electronic medical records, human subjects research, patient safety, risk reduction
Background/Aims
In December 2019, a novel, viral pneumonia was identified in Wuhan, Hubei, China – later found to be a new coronavirus variant, severe acute respiratory syndrome coronavirus 2, and named coronavirus disease 2019 (COVID-19).1 Within weeks, cases were confirmed in multiple Chinese provinces, Thailand, and the United States.2, 3 Since the first domestic case in Washington state on 19 January, 2020,4 103.4 million cases and more than 1.1 million deaths have been reported in the United States as of November 2023 – making the United States the most impacted country in the world.5
By January 2020, there were no approved treatments or vaccines for COVID-19. As a result, under the direction of the U.S. Department of Health and Human Services, the Director of National Institutes of Health (NIH) implemented guidance to reduce the physical presence of staff at the NIH to diminish the risk of transmitting COVID-19 to patients and staff at the NIH Clinical Center on the Bethesda, MD campus. These measures included working remotely for non-clinical, federal staff and contractors, as well as streamlining operations toward COVID-19 research and reducing activities to mission critical only. No clinical research coordinators at NIH were furloughed. NIH clinicians, researchers, and staff adjusted to teleworking, holding team meetings over videoconferencing platforms such as Zoom and Microsoft Teams,6 and working on campus at a reduced occupancy. While beneficial in mitigating the spread of the disease,7 these recommendations, at times, limited the conduct of scientific research with human participants.
Outside of NIH, the COVID-19 pandemic resulted in widespread effects on clinical research, namely: 1) Participants missing scheduled study visits; 2) Monitors having restricted access to research records for source data verification; and 3) Study teams postponing participant recruitment or activation of new trials.8, 9 Each of these events can potentially compromise participant safety or study data. By necessity, most clinical study teams quickly adjusted their operations in an attempt to reduce risks to participants and ensure accurate and complete study data by amending protocols and processes. However, quality improvement research examining systemic pitfalls in neurology clinical trials and their relationship to noncompliance events is rare in the literature.
The purpose of this study was to determine if there were differences in the rates of noncompliance before and after the NIH intramural campus COVID-19 restrictions, based on the protocol’s size, site of protocol procedures, and study type conducted within the National Institute of Neurological Disorders and Stroke (NINDS) intramural program at the NIH. Another purpose was to provide recommendations to reduce noncompliance due to halts or disruptions to clinical research in the future. Furthermore, we sought to describe the nature of the events that resulted due to restrictions enforced to mitigate the spread of COVID-19 to share with other institutions the type of noncompliance we were experiencing in our institute during the pandemic. These results are expected to assist investigators in identifying elements in their protocols which may lead to noncompliance when unexpected events occur – both presently and in the future – and potential solutions that could be implemented when external factors affect a clinical trial’s program.
Methods
Beginning in July 2019, all intramural NINDS investigators were required to report noncompliance events that occurred in clinical research protocols to a central review body on a monthly basis. These reports identified individual noncompliance events and included the: 1) Protocol number; 2) Participant ID; 3) Date of event; 4) Date of event discovery; 5) Date principal investigator was notified; 6) Date reported to sponsor; 7) Date reported to Institutional Review Board; 8) Noncompliance type; 9) Noncompliance primary category; 10) Noncompliance subcategory; and 11) Cause of noncompliance. Reports from July 2019 to August 2021 were reviewed. The World Health Organization declared the COVID-19 outbreak a global pandemic on 11 March 2020.10 This declaration resulted in measures which required a halting of all ancillary research activities at NIH. As a result, data were stratified into two groups – events that occurred: 1) Before 11 March 2020; or 2) On or after 11 March 2020.
Individual noncompliance events were downloaded from the Clinical Informatics System for Trials and Research (CiSTAR), a 21 Code of Federal Regulations Part 11 compliant database, and then collated by protocol. The rate of noncompliance events for each protocol was calculated by dividing the total number of noncompliance events during either time period by the total number of participants enrolled in the protocol at the time of data collection.
Protocol level data, such as the cumulative number of participants, location(s) of study procedures, the type of clinical study, the protocol’s start date, and the protocols termination date (if applicable), were retrieved from an anonymized database at NIH – the Biomedical Translational Research Information System. Protocols were then classified by the size of the protocol, the site of protocol procedures, the category of the protocol, and, for protocols classified as clinical trials, the type of clinical trial. The size of the protocol was classified as either: 1) Small (less than 100 participants); or 2) Large (greater than or equal to 100 participants). The location of the protocol was classified as: 1) Onsite (at NIH); 2) Offsite (institutions outside of NIH); or 3) Multisite (protocol was conducted at both onsite and offsite locations). The study type was classified as either: 1) Natural history; or 2) Clinical trial. Finally, clinical trials were further classified as either: 1) Pharmacokinetics/dynamics; 2) Device or behavioral interventions; 3) Phase 0; 4) Phase I; 5) Phase I/II; 6) Phase II; or 7) Phase III.
In addition to protocol level data, each noncompliance event was classified by the date of the event, the type of event, the primary category of the event, the subcategory of the event, and the cause of the event. Table 1 shows the definitions of all categorizations.
Table 1.
Noncompliance Event Categorizations by Type, Primary Category, Subcategory, and Cause
| Deviation | Definition | |
|---|---|---|
| Type | Major | Potential to negatively influence the safety of the participant or to negatively influence the scientific integrity and validity of the study |
| Minor | No potential to negatively influence the rights, safety, or welfare of participants or others, or the scientific integrity or validity of the study | |
| Unanticipated problem | Any incident that is unexpected, possibly related to research, and suggests that the research places participants at a greater risk of harm | |
| Primary Category | Procedural | Deviates from the IRB approved protocol |
| Eligibility | Failure to comply with the eligibility criteria | |
| Consent | Deviates from protocol with informed consent | |
| Specimen | Deviates from the protocol with collecting research samples | |
| PII | Failure to protect the identity of the participant | |
| NIH policy | Failure to abide by NIH policies | |
| Subcategory | Procedure not performed but does not address safety or outcome measuresa | A research procedure in the protocol not performed, but was not related to safety or primary or secondary measures |
| Study visit not Completeda,b | Required participant visit for the study not completed | |
| Study visit out of timeframea,b | Required participant visit for the study completed out of the timeframe stated in the protocol | |
| Medication/dosing errora | Administering a different dosage of a study drug than what was approved and in the protocol | |
| Lab procedure not addressing safety or outcome measures not performedb | A study task in the protocol which addressed primary or secondary outcome measures not performed | |
| Non-lab procedure addressing outcome measures performed out of timeframeb | A study task which addressed primary or secondary outcome measures performed out of the timeframe stated in the protocol | |
| Lab procedure addressing safety not performedb | A laboratory procedure which addressed safety measures not performed | |
| Othera | Subcategory not satisfying the possible subcategories | |
| Cause | Study team | Principal investigator, research coordinator, or similar staff members |
| Staff member | Protocol navigator or research nurse | |
| Participant | Research participant enrolled in the clinical study | |
| Weather/travel | Weather events that cause travel delays | |
| Service | Service team member (phlebotomy/nursing) | |
| Sponsor | An organization that oversees a study | |
| Technical/equipment | Malfunction with machines or electronic devices | |
| Coronavirus | Caused by direct (the disease itself) or indirect (circumstances of the lockdown) results of COVID-19 |
Definitions of noncompliance event categorizations of type, primary category, subcategory, and cause used in this study to categorize noncompliance events.
COVID-19 = coronavirus disease 2019; IRB = Institutional Review Board; NIH = National Institutes of Health; PII = personally identifiable information; a = one of the top five subcategories overall; b = one of the top five subcategories for COVID-19 related noncompliance.
Furthermore, an analysis of noncompliance events that occurred due to COVID-19, either directly from the disease itself or indirectly from circumstances of the lockdown, was conducted. The raw data set was filtered by events that occurred after the lockdown that were COVID-19 related, and then each event was classified by the type of event, primary category, and subcategory.
Data were excluded if any piece of information was missing from the data set (e.g., number of participants enrolled in the study, location of the study, or study type). In addition, protocols, and their corresponding noncompliance events, were excluded if they started after July 2019 (the earliest noncompliance reports were reviewed) or if they were terminated before August 2021 (the latest noncompliance reports were reviewed). This allowed analyses to account only for protocols that were active during the entire length of the review period.
The Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were used to draft this article.11
This study is exempt from Institutional Review Board approval at NIH/NINDS. The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Statistical analysis
The cumulative database of noncompliance events was stored in a Microsoft Excel (Version 16.49) worksheet and analyzed and created in Prism 9 for macOS (Version 9.0.0 [86]).
Rate of noncompliance was first summarized across protocols and compared within protocol characteristic levels. Median, within protocol change in rates pre- and post-lockdown were compared using the Wilcoxon Signed-Rank Test. Then, noncompliance event rates were pooled by event characteristic, regardless of protocol, and compared using a two-proportion z-test, performed separately for each event characteristic. Data were pooled in this fashion to obtain an institute-level descriptor and due to low event rates within protocols. P-values were considered significant at 0.05.
Results
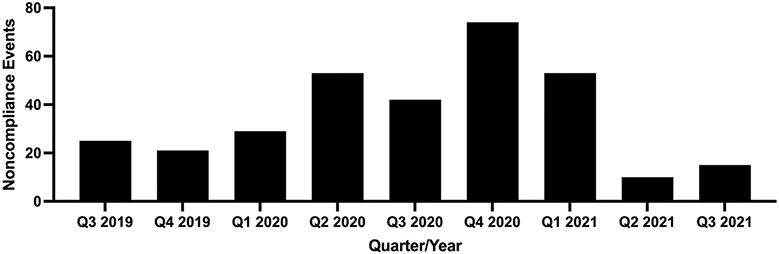
In total, 323 noncompliance events occurred across 35 protocols with 21,146 enrolled participants at risk, including 578 participants enrolled after the enforcement of COVID-19 measures. Trends of noncompliance data over the length of the study are presented in Figure 1. Of the 35 protocols, 18 (51%) were natural history studies and 17 (49%) were clinical trials. Of the 17 clinical trials, 1 (6%) was pharmacokinetics/dynamics, 6 (35%) were device or behavioral interventions, 7 (41%) were phase I, 2 (12%) were phase I/II, and 1 (6%) was phase II.
Figure 1. Raw Noncompliance Event Trends Over Time.
Raw count for noncompliance events. “Q1 2020” indicates the quarter that the COVID-19 lockdown went into effect at NIH. As data were not analyzed after August 2021, “Q3 2021” only contains noncompliance data from July 2021 and August 2021.
COVID-19 = coronavirus disease 2019; NIH = National Institutes of Health; Q1 = quarter one (January, February, March); Q2 = quarter two (April, May, June); Q3 = quarter three (July, August, September); Q4 = quarter four (October, November, December).
Eighty-two events occurred across 10,284 participants at risk before the lockdown (0.008 events per participant). After the lockdown, 241 events occurred across 10,862 participants at risk (0.022 events per participant) – an increase of 0.014 events per participant in the overall rate of noncompliance (p<0.001).
Protocol characteristics
Onsite protocols were most common and more at risk for noncompliance events after the lockdown, compared to before the lockdown, with a median within protocol change in rates of 0.001 (interquartile range (IQR) = 0.141) events per participant (p=0.54). Larger protocols were also more at risk for noncompliance, but this difference was not significant with a median within protocol change in rates of 0.001 (0.017) events per participant (p=0.15).
Natural history protocols were more at risk for noncompliance events after the lockdown, with a nonsignificant, median within protocol change in rates of 0.001 (0.019) events per participant (p=0.34). Table 2 shows a complete list of protocol specific data and key analyses.
Table 2.
Protocol Level Noncompliance Rates by Location, Size, and Category by Time
| Noncompliance events/participants at risk (unique protocols) |
Median within protocol change in rates [Q1-Q3] |
p- value |
|||
|---|---|---|---|---|---|
| Pre-Lockdown N = 35 |
Post-Lockdown N = 35 |
||||
| Location | Onsite | 76/10,284 (33) | 234/10,862 (33) | 0.001 [−0.051-0.090] | 0.54 |
| Size | Small | 67/10,284 (24) | 90/10,862 (24) | −0.015 [−0.091-0.096] | 0.76 |
| Large | 15/10,284 (11) | 151/10,862 (11) | 0.001 [−0.001-0.016] | 0.15 | |
| Category | Natural history | 29/10,284 (18) | 155/10,862 (18) | 0.001 [−0.003-0.016] | 0.34 |
| Clinical trial | 53/10,284 (17) | 86/10,862 (17) | −0.032 [−0.174-0.124] | 0.86 | |
The number of noncompliance events, participants at risk, and unique protocols by time, median change in rate of noncompliance per participant by location, size, and category at the protocol level for 35 protocols. N indicates the number of protocols. P-values are for the median, within protocol change using the Wilcoxon Signed-Rank test.
Q1 = first quartile; Q3 = third quartile.
Event characteristics
Type.
There was a relative risk reduction of 0.20 in major noncompliance events after the lockdown, with 0.001 and 0.0002 events per participant before and after, respectively (p=0.14). The rate of minor noncompliance increased, with minor events increasing three-fold from 0.007 to 0.020 events per participant (p<0.001); the same trend was seen in unanticipated problems, increasing from 0 to 0.001 events per participant (p<0.001).
Primary category.
Procedural noncompliance increased four-fold, with 0.005 and 0.020 events per participant before and after, respectively (p<0.001). Events related to failing to follow NIH policies decreased after the lockdown, with 0.001 and 0.0001 events per participant before and after, respectively (p<0.005).
Subcategory.
Noncompliance related to visit completion significantly increased after the lockdown. For study visits not being completed and study visits occurring outside of the visit window, the rate of events per participant increased from 0.001 to 0.012 (relative risk increase of 12) and 0.001 to 0.003 (relative risk increase of 3), respectively (both p<0.001). The rate of events related to medication/dosing errors increased significantly from 0 to 0.002 (p<0.001). Research study procedures not being performed, but not addressing safety or outcome measures, remained relatively consistent with 0.001 events per participant both before and after the lockdown (p=0.16), with a relative risk increase of 2. The rate of noncompliance per participant related to other factors decreased after the lockdown, with 0.002 events per participant occurring before and 0.001 events per participant occurring after (p=0.08) and a relative risk reduction of 0.5.
Cause.
Events caused by study teams decreased in the rate of noncompliance per participant after the lockdown, with 0.003 events per participant occurring before and 0.002 events per participant occurring afterwards (p=0.13). Table 3 shows a complete list of event specific data and exploratory analyses.
Table 3.
Event Level Noncompliance Rates by Type, Primary Category, Subcategory, and Cause for All Events by Time
| Noncompliance events/participants at risk (Rate of events per participant of pooled noncompliance) |
|||||
|---|---|---|---|---|---|
| Pre- Lockdown n = 82 |
Post- Lockdown n = 241 |
Change in rates |
p- value |
||
| Type | Major | 6/10,284 (0.001) |
2/10,862 (0.001) |
−0.0008 | 0.14 |
| Minor | 75/10,284 (0.007) |
217/10,862 (0.020) |
0.013 | < 0.001 | |
| Unanticipated problem | 0/10,284 (0) |
14/10,862 (0.001) |
0.001 | < 0.001 | |
| Other | 1/10,284 (0.0001) |
8/10,862 (0.001) |
0.0009 | 0.02 | |
| Primary category | Procedural | 47/10,284 (0.005) |
221/10,862 (0.020) |
0.015 | < 0.001 |
| Eligibility | 0/10,284 (0) |
0/10,862 (0) |
0 | 1.0 | |
| Consent | 10/10,284 (0.001) |
5/10,862 (0.001) |
0 | 0.16 | |
| Specimen | 13/10,284 (0.001) |
10/10,862 (0.001) |
0 | 0.45 | |
| Personally identifiable information | 1/10,284 (0.0001) |
4/10,862 (0.0004) |
0.0003 | 0.20 | |
| NIH policy | 11/10,284 (0.001) |
1/10,862 (0.0001) |
−0.0009 | < 0.005 | |
| Subcategory | Medication/dosing error | 0/10,284 (0) |
17/10,862 (0.002) |
0.002 | < 0.001 |
| Procedure not performed but does not address safety or outcome measures | 5/10,284 (0.001) |
11/10,862 (0.001) |
0 | 0.16 | |
| Other | 16/10,284 (0.002) |
8/10,862 (0.001) |
−0.001 | 0.08 | |
| Visit not completed | 9/10,284 (0.001) |
125/10,862 (0.012) |
0.011 | < 0.001 | |
| Visit out of timeframe | 7/10,284 (0.001) |
37/10,862 (0.003) |
0.002 | < 0.001 | |
| Cause | Study team | 28/10,284 (0.003) |
19/10,862 (0.002) |
−0.001 | 0.13 |
| Staff member | 2/10,284 (0.0002) |
6/10,862 (0.001) |
0.0008 | 0.18 | |
| Participant | 23/10,284 (0.002) |
21/10,862 (0.002) |
0 | 0.63 | |
| Weather/travel | 1/10,284 (0.0001) |
0/10,862 (0) |
−0.0001 | 0.30 | |
| Service | 9/10,284 (0.001) |
20/10,862 (0.002) |
0.001 | 0.06 | |
| Sponsor | 1/10,284 (0.0001) |
0/10,862 (0) |
−0.0001 | 0.30 | |
| Technical/equipment | 6/10,284 (0.001) |
4/10,862 (0.0004) |
−0.001 | 0.47 | |
| Coronavirus | 2/10,284 (0.0002) |
162/10,862 (0.015) |
0.0148 | < 0.001 | |
| Other | 10/10,284 (0.001) |
9/10,862 (0.001) |
0 | 0.73 | |
The pooled rates of noncompliance of events per participant by type, primary category, subcategory, and cause and by time at the event level. n indicates the number of noncompliance events. P-values are for the change in noncompliance rate using two-proportion z-test.
COVID-19 characteristics
For noncompliance events reported as being COVID-19 related, 162 events occurred with 2,774 enrolled participants (0.058 events per participant) after the lockdown. Focusing on COVID-19 related noncompliance events which influenced participant safety, one major noncompliance event occurred in a small sized clinical trial conducted onsite. This event involved failing to perform a procedure which addressed the study’s primary or secondary outcome measures. More specifically, a blood draw was not performed due to the participant’s disease progression and restrictions in their assisted living facility due to COVID-19.
Type.
One noncompliance event was classified as major noncompliance, with an event per participant rate of 0.0004. Most noncompliance events were classified as minor events, with 160 events occurring from a rate of 0.058 events per participant. Finally, one event was classified as other, at a rate of 0.0004 events per participant.
Primary category.
All but one noncompliance event was classified as procedural noncompliance. More specifically, 161 events were related to procedural noncompliance with a rate of 0.058 events per participant and one event was related to specimen collection with a rate of 0.0004 events per participant.
Subcategory.
The highest frequencies of noncompliance events were related to study visits not being completed, study visits being completed out of timeframe, laboratory procedures not addressing safety or outcome measures not being performed, nonlaboratory procedures addressing outcome measures being performed out of timeframe, and laboratory procedures addressing safety measures not being performed. Study visits not being completed occurred 124 times with a rate of 0.045 events per participant. Study visits being completed out of timeframe occurred 34 times with a rate of 0.012. Laboratory procedures not addressing safety or outcome measures not being performed happened 11 separate times with a 0.004 rate. Non-laboratory procedures addressing outcome measures being performed out of timeframe occurred for seven instances with a 0.003 rate. Finally, laboratory procedures addressing safety measures not being performed caused six events of noncompliance with a rate of 0.002 events per participant.
Discussion
COVID-19 is becoming one of the most widespread pandemics in modern history and it is important to understand its impact on clinical human subjects research. In March of 2020, the United States Food and Drug Administration released guidance12 to help sponsors and investigators navigate challenges with carrying out trials and maintaining safety and data integrity. Many of these guidelines, such as delaying assessments for ongoing trials, considering alternative methods to participant site visits, and utilizing remote monitoring plans when onsite monitoring is unfeasible, are supported by our noncompliance data at NINDS. Our data show the validity of instituting these recommendations to optimize patient safety and support successful completion of trials when external factors unexpectedly interfere with protocol adherence.
This article investigated the noncompliance in clinical research in intramural clinical human subjects protocols at the NIH Clinical Center from July 2019 to August 2021 (25 months) during the COVID-19 pandemic and ensuing mitigation measures. The overall rate of noncompliance events per participant increased significantly in protocols after the lockdown began, with a relative risk increase of 2.75. Importantly in our study, major deviations showed a relative risk reduction of 0.20 following mitigation measures, while minor noncompliance events experienced a relative risk increase of 2.86. Major noncompliance in clinical trials involved study visits not being completed or being completed out of timeframe, often caused by the participant (i.e., loss of contact or recovering from an unrelated surgery), whereas major noncompliance in natural history studies involved research procedures being performed that were not included in the protocol (i.e., a questionnaire that was not part of the protocol). It is important to discuss that while protocol deviations are a common occurrence in the trial landscape, cumulative minor events can compromise the overall integrity of a scientific program and should be regarded as an important target of a protocol’s quality assurance measures.
Protocols that were only conducted onsite at the NIH Clinical Center experienced the most significant increase in the rate of noncompliance events after the lockdown. This difference in compliance is perhaps due to flexible procedures already being in place for protocols seeing participants in multiple locations, as opposed to protocols that needed to design new procedures and locations for protocol-specific events ad hoc, which exposes a protocol to more risk of noncompliance. Small-sized protocols experienced a nonsignificant decrease in the rate of noncompliance per participant, while large-sized protocols experienced a nonsignificant increase in the rate of noncompliance. Although clinical trials experienced a nonsignificant decrease in noncompliance rates after the lockdown, natural history studies experienced a nonsignificant increase in noncompliance after the lockdown. At the NIH, natural history protocols, on average, have more participants than early-phase clinical trials, and a larger infrastructure might indirectly increase the chance of noncompliance events compared to smaller, early-phase clinical trials.
Two of the primary categories of events occurred with higher frequency after the lockdown – specifically, procedural noncompliance and breach of personally identifiable information, with the former increasing with a significantly higher frequency. The magnitude of this increased frequency suggests that participants in the post-lockdown period were at a greater risk of experiencing these types of noncompliance. Increased rates of noncompliance can jeopardize patient safety, patient trust, and protocol success. Therefore, finding ways to amend protocols and prevent future noncompliance are imperative. The rate of noncompliance for consent events and failing to follow NIH policy decreased after the lockdown, with the latter showing a greater, significant decrease in occurrence. Of note, there was a nonsignificant decrease in major noncompliance after the lockdown. Whether this decrease in major noncompliance was due to the reduction in participant events and enrollment in active protocols during lockdown, or whether this decrease was due to increased procedural vigilance from sponsors and principal investigators, was unable to be determined in this study.
Noncompliance that occurred because of COVID-19 was primarily categorized as minor noncompliance, related to procedural noncompliance, and further categorized by study participants not being able to complete study visits or having these study visits outside of the study visit window described in the Institutional Review Board approved protocol.
This work has three notable strengths. First, the size of the study, including the number of noncompliance events and the total number of participants, permitted more accurate rates for the variables. Second, our recommendations, listed in Table 4, have broad applicability in human clinical trials. The third strength is that although rates are presented, these can be interpreted in a more practical point of view. For example, a rate of 0.001 noncompliance events per participant can be interpreted as, and mean, one noncompliance event per 1,000 participants. Although there are strengths, there are also limitations of this study. One limitation is that the noncompliance data are manually entered by our institute’s staff members. As such, information about these data and events are subject to human error while saving new information. To minimize and adjust for this limitation, noncompliance data were verified in CiSTAR to avoid data discrepancies.Another limitation is that although some of the protocols we have reported on were approved by our Institutional Review Board within the past few years, some protocols were approved many years ago and, while there have been events before and after the lockdown, these protocols may not be actively recruiting participants. A limitation of our fourth recommendation in Table 4, modifying protocols to include broad study visit windows, is that while this may reduce noncompliance, it may inadvertently compromise the integrity of the protocol’s outcome measures and the safety of patients. Finally, an inherent limitation is that the outcomes in neurology studies may often be subjective measures, which may bias noncompliance and impact data integrity.
Table 4.
Recommendations to Reduce Human Subjects Research Noncompliance
| 1. Collect safety labs remotely, such as in the community, and access safety labs electronically |
| 2. Eliminate the use of fax machines while handling participant records |
| 3. Implement the electronic consent process |
| 4. Modify protocols to include broad study visit windows |
| 5. Perform remote monitoring visits |
| 6. Record televisits and incorporate televisit documentation into the electronic health records |
| 7. Shift protocol design to emphasize pragmatic clinical trials |
| 8. Use secure email |
Recommendations for clinical research teams to reduce noncompliance with research participants in the future.
Conclusion
Since the beginning of the COVID-19 pandemic, there has been a growing body of literature for the noncompliance of safety measures,13 personal protective equipment,14 and prevention protocols15 related to the pandemic. An additional study proposed practical solutions for continuing clinical research and improving participant safety during the COVID-19 pandemic. This study recommended telemedicine to reduce in person visits and permit workforce adaptability, remote monitoring visits as an alternative for oversight and monitoring, flexibility with obtaining laboratory tests, and remote audits and meetings.16 Literature detailing qualitative and quantitative data about the change in the rate of noncompliance events related to the pandemic as well – as presented here – is sparse, especially pertaining to neurologic human subjects research. While our data are specific to NINDS, our data and recommendations ideally would be broadly applied to clinical research protocols in all medical sub-specialties. Additional studies are warranted to assess the differences in rates of noncompliance for protocols that are actively recruiting participants versus protocols that are not actively recruiting participants. These data would help elucidate differences in the rates of noncompliance based on activity – thereby providing additional information on safety measures that can be enforced for both high activity protocols and low activity protocols.
These results have implications for the conduct of human subjects research as the field looks into the future. We propose that, where possible, protocols be developed with, and actively amended to include, maximum flexibility to facilitate the capture of all safety data, such as enabling broader study visit windows, blood draws in the community or with partnering centers, and telehealth visits when physical presence is not required, without compromising scientific quality or participant safety. While these measures might be most applicable to longer natural history or observational studies, early development and creation of flexible structure in a clinical trial protocol would allow those studies to pivot as needed to maintain participant safety and data integrity to avoid noncompliance during times of protocol “stress” – such as COVID-19. However, this would apply more broadly to allowing participants more flexibility in testing arrangements and would be expected to facilitate protocol compliance in broad measures.
In addition, partnership with clinical research organizations, or units within organizations that perform the functions of a centralized research support organization, can provide a safety net for study teams lacking adequate staffing and resources to implement real-time changes or additions to human subjects research. Within NINDS, the Clinical Trials Unit is a support infrastructure with personnel familiar with conducting human subjects research analogous to a small clinical research organization. This model is gaining popularity across the NIH intramural institutes and centers.
Specifically, internal training of investigators and staff on clinical protocols, focusing on Human Subjects Research Training and Events Reporting Training, and site initiation visits, or meetings to ensure all investigators are aware of their roles and responsibilities in the conduct of the clinical study, are encouraged for all researchers on the study team and have been shown to increase protocol compliance.17 These recommendations should be considered when changes happen to existing procedures and protocols that are outside of the principal investigator’s control. The COVID-19 pandemic, which resulted in numerous changes across the clinical research landscape, highlights the need for clear, flexible language in the protocol design that allow for study procedures to be captured with an eye toward maximal, practical design.
Grant Support:
NINDS IRP and grant number R13 NS127635-01A1
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program (NIH, Bethesda, MD) and the Neurotherapeutics Symposium 2022: Integrating Equity within Translational Research [grant number R13 NS127635-01A1] from the National Institute of Neurological Disorders and Stroke.
Footnotes
Declaration of conflicting interests
Lauren Reoma is the incoming Vice Chair of the American Academy of Neurology’s Section on Experimental Neurotherapeutics and is the Intramural NINDS Program Director for the NINDS-FDA Fellowship in Clinical Trial Methodology and Regulatory Science.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Novel Coronavirus – Thailand (ex-China), https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON234 (2020, acessed 13 March 2023). [Google Scholar]
- 3.Centers for Disease Control and Prevention. First Travel-related Cae of 2019 Novel Coronavirus Detected in United States, https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html (2020, accessed 13 March 2023). [Google Scholar]
- 4.Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020; 382: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO COVID-19 Dashboard, https://covid19.who.int/region/amro/country/us (accessed 28 November 2023).
- 6.Sidpra J, Gaier C, Reddy N, et al. Sustaining education in the age of COVID-19: a survey of synchronous web-based platforms. Quant Imaging Med Surg 2020; 10: 1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atalan A. Is the lockdown important to prevent the COVID-19 pandemic? Effects on psychology, environment and economy-perspective. Ann Med Surg (Lond) 2020; 56: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon J, Kim H and Yu KS. The Impact of COVID-19 on the Conduct of Clinical Trials for Medical Products in Korea. J Korean Med Sci 2020; 35: e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sathian B, Asim M, Banerjee I, et al. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal J Epidemiol 2020; 10: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucinotta D and Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016; 25: 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA Guidance on Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency, https://www.fda.gov/media/172258/download (2021, accessed 30 November 2023). [Google Scholar]
- 13.Banerjee R, Bhattacharya J and Majumdar P. Exponential-growth prediction bias and compliance with safety measures related to COVID-19. Soc Sci Med 2021; 268: 113473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbeek JH, Rajamaki B, Ijaz S, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev 2020; 5: Cd011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinyo DGA, Ahmadi A, Okereke M, et al. South Sudan: a young country's fight against COVID-19. Pan Afr Med J 2020; 37: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorusso D, Ray-Coquard I, Oaknin A, et al. Clinical research disruption in the post-COVID-19 era: will the pandemic lead to change? ESMO Open 2020; 5: e000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooden MJ, Norato G, Martin SB, et al. Reducing Events of Noncompliance in Neurology Human Subjects Research: the Effect of Human Subjects Research Protection Training and Site Initiation Visits. Neurotherapeutics 2021; 18: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]