Abstract
The attachment (G) protein of respiratory syncytial virus (RSV) is synthesized as two mature forms: a membrane-anchored form and a smaller secreted form. BALB/c mice scarified with vaccinia virus (VV) expressing the secreted form develop a greater pulmonary eosinophilic influx following RSV challenge than do mice scarified with VV expressing the membrane-anchored form. To determine if a soluble form of an RSV protein was sufficient to induce eosinophilia following RSV challenge, a cDNA that encoded a secreted form of the fusion (F) protein of RSV was constructed and expressed in VV (VV-Ftm−). Splenocytes and lung lymphocytes from mice primed with VV-Ftm− produced significantly more of the Th2 cytokines interleukin-4 (IL-4) and IL-5 than did mice vaccinated with VV expressing either the native (membrane-anchored) form of the F protein or the G protein. Although mice scarified with VV-Ftm− developed a slight increase in the number of pulmonary eosinophils following RSV infection, the increase was not as great as that seen in VV-G-primed mice. Despite the increased IL-4 and IL-5 production and in contrast to mice primed with VV-G, mice primed with VV-Ftm− developed RSV-specific cytotoxic T lymphocytes (CTL) and maintained high levels of gamma interferon production. These data demonstrate that recombinant VV strains expressing soluble forms of RSV proteins induce immune responses that are more Th2-like. However, this change alone does not appear sufficient to induce vaccine-augmented disease in the face of active CD8+ CTL populations.
Identification of strategies which preferentially induce specific types of immune responses is critical for the development of improved vaccines and will allow a more targeted approach to the development of antigen delivery systems. Respiratory syncytial virus (RSV), a pneumovirus within the Paramyxoviridae family, has a worldwide distribution and is the major viral pathogen of the pediatric respiratory tract. Despite years of active research, no effective vaccine against human RSV is currently available. Previous attempts at vaccination with a formalin-inactivated, alum-precipitated whole virus vaccine increased the severity of disease during primary RSV infection, and up to 80% of vaccinees required hospitalization (9, 15). A growing body of evidence from animal models suggests that RSV vaccine-enhanced illness is caused by the selective activation of virus-specific Th2 cells.
The BALB/c mouse model has been used extensively to investigate how the route and formulation of RSV antigens affect disease outcome in primed animals. Distinct immunopathological responses to RSV infection are induced in mice sensitized to different RSV proteins (18). Thus, scarification of mice with recombinant vaccinia viruses (rVV) expressing the fusion (F) protein of RSV induces a Th1-like immune response, characterized by lymphocyte and neutrophil efflux into the lungs following RSV challenge (1, 18). In contrast, mice scarified with rVV expressing the attachment (G) protein of RSV are primed for a Th2-like immune response and develop a characteristic pulmonary eosinophilia following RSV challenge (1, 18). The F and the G proteins of RSV differ in both the form and extent of their glycosylation. The F protein has five or six potential sites for N glycosylation (17) whereas the G protein is glycosylated by both N- and O-linked carbohydrate (11, 16, 32). Indeed, nearly two-thirds of the mass of the G protein is due to glycosylation (21, 31). The F and G proteins also differ in their subcellular site of expression in virus-infected cells. The F protein is a type I, membrane-anchored glycoprotein that mediates fusion of the viral membrane with that of the host cell to initiate a new infective cycle (30). The G protein is naturally synthesized as a type II, membrane-anchored glycoprotein in addition to a smaller soluble form which lacks the cytoplasmic domain and part of the membrane anchor domain (19). We and others have shown previously that mice sensitized with rVV expressing the soluble form of the G protein have a greater eosinophilic influx into the lungs following RSV challenge than do mice sensitized with rVV expressing only the membrane-anchored form (4, 14). To determine if the soluble nature of an RSV glycoprotein is sufficient to induce a Th2-like response in vaccinated mice following challenge, we have constructed an rVV expressing a transmembrane deletion mutant of the F protein that is secreted from VV-infected cells. In addition, we have analyzed the effect of retaining the F protein within the cytosol of infected cells in an attempt to improve upon cytotoxic T-lymphocyte (CTL) and Th1 priming. This approach allowed us to further investigate the role of Th subsets in the pathogenesis of exacerbated RSV infection in BALB/c mice. In the wider context of antigen delivery systems, this model allows the investigation of strategies that can be used to prime different T-cell subsets.
MATERIALS AND METHODS
Viruses.
rVV strains were constructed by standard methods as briefly described below. Plasmid LF1 (7) contains the F gene of the Long strain of human RSV inserted into the pGEM-4 vector under control of the T7 promoter. The F gene inserted into this plasmid was mutagenized by PCR to encode the Ile525Stop (ATC to TAA) mutation, following the procedure of Higuchi et al. (12) as described previously (3), to create LFtrans−. In a similar manner, LF1 was mutagenized to create LFsig−, by eliminating the sequence encoding the first 21 amino acids and introducing a new initiation codon (ATG) before the sequence encoding amino acid Phe 22. The LF1, LF trans−, and LFsig− inserts were subcloned into plasmid pRB21 (6). rVV strains were selected in CV-1 cells infected with VV vRB12 and transfected with the pRB21-derived plasmids as described previously (5). VV-G contained the full-length G gene of the Long strain of RSV and has been described elsewhere (4).
Immunofluorescence and radioimmunoprecipitation.
Expression of the F protein by the rVV was analyzed by immunofluorescence and Western blotting. HEp-2 cells were grown in tissue culture chamber slides (Nunc) and infected with VV. At 24 h later, the cells were fixed with either cold methanol for 5 min and acetone for 30 s or 3.5% formaldehyde in phosphate-buffered saline (PBS) for 30 min. Cells were processed for indirect immunofluorescence with a pool of anti-F monoclonal antibodies (20). CV-1 cells growing in 60-mm petri dishes were infected with rVV (multiplicity of infection, 5 PFU/cell) in Dulbecco’s modified Eagle’s medium supplemented with 2.5% fetal calf serum (FCS). Tran35S-Label (Amersham) was added 8 h later in methionine-free medium, and the cultures were incubated for a further 4 h. Then supernatants were collected, and cell extracts were made as described previously (17). Immunoprecipitation of extracts (2 × 107 cpm) and supernatants (5 × 106 cpm) was done with a pool of anti-F monoclonal antibodies (MAbs) and protein A-agarose. Proteins bound to the agarose resin were eluted in sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. rVV strains were propagated on CV-1 cells and subjected to titer determination on HTK− cells as described previously (25). The A2 strain of human RSV was grown in fetal calf kidney cells and stored in liquid nitrogen.
Mice.
Six-week-old, specific-pathogen-free, female BALB/c mice were vaccinated intraperitoneally (i.p.) or scarified on the rump with 2 × 106 PFU of rVV. Alternatively, mice were vaccinated intranasally (i.n.) with 2 × 105 PFU of rVV. Serum samples were taken 3 weeks postvaccination and postmortem, 5 days after challenge. At 3 weeks postvaccination, the mice were challenged i.n. with approximately 105 PFU of RSV in 50 μl. Groups of four or five mice were killed 5 days after challenge, lungs were removed, and virus titers were determined by the plaque assay (28).
Antibody assays.
The presence of serum antibodies to RSV was determined by enzyme-linked immunosorbent assay (ELISA) (26), and the presence of isotype-specific antibodies was determined by using horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G1 (IgG1) or IgG2a (ICN Biomedicals Inc., Thame, United Kingdom).
Flow-cytometric and morphological analysis of BAL fluid.
Five days after challenge, groups of five mice were killed and subjected to repeated bronchoalveolar lavage (BAL) with 12 mM lidocaine in PBS. The fluid obtained during the first round of BAL was used in the determination of an index of cell numbers infiltrating the lungs and the formation of cytocentrifuge preparations which were stained with May-Grunwald Giemsa stain. Differential cell counts of between 300 and 400 cells/mouse were examined by oil immersion microscopy. Further cells were collected from the lungs of each mouse by two further rounds of BAL and pooled in ice-cold RPMI with 10% heat-inactivated FCS for flow-cytometric analysis. Cells were resuspended at 1 × 106 to 5 × 106 per ml in PBS containing 1% normal mouse serum and 0.5% bovine serum albumin. Two-color flow-cytometric analyses were performed with rat anti-mouse CD4 coupled to fluorescein isothiocyanate (Sigma, Poole, United Kingdom) and biotinylated rat anti-mouse CD8 (Pharmingen, San Diego, Calif.) followed by streptavidin-phycoerythrin (Southern Biotechnology Associates, Birmingham, Ala.). The staining was analyzed on a FACscan instrument (Becton Dickinson, Mountain View, Calif.).
CTL assays.
Splenocytes from mice immunized 4 to 5 weeks previously with rVV were restimulated in vitro with RSV-infected autologous splenocytes for 5 days (10). Briefly, 1.5 × 107 cells were cultured in 5 ml of RPMI 1640 containing 10% heat-inactivated FCS, 2 mM glutamine, 5 × 10−5 M β-mercaptoethanol, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 3 × 106 irradiated RSV-infected naïve splenocytes. The target cells for cytotoxicity assays were BCH4 cells, which are a BALB/c fibroblast cell line (H-2d) persistently infected with the Long strain of RSV (8), and uninfected BALB/c fibroblasts (27). To demonstrate major histocompatibility complex (MHC) class I restriction of CTL activity, the mouse fibroblast cell line L-929 (H-2k) was used uninfected or infected with the A2 strain of RSV. Target cells were labelled with 51Cr (10).
Cytokine assays.
Splenocytes were taken from vaccinated mice 5 days after RSV challenge and restimulated with RSV-infected autologous splenocytes as described above. In addition, groups of vaccinated mice were killed 5 days after challenge and their lungs were removed for the isolation of lymphocytes. This was achieved by sieving finely chopped lung tissue through a 70-μm Falcon cell strainer (Becton Dickinson, Oxford, United Kingdom) and isolating viable cells following density gradient centrifugation on Ficoll Histopaque 1083 (Sigma). Lung lymphocytes at 2 × 106 ml−1 were restimulated with 4 × 105 RSV-infected autologous splenocytes in Nunc 24-well plates (Life Technologies, Paisley, United Kingdom) in a total volume of 2 ml. Culture supernatants (CS) were harvested daily and stored at −20°C until assayed for cytokines. The presence of interleukin-2 (IL-2), IL-4, IL-5, IL-10, and gamma interferon (IFN-γ) in the CS was assessed by cytokine-specific antigen capture ELISA by using antibody pairs and standard methods (Pharmingen).
RESULTS
Isolation and characterization of rVV expressing different forms of the F protein.
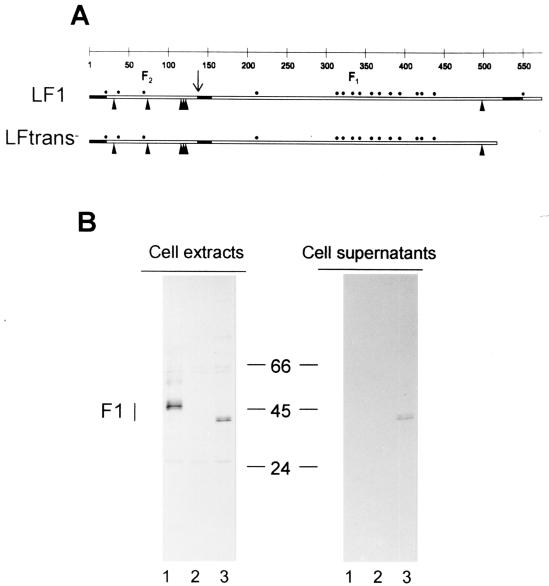
The rVV strains encoding different forms of the F protein are listed in Table 1. Recombinant VV-LF1 (hereafter referred to as VV-F) contains the wild-type gene and encodes the membrane-anchored F protein. The recombinant VV-LFtrans−, containing a form of the F protein gene in which the transmembrane region was deleted by eliminating the last 50 amino acids (Fig. 1A), is predicted to encode a soluble form of the F protein and is referred to as VV-Ftm−. The recombinant VV-LFsig− (referred to as VV-Fsig−) contains an F gene in which the sequence encoding the first 21 amino acids corresponding to the signal peptide was deleted and a new AUG codon was introduced before the sequence encoding Phe 22. This recombinant is predicted to encode an F protein that is retained within the cytoplasm of infected cells. The F protein produced in cells infected with the various rVV strains was analyzed by SDS-PAGE (Fig. 1B). The F1 subunit was readily identified in cell extracts of CV-1 cells infected with VV-F but not in the supernatants from infected cells (Fig. 1B). In contrast, the F1 subunit expressed by VV-Ftm− was detected in both cell extracts and cell supernatants (Fig. 1B). In addition, the F1 subunit expressed by VV-Ftm− had a higher mobility than did wild type F, in agreement with its smaller size. In contrast, the F protein produced in cells infected with VV-Fsig− was not detectable by the radioimmunoprecipitation assay (data not shown).
TABLE 1.
Serum antibody responses and protection against RSV induced by rVV
| Vaccinea | Serum antibody response to RSV 5 days after challengeb
|
RSV titer in lungs (Log10 PFU/g)c | ||
|---|---|---|---|---|
| Ig | IgG1 | IgG2a | ||
| VV-G | 4.1 ± 0.1 | 2.29 ± 0.52 | 3.61 ± 0.18 | <1.5d |
| VV-F | 4.4 ± 0.1 | 3.57 ± 0.25 | 4.22 ± 0.06 | <1.5 |
| VV-Ftm− | 4.5 ± 0.2 | 3.84 ± 0.18 | 4.42 ± 0.08 | <1.5 |
| VV-Fsig− | <1.5 | <1.5 | <1.5 | 3.30 ± 0.2e |
| VV-βgal | <1.5 | <1.5 | <1.5 | 3.80 ± 0.1 |
Mice were vaccinated i.p. with 2 × 106 PFU of rVV and challenged i.n. with RSV 3 weeks later. At 5 days after challenge, the mice were sampled for virus titers in the lungs and serum antibody responses. RSV-specific serum antibody responses were determined by ELISA with goat anti-mouse Ig or rat anti-mouse IgG1 or IgG2a second-step reagents.
Log10 titer of antibody to RSV (mean ± standard deviation) as determined by ELISA.
Log10 PFU of RSV per gram (mean ± standard deviation) in the lungs of vaccinated mice 5 days after virus challenge.
Virus titers in mice vaccinated with VV-G, VV-F, or VV-Ftm− were significantly different from those in mice immunized with VV-βgal (P < 0.0001).
Virus titers from mice vaccinated with VV-Fsig− were significantly different from those in mice vaccinated with VV-βgal (P < 0.004).
FIG. 1.
Construction of rVV strains and expression of F proteins. (A) Scheme of F protein insertion. The locations of hydrophobic regions (black rectangles), cleavage site for the generation of F2 and F1 subunits (↓), potential sites for N glycosylations (▴), and cysteine residues (●) are all denoted. (B) Expression of F proteins encoded by rVV strains. F proteins expressed by rVV were immunoprecipitated and analyzed by SDS-PAGE as described in Materials and Methods. The position of the F1 subunit is indicated on the left. Numbers in the middle refer to molecular weight markers (in thousands). The F2 subunit is not detected under these conditions. Note that the F1 subunit of the Ftm− mutant has a higher mobility than wild-type F, in agreement with its smaller size. Lanes: 1, cells infected with VV-F; 2, cells infected with control vRB12; 3, cells infected with VV-Ftm−.
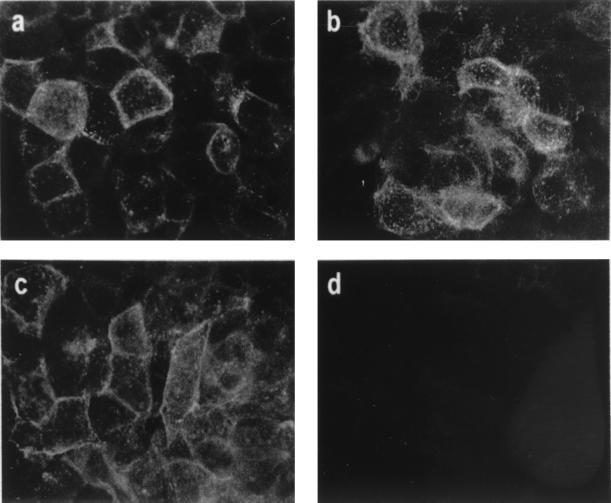
Immunofluorescence was used to examine the sites of expression of the F protein encoded by the different rVV in HEp-2 cells. Anti-F MAbs stained both VV-F and VV-Ftm−-infected cells when fixed with methanol/acetone (Fig. 2a and b). However, only VV-F expressed detectable F protein on the surface of infected cells fixed with formaldehyde to preserve cell membrane impermeability to antibodies (Fig. 2c and d). Very light cytoplasmic immunofluorescence of cells infected with VV-Fsig− and fixed with methanol-acetone was observed after staining with anti-F MAbs (data not shown).
FIG. 2.
Immunofluorescence of rVV-infected cells. HEp-2 cells were infected with rVV expressing either wild-type F (a and c) or soluble F (b and d) proteins. At 24 h later, the cells were fixed with methanol-acetone (a and b) or formaldehyde (c and d) and stained by indirect immunofluorescence with a pool of anti-F MAbs.
Antibody responses and protection against RSV challenge in mice vaccinated with VV-G, VV-F, VV-Ftm−, VV-Fsig−, or VV-βgal.
The site of expression of the F protein influenced the humoral immune response as assessed by RSV-specific ELISA following i.p. vaccination. Similar titers of RSV-specific serum antibodies were detected in mice vaccinated with VV-F, VV-Ftm−, or VV-G 3 weeks after vaccination or 5 days postchallenge (Table 1). In contrast, VV-Fsig− did not induce detectable RSV-specific antibodies at this time and was no different from the control group vaccinated with vaccinia virus-β-galactosidase (βgal). No significant differences in IgG1 or IgG2a antibody titers were detected in sera from mice vaccinated with VV-F or VV-Ftm−. Protection against i.n. challenge with RSV was examined 3 to 4 weeks after vaccination with the various rVVs (Table 1). RSV was not recovered from the lungs of mice vaccinated with VV-F, VV-Ftm−, or VV-G 5 days postchallenge (Table 1). However, mice vaccinated with VV-Fsig− or VV-βgal were not protected against subsequent RSV infection (Table 1).
Kinetics of RSV clearance from the lungs of mice vaccinated with VV-F, VV-Fsig−, or VV-βgal.
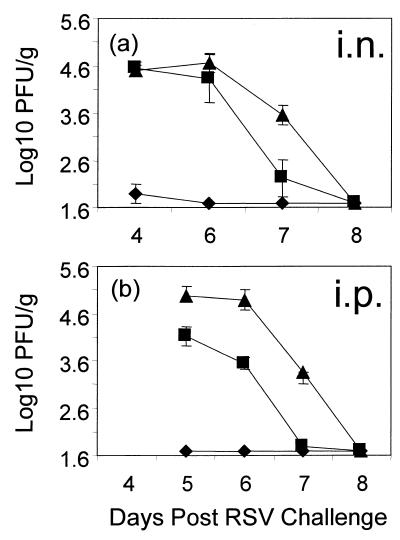
Although RSV was readily recovered 5 days after challenge from the lungs of mice vaccinated i.p. with VV-Fsig−, the titers of virus were generally lower than those recovered from mice vaccinated with VV-βgal (Table 1). To determine whether mice vaccinated with VV-Fsig− cleared RSV more rapidly from the lungs than did the control (VV-βgal) group, mice were killed on days 4 to 8 after RSV challenge and virus titers in the lungs were determined. In addition, the possibility that immunization with VV-Fsig− via the respiratory tract induced greater immunity than that induced following i.p. vaccination was investigated. The data from these experiments are summarized in Fig. 3. Virus titers in the lungs were significantly reduced in mice vaccinated i.n. or i.p. with VV-F compared with mice vaccinated with VV-βgal on days 4 and 5, (P < 0.0001) and on days 6 and 7, (P < 0.006). The titers were significantly reduced in mice vaccinated i.p. with VV-Fsig− but not in those vaccinated i.n. on day 6 (P = 0.0025 and P = 0.36, respectively). However, by day 7 after challenge, RSV titers in the lungs were significantly reduced in mice vaccinated either i.p. or i.n. with rVV-Fsig− compared with controls (P < 0.01).
FIG. 3.
Kinetics of RSV clearance from the lungs of mice vaccinated with VV-F, VV-Fsig−, or VV-βgal. To determine if i.n. vaccination with VV-Fsig− induced better immunity than that induced by i.p. vaccination, mice were vaccinated with 2 × 105 PFU of rVV i.n. (a) or 2 × 106 PFU of rVV i.p. (b) with VV-F (⧫), VV-Fsig− (■), or VV-βgal (▴). The mice were challenged i.n. with RSV 3 weeks later. RSV titers in the lungs of the mice were assayed on days 4 to 8 after challenge.
Pulmonary inflammatory response in vaccinated mice.
Following RSV challenge, there was a significant increase in the number of cells isolated after a single BAL from mice vaccinated by scarification with VV-F, VV-Ftm−, or VV-G compared with VV-βgal controls (P < 0.0001) (Table 2). However, the number of cells in the BAL from VV-Fsig−-primed mice was similar to that in BAL from VV-βgal controls following challenge (Table 2).
TABLE 2.
Pulmonary inflammatory response in vaccinated mice after RSV challenge
| Vaccinea | BAL cell counts (Log10 cells/ml)b | Time (wk) after vaccination | % of T-cell populations in BAL fluidc
|
|||
|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD4− CD8− | CD4+/CD8+ ratio | |||
| VV-G | 5.9 ± 0.1 | 3 | 36 | 27 | 35 | 1.3:1 |
| 10 | 47 | 27 | 25 | 1.7:1 | ||
| VV-F | 5.6 ± 0.1 | 3 | 36 | 36 | 26 | 1:1 |
| 10 | 21 | 65 | 13 | 1:3 | ||
| VV-Ftm− | 5.7 ± 0.1 | 3 | 42 | 33 | 22 | 1.3:1 |
| 10 | 26 | 55 | 16 | 1:2 | ||
| VV-Fsig− | 5.0 ± 0.1 | 3 | 29 | 33 | 33 | 1:1 |
| 10 | NDd | ND | ND | ND | ||
| VV-βgal | 4.8 ± 0.1 | 3 | 33 | 48 | 18 | 1.5:1 |
| 10 | 26 | 39 | 32 | 1.5:1 | ||
Mice were vaccinated by dermal scarification with 2 × 106 PFU of rVV and challenged i.n. with RSV 3 or 10 weeks later. At 5 days after RSV challenge, the mice were killed and the lungs subjected to three rounds of BAL. The counts in the fluid from the first round of BAL were used to give an index of cell numbers.
Mean ± standard deviation.
Cells obtained by repeated BAL of groups of five mice were pooled, stained for CD4 and CD8 expression, and analyzed by flow cytometry. Representative data from one of three independent experiments are shown.
ND, not determined.
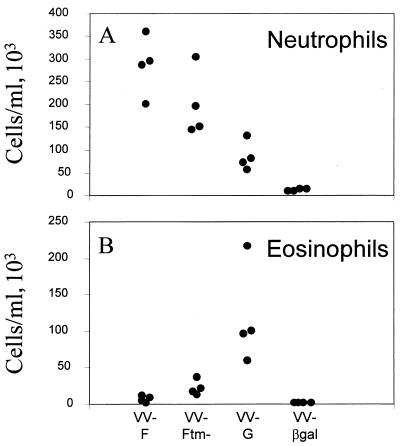
To determine if the site of expression of the F protein influenced the pulmonary inflammatory response, cytocentrifuge preparations made from fluid obtained from a single BAL, 5 days after challenge, were examined for changes in polymorphonuclear granulocyte content. Mice scarified with VV-F had a characteristic influx of neutrophils into the lungs following RSV challenge (median, 278 × 103 cells/ml) (Fig. 4A) and few eosinophils (Fig. 4B). Indeed, the levels of eosinophils were usually <1% of the total cell population in BAL fluid. Neutrophils were also present in the BAL fluid from VV-Ftm−-primed mice following challenge, although the numbers were slightly reduced compared with those from VV-F-primed mice (median, 221 × 103 cells/ml) (Fig. 4A). There was a significant increase (P < 0.007) in the numbers of eosinophils (2.5% ± 0.5% of the total population in BAL fluid) in the BAL fluid from VV-Ftm−-primed mice compared with those in the BAL fluid from VV-F-primed animals (0.4% ± 0.3% of the total population in BAL fluid) (Fig. 4B). However, the pulmonary eosinophilic response in VV-Ftm−-primed mice was not as great as that in mice primed with VV-G (14% ± 2.1% of the total population in BAL fluid). Fewer neutrophils were present in the BAL fluid from VV-G-primed mice (median, 90 × 103 cells/ml) compared with the BAL fluid from mice primed with VV-F or VV-Ftm− (Fig. 4A). The predominant cell type in the BAL fluid from VV-βgal-primed mice, following challenge, were lymphocytes and macrophages with few neutrophils (median, 9 × 103 cells/ml) and few detectable eosinophils (Fig. 4).
FIG. 4.
Effect of the site of F protein expression on neutrophil and eosinophil recruitment into the lungs of mice 5 days after RSV challenge. Cytospin preparations and differential cell counts of the cell population in BAL fluid were made. The percentage of each cell type was converted into cells per milliliter based on the total cell count in each BAL sample. Significant differences in the numbers of eosinophils between the different groups were observed (see the text).
To determine if the site of expression of the F protein influenced the recruitment of CD4+ and CD8+ cells into the lungs, cells obtained by repeated rounds of BAL were pooled and analyzed by flow cytometry (Table 2). When mice were challenged with RSV 3 to 4 weeks after vaccination, CD4+ T cells predominated in BAL fluid from VV-G-, VV-Ftm−-, and VV-βgal-vaccinated mice and equal proportions of CD4 and CD8 cells were found in the BAL fluid of VV-F- and VV-Fsig−-vaccinated mice (Table 2). The lack of protection afforded by VV-Fsig−, undetectable antibody response, and low level of cellular infiltration into the lungs following challenge suggested that these mice were similar to the control group vaccinated with VV-βgal, and further studies of the pulmonary inflammatory response did not include this group. If RSV challenge was delayed until 10 weeks after vaccination, the bias toward CD8+ T cells in BAL fluid from mice primed with VV-F or VV-Ftm− and toward CD4+ T cells in mice primed with VV-G was more pronounced. Thus, CD8+ T cells outnumbered CD4+ T cells in BAL fluid from mice scarified with VV-F or VV-Ftm− whereas CD4+ cells outnumbered CD8+ cells in BAL fluid from mice scarified with VV-G (Table 2).
Generation of a CTL response in mice vaccinated i.p. with VV-F, VV-Ftm−, VV-Fsig−, VV-G, or VV-βgal.
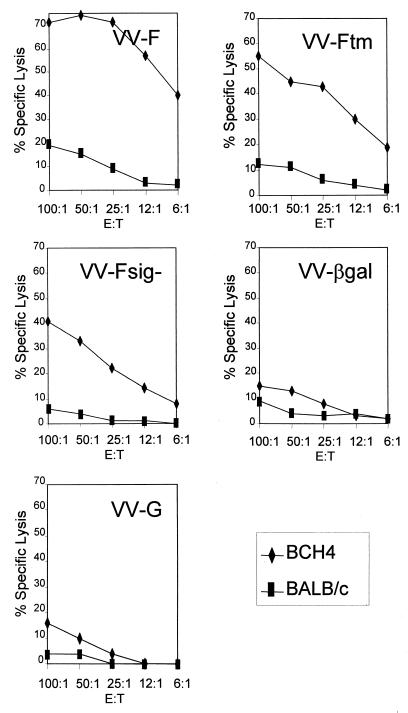
Effector CTLs were generated by in vitro RSV restimulation of splenocytes from mice 4 to 5 weeks after scarification and prior to RSV challenge. Lymphocytes from mice vaccinated with VV-F or VV-Ftm− specifically lysed BCH4 or RSV-infected BALB/c fibroblasts but not uninfected BALB/c fibroblasts (Fig. 5) or virus-infected, MHC-mismatched L-929 cells (H-2k) (data not shown). Mice vaccinated with VV-Fsig− had a reduced RSV-specific CTL response in comparison with that generated from splenocytes of VV-F- or VV-Ftm− vaccinated mice. Nevertheless, the level of CTL activity in these splenocytes was greater than that observed in splenocytes from mice vaccinated with VV-βgal or VV-G (Fig. 5).
FIG. 5.
Effect of the subcellular site of expression of the F protein on priming for RSV-specific CTL. Splenocytes from mice immunized by dermal scarification with VV-F, VV-Ftm−, VV-Fsig−, VV-G, or VV-βgal were stimulated in vitro with RSV-infected naive splenocytes for 5 days. The RSV-specific cytolytic activity of these cultures was assessed by a standard Cr51 release assay with labelled BCH4 cells or uninfected BALB/c fibroblasts at different effector-cell-to-target-cell (E:T) ratios.
Cytokine production by RSV-restimulated immune splenocytes from mice scarified with VV-F, VV-Ftm−, VV-Fsig−, or VV-βgal.
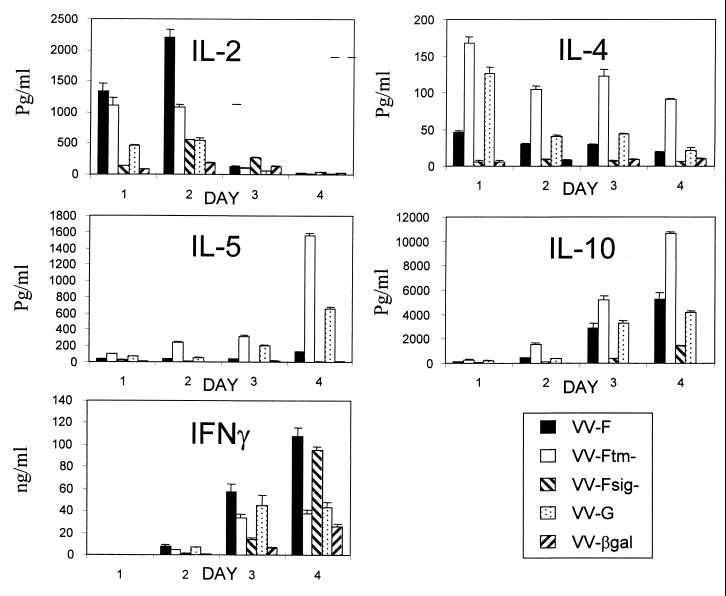
The differences in pulmonary pathology following RSV challenge in mice scarified with VV-F or VV-G have been explained, in part, by differences in Th priming (1). To determine if the site of expression of the F protein within infected cells influences the cytokine response, spleen cells were restimulated in vitro with RSV and CS were analyzed by ELISA for the presence of IFN-γ, IL-2, IL-4, IL-5, and IL-10. Lymphocytes from mice vaccinated with rVV expressing the soluble form of the F protein (VV-Ftm−) produced more IL-4, IL-5, and IL-10 and less IL-2 and IFN-γ than did lymphocytes from VV-F-, VV-Fsig−-, VV-G-, or VV-βgal-primed mice (Fig. 6). In this respect, the pattern of cytokine production by lymphocytes from VV-Ftm−-vaccinated mice more closely resembled that of cytokine production by lymphocytes from VV-G-vaccinated mice than that of cytokine production by lymphocytes from VV-F-primed mice (Fig. 6). In contrast, lymphocytes from VV-F-primed mice produced predominantly IL-2 and IFN-γ, with little or no IL-4 or IL-5. Lymphocytes from VV-Ftm−- or VV-G-vaccinated mice produced similar levels of IFN-γ, which were approximately threefold lower than those from mice vaccinated with either VV-F or VV-Fsig−. Indeed, IFN-γ was the predominant cytokine produced by spleen cells from VV-Fsig−-vaccinated mice. Thus, rVV expressing the soluble form of the F protein primed Th2 cells whereas rVV expressing a cytoplasmic form of the F protein primed Th1 cells (Fig. 6).
FIG. 6.
Cytokine production by spleen cells. Mice were vaccinated by scarification and challenged i.n. with RSV 3 to 4 weeks later. At 5 days after challenge, lymphocytes were isolated from the spleen and 1.5 × 107 cells were stimulated in vitro with RSV. Supernatants were harvested daily (days 1 to 4) and assayed for cytokines by capture ELISA. Data from one representative experiment of two are shown as the mean and standard deviation of triplicate samples from cultures of RSV-restimulated splenocytes.
Cytokine production by RSV-restimulated lung lymphocytes.
The predominant Th2 response of spleen cells from VV-Ftm−-vaccinated mice and the reduced pulmonary eosinophil response compared with that in VV-G-vaccinated mice (Fig. 4) suggests that the pattern of cytokine production by spleen cells does not accurately reflect that in the lungs. Therefore, the cytokine profiles of lymphocytes isolated from the lungs of vaccinated mice 5 days after challenge were analyzed after restimulation in vitro with RSV-infected autologous splenocytes. Supernatants were harvested on a daily basis for cytokine analysis by ELISA. Lung lymphocytes from VV-F-vaccinated mice produced the highest levels of IL-2 and IFN-γ and only low levels of IL-4 and IL-5 (Table 3). In contrast, lung lymphocytes from VV-Ftm−-vaccinated mice produced less IL-2 and IFN-γ and significantly more IL-4 and IL-5 than did those from VV-F-vaccinated mice. However, lymphocytes from VV-G-primed mice produced significantly less IL-2 and IFN-γ than did lymphocytes from either the VV-F- or VV-Ftm−-primed mice. Although the levels of IL-4 and IL-5 in CS from lung lymphocytes of VV-G-primed mice were higher than those in CS from lymphocytes of VV-F-primed mice, they were not as high as those in CS from lymphocytes of VV-Ftm−-vaccinated mice (Table 3). Thus, in contrast to the Th2-biased cytokine response by spleen cells from VV-Ftm−-primed mice, the lung lymphocytes exhibited a mixed response.
TABLE 3.
Cytokine production by lung lymphocytes
| Vaccinea | Peak levels of cytokines (pg/ml)
|
|||
|---|---|---|---|---|
| IL-2 (24 h)b | IL-4 (24 h)c | IL-5 (96 h)d | IFN-γ (48 h)e | |
| VV-G | 42 ± 7 | 75 ± 0.6 | 82 ± 6 | 7,901 ± 760 |
| VV-F | 394 ± 15 | 50 ± 3.0 | 25 ± 7 | 62,512 ± 10,729 |
| VV-Ftm− | 249 ± 5 | 140 ± 1.5 | 245 ± 9 | 35,619 ± 4,177 |
| VV-βgal | 250 ± 29 | 22 ± 1 | 26 ± 3 | 12,666 ± 1,279 |
Mice were vaccinated by dermal scarification with 2 × 106 PFU of rVV and challenged i.n. with RSV 3 weeks later. At 5 days after the challenge, lung lymphocytes from groups of five mice were pooled and 2 × 106 cells were restimulated in vitro with RSV-infected autologous splenocytes.
Statistically significant differences were found as follows: VV-F compared to all groups (P < 0.008) and all groups compared to VV-G (P < 0.01).
Statistically significant differences were found as follows: VV-βgal compared to all other groups (P < 0.004) and VV-Ftm− compared to VV-G and VV-F (P < 0.00075).
Statistically significant differences were found as follows: VV-G compared to VV-F and VV-βgal (P < 0.004), and VV-Ftm− compared to all other groups (P < 0.004).
Statistically significant differences were found as follows: VV-F compared to all other groups (P < 0.05), and VV-βgal compared to VV-G (P = 0.005).
DISCUSSION
Altering the subcellular location of the F protein in rVV-infected cells had a profound effect both on priming of the immune response and on protection against RSV challenge. As seen previously with a form of the F protein that is retained within the cytoplasm of infected cells (10), rVV expressing a signal peptide deletion mutant of the F protein primed RSV-specific CTLs but failed to elicit detectable serum antibody. However, the CTL response in VV-Fsig−-vaccinated mice was not as great as that induced by vaccination with the membrane-anchored form of the protein. This contrasts with CTLs from mice primed with rVV expressing a signal peptide-deleted hemagglutinin (HA) of influenza virus (29). Thus, CTL recognition of HA could be enhanced by altering the subcellular location of the protein in infected cells. The failure to detect Fsig− by RIPA, together with low levels of fluorescence, suggests that Fsig− may be rapidly degraded within infected cells. By deleting the signal sequence, the entry of the F or HA proteins into the endoplasmic reticulum is inhibited, and this should increase their availability as substrates for cytoplasmic proteases and presentation by class I MHC. This would be expected to result in enhanced priming of CTL and therefore to more rapid virus clearance. Although CTL priming was less pronounced in mice vaccinated with VV-Ftm− than in those vaccinated with VV-F, RSV was cleared more rapidly from the lungs compared with the clearance from the lungs of control mice vaccinated with VV-βgal. However, we found no significant difference in virus clearance if CTLs were primed by the i.n. rather than the i.p. route of vaccination.
Mice scarified with rVV expressing individual RSV proteins show characteristic patterns of pulmonary pathology, reflected in the cellular infiltrate present in the BAL fluid, and Th priming following RSV challenge. Thus, mice scarified with VV-F have a neutrophilic efflux into the lungs, and splenocytes restimulated in vitro with RSV produce predominantly IL-2 and IFN-γ (1, 4, 14, 24; also see above). Lung lesions in mice primed with VV-G are characterized by lymphocytes and an extensive eosinophilic infiltration (18), while restimulated splenocytes produce significant levels of IL-4 and IL-5 (1). Following RSV challenge, pulmonary eosinophilia was not observed in mice scarified with rVV expressing a soluble form of the F protein to the same extent as that observed in mice scarified with rVV expressing the G protein. However, a comparison of the cytokine response of spleen cells from either VV-Ftm−- or VV-G-scarified mice revealed that both groups produced high levels of IL-4 and IL-5 and only low levels of IFN-γ. In fact, priming for IL-4, IL-5, and IL-10 was more marked in VV-Ftm−-primed mice than in those primed with VV-G. Therefore, the pattern of cytokines produced by the spleen cells did not explain the reduced numbers of eosinophils in BAL fluid from VV-Ftm−-primed mice compared with those in BAL fluid from animals primed with VV-G. Analysis of the pattern of cytokine production of lung lymphocytes obtained 5 days postinfection and restimulated in vitro with RSV also showed higher levels of IL-4 and IL-5 from VV-Ftm−-primed mice than from any of the other vaccinated groups. Despite the abundant Th2 cytokine production by lung lymphocytes, VV-Ftm−-primed mice did not develop the same level of pulmonary eosinophilia as that observed in VV-G-primed mice following RSV challenge. Although IFN-γ is expressed more abundantly than Th2 cytokines from cells isolated from the lungs of VV-G-primed mice during pulmonary eosinophilia (21, 23), the present study indicates that they produce significantly lower levels of IFN-γ than do lung lymphocytes from any of the other groups of vaccinated mice. Indeed, we consistently found that lung lymphocytes from RSV-infected control mice (VV-βgal) produce more IFN-γ than did lung lymphocytes from VV-G primed mice. It may be that priming with VV-G actively down regulates IFN-γ production. Our results contrast with those of Srikiatkchorn and Braciale (24), who found higher levels of IFN-γ produced by lung lymphocytes from VV-G-primed mice than from VV-F-primed mice during an acute infection. Similarly, flow-cytometric and quantitative PCR analysis demonstrated that IFN-γ is a predominant cytokine in lung lymphocytes from VV-G-primed mice following RSV challenge (22). However, IFN-γ mRNA in BAL fluid cell populations was more abundant in VV-F-primed mice than in VV-G-primed mice 4 days after RSV challenge and the numbers of IFN-γ+ cells in BAL fluid declined more rapidly in VV-G-primed mice than in VV-F-primed mice after RSV challenge. The relative paucity of Th2 cytokines in lung lymphocytes, whether analyzed after restimulation in vitro with RSV (24; see above) or without restimulation (22) by flow cytometry or quantitative PCR has led to the suggestion that modest increases in the numbers of Th2 cytokines can result in lung eosinophilia even with abundant IFN-γ from local T cells. However, this does not seem to apply to mice primed with the soluble form of the F protein (VV-Ftm−). In these mice, the high levels of IL-4 and IL-5 were accompanied by a decrease in the level of IFN-γ production compared with that in the VV-F group, and the ratios of IFN-γ to IL-5 in pulmonary lymphocytes from VV-G- and VV-Ftm−-primed mice were similar (96:1 and 145:1, respectively). However, lung lymphocytes from VV-Ftm−-primed mice consistently produced more IL-2 (sixfold) and more IFN-γ (fourfold) than did lung lymphocytes from VV-G-primed mice. It appears that priming with VV-Ftm− resulted in a cytokine profile that was intermediate between those obtained after priming with VV-F or VV-G. The relative lack of pulmonary eosinophilia, compared with that seen in VV-G-primed mice, suggests that the increased IL-4 and IL-5 levels are insufficient for the induction of pulmonary eosinophilia after RSV challenge. IFN-γ has been implicated in the control of pulmonary eosinophilia in this model (12), and it is possible that the levels of IFN-γ in VV-Ftm−-primed mice are sufficient to prevent eosinophilia whereas the levels in VV-G-primed mice are not.
CD8+ T cells have been strongly implicated in the control of pulmonary eosinophilia (13, 23), and this is supported by the observation that VV-G-primed mice fail to elicit a CD8+ CTL response (2, 23; see above). Mice vaccinated with VV-F develop pulmonary eosinophilia during RSV infection if they are depleted of CD8+ T cells or IFN-γ (13). In contrast, BALB/c mice vaccinated with VV-G incorporating a CTL epitope from the matrix (M2) protein of RSV do not succumb to pulmonary eosinophilia following RSV infection (23). We have shown previously that altering the route of vaccination with VV-G can result in a predominant CD8+-T-cell response in the lungs after RSV challenge and that pulmonary eosinophilia is not observed (4). Furthermore, there is a preferential accumulation of CD8+ T cells in BAL fluid from VV-F-primed mice that do not succumb to pulmonary eosinophilia, whereas there is an accumulation of CD4+ T cells in the BAL fluid from VV-G-primed mice (22). Similarly, we have observed a predominant CD8+-T-cell accumulation in the BAL fluid from both VV-F- and VV-Ftm−-primed mice compared with that in VV-G-primed mice when the animals are challenged 10 weeks or more after vaccination. Although vaccination with VV-Ftm− induces high levels of IL-4 and IL-5, it also primes CD8+ CTLs. It is likely that this capacity to prime CD8+ T cells controls the potential development of lung eosinophilia in mice scarified with VV-Ftm−.
It is becoming increasingly apparent that the ability to effectively prime CD8+ T cells can counter the Th2-driven pathology during RSV infection. Vaccine candidates that prime this population of cells should serve to limit the potential problems of vaccine-augmented disease. However, priming of RSV-specific CD8+ CTLs alone is not sufficient to protect against RSV infection in BALB/c mice, as demonstrated by the results obtained with VV-Fsig−.
ACKNOWLEDGMENTS
This work was supported by the grants from the European Union (PL960637), Toudo de Investigacienes Sanitarias (98/1086), and the Ministry of Agriculture Fisheries and Food.
REFERENCES
- 1.Alwan W H, Openshaw P J. Distinct patterns of T- and B-cell immunity to respiratory syncytial virus induced by individual viral proteins. Vaccine. 1993;11:431–437. doi: 10.1016/0264-410x(93)90284-5. [DOI] [PubMed] [Google Scholar]
- 2.Alwan W H, Record F M, Openshaw P J M. Phenotypic and functional characterisation of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- 3.Arbiza J, Taylor G, Lopez J A, Furze J, Wyld S, Whyte P, Stott E J, Wertz G, Sullender W, Trudel M, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73:2225–2234. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 4.Bembridge G P, Garcia Beato R, Lopez J A, Melero J A, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J Immunol. 1998;161:2473–2480. [PubMed] [Google Scholar]
- 5.Bembridge G P, Lopez J A, Cook R, Melero J A, Taylor G. Recombinant vaccinia virus coexpressing the F protein of respiratory syncytial virus (RSV) and interleukin-4 (IL-4) does not inhibit the development of RSV-specific memory cytotoxic T lymphocytes, whereas priming is diminished in the presence of high levels of IL-2 or gamma interferon. J Virol. 1998;72:4080–4087. doi: 10.1128/jvi.72.5.4080-4087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasco R, Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157–162. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- 7.Cristina J, Lopez J A, Albo C, Garciabarreno B, Garcia J, Melero J A, Portela A. Analysis of genetic-variability in human respiratory syncytial virus by the RNase-a mismatch cleavage method—subtype divergence and heterogeneity. Virology. 1990;174:126–134. doi: 10.1016/0042-6822(90)90061-u. [DOI] [PubMed] [Google Scholar]
- 8.Fernie B F, Ford E C, Gerin J L. The development of BALB/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981;167:83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- 9.Fulginiti V A, Eller J J, Sieber O F, Joyner J W, Minamihani M, Meikeiejohn G. Respiratory virus immunisation. I. A field trial of two inactivated respiratory vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 10.Gaddum R M, Cook R S, Wyld S G, Lopez J A, Bustos R, Melero J A, Taylor G. Mutant forms of the F protein of human respiratory syncytial (RS) virus induce a cytotoxic T lymphocyte response but not a neutralizing antibody response and only transient resistance to RS virus infection. J Gen Virol. 1996;77:1239–1248. doi: 10.1099/0022-1317-77-6-1239. [DOI] [PubMed] [Google Scholar]
- 11.Gruber C, Levine S. Respiratory syncytial virus polypeptides. IV. The oligosaccharides of the glycoproteins. J Gen Virol. 1985;66:417–432. doi: 10.1099/0022-1317-66-3-417. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi R, Krummel B, Saiki R K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussell T, Baldwin C J, A. O G, Openshaw P J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 14.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H W, Canchola J G, Brandt C C, Jensen K P, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 16.Lambert D M. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology. 1988;164:458–466. doi: 10.1016/0042-6822(88)90560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez J A, Bustos R, Portela A, Garcia Barreno B, Melero J A. A point mutation in the F1 subunit of human respiratory syncytial virus fusion glycoprotein blocks its cell surface transport at an early stage of the exocytic pathway. J Gen Virol. 1996;77:649–660. doi: 10.1099/0022-1317-77-4-649. [DOI] [PubMed] [Google Scholar]
- 18.Openshaw P J, Clarke S L, Record F M. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 19.Roberts S R, Lichtenstein D, Ball L A, Wertz G W. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol. 1994;68:4538–4546. doi: 10.1128/jvi.68.7.4538-4546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rueda P, Garcia Barreno B, Melero J A. Loss of conserved cysteine residues in the attachment (G) glycoprotein of two human respiratory syncytial virus escape mutants that contain multiple A-G substitutions (hypermutations) Virology. 1994;198:653–662. doi: 10.1006/viro.1994.1077. [DOI] [PubMed] [Google Scholar]
- 21.Satake M, Coligan J E, Elango N, Norrby E, Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985;13:7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spender L C, Hussell T, Openshaw P J. Abundant IFN-gamma production by local T cells in respiratory syncytial virus-induced eosinophilic lung disease. J Gen Virol. 1998;79:1751–1758. doi: 10.1099/0022-1317-79-7-1751. [DOI] [PubMed] [Google Scholar]
- 23.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikiatkhachorn A, Braciale T J. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stott E J, Taylor G, Ball L A, Anderson K, Young K K, King A M, Wertz G W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor G, Stott E J, Furze J, Ford J, Sopp P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J Gen Virol. 1992;73:2217–2223. doi: 10.1099/0022-1317-73-9-2217. [DOI] [PubMed] [Google Scholar]
- 27.Taylor G, Stott E J, Hayle A J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985;66:2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- 28.Taylor G, Stott E J, Hughes M, Collins A P. Respiratory syncytial virus infection in mice. Infect Immun. 1984;43:649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend A R, Bastin J, Gould K, Brownlee G G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986;324:575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- 30.Walsh E E, Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983;47:171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wertz G W, Collins P L, Huang Y, Gruber C, Levine S, Ball L A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci USA. 1985;82:4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wertz G W, Krieger M, Ball L A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989;63:4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]