Abstract
Objective
To evaluate the efficacy and safety of thermal ablation in treating solitary low-risk T2N0M0 papillary thyroid cancer (PTC) and compare the outcomes of microwave ablation (MWA) and radiofrequency ablation (RFA).
Materials and Methods
This retrospective, single center study involved 34 patients (age: 40.0 ± 13.9 years; 28 female) who had low-risk T2N0M0 PTC with a maximum diameter >2 cm and ≤4 cm and underwent MWA (n = 15) or RFA (n = 19) from November 2016 to April 2023. The primary outcomes were the cumulative rate of disease progression and delayed surgery rates. In contrast, the secondary outcomes included changes in tumor size, cumulative rate of complete tumor disappearance, and complication rates.
Results
The median follow-up period was 18.0 months (interquartile range [IQR]: 9.0–40.0 months). At 12 months, the median volume reduction rate of the ablation zone was 74.2% (IQR: 53.7%–86.0%). Disease progression was noted in two patients within 1 year, including one patient with local tumor progression post-RFA and one with a new tumor post-MWA, resulting in a constant cumulative disease progression rate of 8.8% (95% confidence interval [CI]: 0%–19.8%) throughout the remaining follow-up period. Both patients were subsequently treated with additional ablation and did not require surgery. The cumulative rates of complete tumor disappearance at 1, 3, and 5 years were 4.0% (95% CI: 0%–11.4%), 26.8% (95% CI: 2.7%–44.9%), and 51.2% (95% CI: 0%–79.1%), respectively. No significant differences were observed in the disease progression (P = 0.829) or complete tumor disappearance (P = 0.633) rates between the MWA and RFA groups. Complications occurred in 14.7% (5/34) of patients presenting with transient hoarseness. RFA had a higher but not statistically significant complication rate than MWA did (21.1% [4/19] vs. 6.7% [1/15]; P = 0.355).
Conclusion
Both MWA and RFA demonstrated promising short-term outcomes in terms of efficacy and safety in treating solitary low-risk T2N0M0 PTC, with no significant differences.
Keywords: Thyroid cancer, Papillary thyroid carcinoma, Radiofrequency ablation, Microwave ablation, Ultrasound
INTRODUCTION
Papillary thyroid cancer (PTC) is the most prevalent type of thyroid cancer, accounting for 83.6% of all cancer cases [1]. From 1974 to 2013, the incidence of T2 PTC consistently increased at an annual rate of 1.6% [2]. Despite this increase, T2N0M0 PTC, categorized as prognostic stage I, has an exceptionally high disease-specific survival rate over 10 years, ranging from 98% to 100% [3]. Standard treatments for T2 PTC include thyroid lobectomy and total thyroidectomy [4,5,6]; however, its high incidence in individuals aged <45 years raises concerns regarding surgical complications [7]. These include long-term functional issues, such as hypothyroidism, hypoparathyroidism, and laryngeal nerve injury, as well as impact on quality of life due to fatigue, sleep disturbances, and cosmetic concerns [8,9,10].
Consequently, there has been a shift toward considering minimally invasive alternatives such as ultrasound (US)-guided thermal ablation techniques for treating PTC [11,12]. In recent years, radiofrequency ablation (RFA) and microwave ablation (MWA) have shown promising results in managing early-stage PTC, with efficacies comparable to that of traditional surgery but with potentially fewer complications [13,14,15]. Furthermore, a recent review demonstrated that PTCs of different sizes showed similar efficacy post-RFA, indicating that RFA may be a viable alternative therapy for patients with large PTCs [12].
However, research on thermal ablation for T2N0M0 PTC remains sparse, with only one known small-scale study on RFA (involving 12 patients) [16], and no known studies on MWA for this specific condition. Therefore, this study aimed to fill this gap by assessing the efficacy and safety of thermal ablation, including MWA and RFA, in a broad group of patients with solitary, low-risk T2N0M0 PTC. We also aimed to compare the outcomes of the two ablation techniques additionally.
MATERIALS AND METHODS
Patients
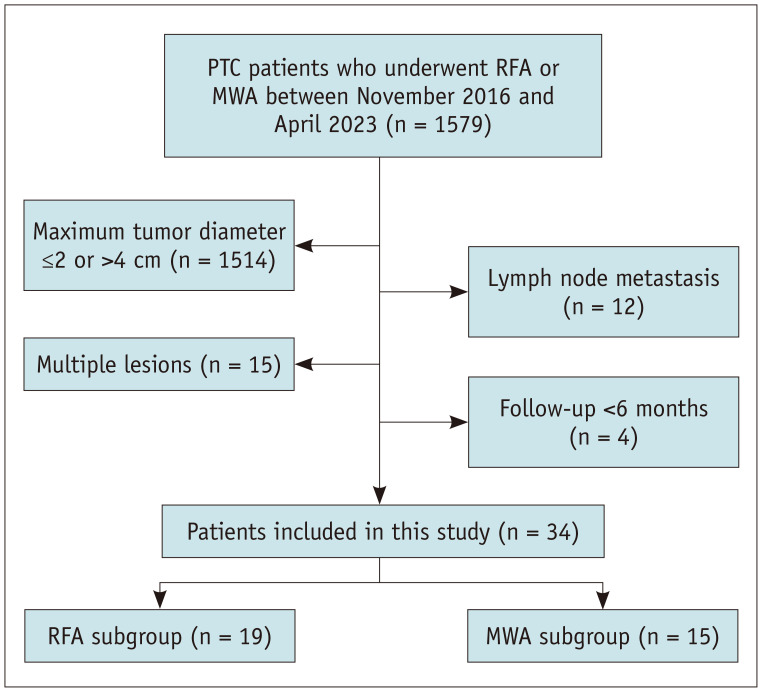
This retrospective, single-center study consecutively enrolled 34 patients (mean age: 40.0 ± 13.9 years [standard deviation]; 28 female) with low-risk T2N0M0 PTC who underwent RFA or MWA between November 2016 and April 2023 (Fig. 1). The study protocol (IRB No. S2019-283-02) was approved by the hospital’s Human Ethics Committee. The requirement for informed consent for inclusion in the study was waived, given the study’s retrospective design and the anonymity of the personal data involved.
Fig. 1. Flowhart of patient selection. PTC = papillary thyroid carcinoma, RFA = radiofrequency ablation, MWA = microwave ablation.
The inclusion criteria were as follows: a) PTC confirmed by US-guided biopsy, b) tumor with a maximum diameter >2 cm and ≤4 cm, c) no sonographic evidence of extrathyroidal extension into the strap muscles, trachea, esophagus, recurrent laryngeal nerve (RLN), and carotid artery [17], d) no evidence of cervical lymph node metastasis (LNM) or distant metastasis on both the US and computed tomography (CT) [18,19,20], and e) ineligibility or refusal of surgery [21]. The exclusion criteria were a) multiple malignancies or b) insufficient follow-up of less than 6 months. Patients were divided into the MWA and RFA groups based on the ablation method.
Pre-Ablation Assessment
Comprehensive diagnostic assessments were conducted before ablation, including using routine US to evaluate tumor dimensions, volume, location, US characteristics, and vascularity [21]. Contrast-enhanced ultrasonography (CEUS) was used to examine the enhancement patterns and boundaries of the thyroid nodules. The enhancement pattern was categorized as hyperenhancement, isoenhancement, hypoenhancement, or non-enhancement relative to the adjacent thyroid tissue [22]. Neck and chest CT scans were performed to detect lymph node involvement or distant metastases [19,23]. Laboratory test results included routine blood tests, coagulation function, thyroid function, and calcitonin levels. The pathological diagnosis was made using US-guided biopsy.
The tumor volume was calculated using the formula V = abc × 0.524 (where V represents the volume, and a, b, and c are orthogonal diameters) [24]. The volume reduction ratio (VRR) was calculated using the following formula: VRR = ([initial volume – final volume]/initial volume) × 100. Three radiologists, each with over 5 years of experience in thyroid imaging and intervention, assessed the data.
Ablation Procedure
This study used a LOGIQ E9 system (GE Healthcare, Chicago, IL, USA) with a 6–9-MHz linear probe for US guidance. A 17-gauge cooled MWA antenna with a 0.35-cm active tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China) or a 17-gauge RFA electrode with a 0.5- or 0.7-cm active tip (Cooltip Radiofrequency Ablation System, Covidien, Dublin, Ireland) was used for thermal ablation. All procedures were conducted in an inpatient US interventional setting by three radiologists, each with 5 years of experience in RFA, MWA, or both. Given that previous studies have not reported a clear technical superiority of either RFA and MWA over the other for treating PTC, the choice of ablation technique was based on each practitioner’s proficiency in performing RFA and MWA.
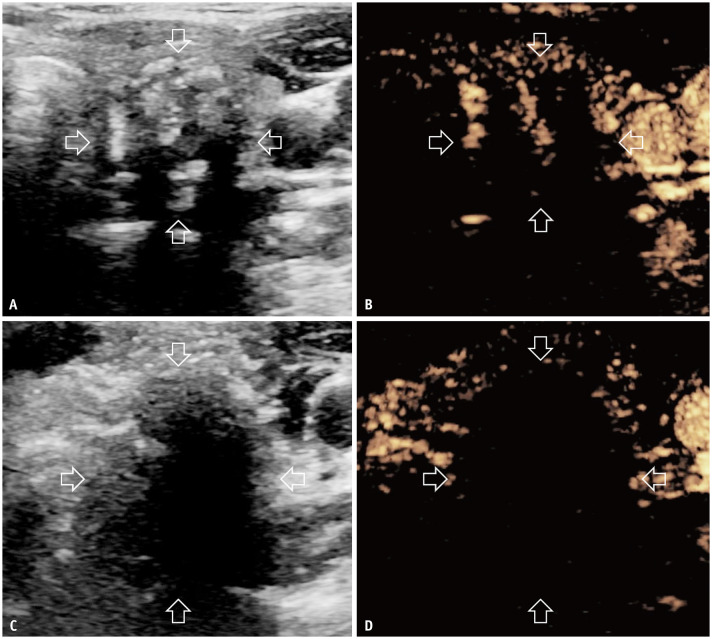
To begin the procedure, the patients were placed supine with their necks extended. After neck sterilization, 0.5% lidocaine was administered at the puncture site under local anesthesia. Subsequently, hydro-dissection was performed using an 18-gauge needle to safely separate the thyroid from critical structures using saline for MWA and sterilized water for RFA. Continuous injections were administered during the ablation to ensure a safe distance of at least 5 mm. The moving-shot technique was used for ablation [25]. The power was set at 30 W in MWA and adjusted from 30 W to 70 W in RFA, depending on the size of the active tip. Ablation was terminated after the hyperechoic zone covered the entire tumor and extended at least 2 mm beyond the original margin [26]. For tumors close to the thyroid capsule (<2 mm), ablation was performed after a thorough treatment of the adjacent capsule. CEUS was performed immediately after ablation to evaluate its effectiveness. If any residual enhancement areas were detected or the safety margin was inadequate, complementary ablation was immediately performed to ensure comprehensive treatment (Fig. 2).
Fig. 2. Images of a 40-year-old female with T2N0M0 papillary thyroid carcinoma in the left lobe treated with RFA. A, B: Heterogeneous hypoechoic ultrasound and hypoenhanced CEUS images of the tumor before RFA (transverse view, arrows). C, D: Hypoechoic and non-enhanced CEUS images of the tumor immediately after RFA (transverse view; arrows). RFA = radiofrequency ablation, CEUS = contrast-enhanced ultrasonography.
Post-ablation Assessment
Post-ablation RLN function was evaluated by monitoring vocal cord movements using US [27]. Laryngoscopy was performed in patients with abnormal vocal cord movements on US, hoarseness, or history of neck surgery. Adverse events during or after the procedure were carefully recorded.
Follow-up evaluations were scheduled for the first and third months post-ablation, with subsequent visits every 6–12 months. Each follow-up visit included a routine US examination and thyroid function tests, with annual CT scans to check for changes in the cervical lymph nodes or distant metastases. In cases where new suspicious lesions were detected, fine-needle aspiration (FNA) or core-needle biopsy was performed for further diagnosis.
Outcomes
This study defined technical success as complete non-enhancement on CEUS and hyperechogenicity on grayscale US of the target tumor immediately after ablation according to the planned procedure [28]. The primary outcomes were the cumulative rate of disease progression (pathologically confirmed local tumor progression, new tumors or LNM, distant metastasis identified using CT, and PTC-related mortality) and delayed surgery rate [29]. Local tumor progression was defined as the appearance of a PTC lesion at the edge of the ablation zone, and a new tumor was defined as a PTC lesion emerging in a previously uninvolved thyroid region.
Secondary outcomes included changes in ablation zone size, cumulative rate of complete tumor disappearance, and complication rates. Complications were classified following the reporting standards established by the Society of Interventional Radiology [29,30]. Permanent RLN injury was characterized by hoarseness lasting for over 6 months post-ablation.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics (version 25.0, IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 9.0.0, GraphPad Software, San Diego, CA, USA). Continuous variables are presented as means ± standard deviations or medians (interquartile ranges [IQRs]) based on the normality of the distribution, and categorical variables are present as frequencies (percentages). t-tests or Mann–Whitney U tests were used for continuous data, whereas chi-square or Fisher’s exact tests were used for categorical data. Changes in tumor size before and after ablation were analyzed using paired t-tests, whereas differences between groups were assessed using the Mann–Whitney U test. Kaplan–Meier curves were used to estimate the cumulative rates of disease progression and complete tumor disappearance, and the log-rank test was used to compare the RFA and MWA groups in terms of these outcomes. All tests were two-tailed, with a significance threshold of P < 0.05.
RESULTS
Patient And Tumor Characteristics
Between November 2016 and April 2023, 1579 patients with PTC who underwent RFA or MWA were reviewed. After applying the exclusion criteria, 34 patients with T2N0M0 PTC were included in the study (Fig. 1). Among these patients, seven were ineligible for surgery due to renal insufficiency (n = 1), cardiovascular disease (n = 4), or the presence of other malignant tumors (n = 2). Moreover, 27 patients opted out of surgery because of concerns regarding surgical risks and cosmetic issues. The median follow-up period was 18.0 months (IQR: 9.0–40.0 months), and the median maximum diameter of the PTC lesions was 2.5 cm (IQR: 2.1–2.8 cm). Table 1 shows no significant differences in the baseline characteristics between the RFA and MWA groups.
Table 1. Patient and tumor characteristics.
| Variable | Total (n = 34) | RFA (n = 19) | MWA (n = 15) | P |
|---|---|---|---|---|
| Age, yrs* | 40.0 ± 13.9 | 41.6 ± 14.2 | 38.1 ± 13.6 | 0.478 |
| Sex, male:female | 6:28 | 3:16 | 3:12 | >0.999 |
| Maximum diameter, cm† | 2.5 (2.1, 2.8) | 2.5 (2.1, 3.1) | 2.3 (2.1, 2.7) | 0.461 |
| Volume, mL† | 3.3 (2.5, 4.9) | 4.0 (2.8, 5.7) | 3.0 (2.0, 4.0) | 0.160 |
| Location, right:left | 19:15 | 12:7 | 7:8 | 0.489 |
| CEUS, hypo enhancement:hyper enhancement | 8:26 | 3:16 | 5:10 | 0.417 |
| Diagnosis, CNB:FNA | 4:30 | 2:17 | 2:13 | >0.999 |
| Ablation time, s* | 337.9 ± 133.9 | 370.0 ± 143.1 | 297.3 ± 113.2 | 0.118 |
| Follow-up time, mos† | 18.0 (9.0, 40.0) | 18.0 (9.0, 41.0) | 17.0 (7.0, 36.0) | 0.689 |
*Data are presented as means ± standard deviations, †Data are presented as medians (interquartile range).
RFA = radiofrequency ablation, MWA = microwave ablation, CEUS = contrast-enhanced ultrasonography, CNB = core-needle biopsy, FNA = fine-needle aspiration
Disease Progression After Thermal Ablation
The technical success rate of the procedure was 100%, and complete ablation was achieved in all participants in a single session.
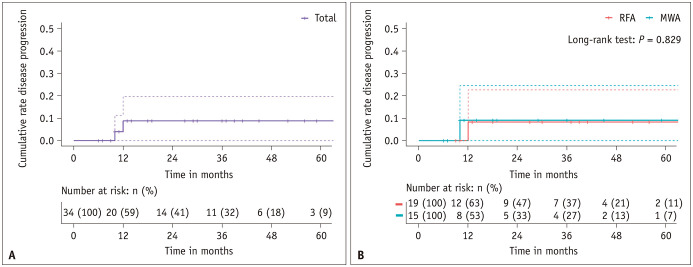
At the end of the follow-up period, this study documented two instances of disease progression, involving one case of new tumor appearing 10 months post-MWA and one case of local tumor progression 12 months post-RFA. No disease-related fatalities were observed. The cumulative disease progression rate was 0% at 6 months and remained constant at 8.8% (95% confidence interval [CI]: 0%–19.8%) at 1, 3, and 5 years (Fig. 3A). Specifically, the rates of new tumor occurrence and local progression at 1 year were 4.0% (95% CI: 0%–19.8%) and 4.8% (95% CI: 0%–19.8%), respectively. The 1-year disease progression rates were 8.3% (95% CI: 0%–22.7%) in the RFA group and 9.1% (95% CI: 0%–24.6%) in the MWA group (Fig. 3B). A comparison of cumulative disease progression between the MWA and RFA groups revealed no significant differences (P = 0.829).
Fig. 3. Kaplan–Meier curves for disease progression. A: Graph illustrating the Kaplan–Meier curves for cumulative disease progression in 34 patients with T2N0M0 papillary thyroid cancer. B: Graph showing the Kaplan–Meier curves for cumulative disease progression in the RFA and MWA groups. RFA = radiofrequency ablation, MWA = microwave ablation.
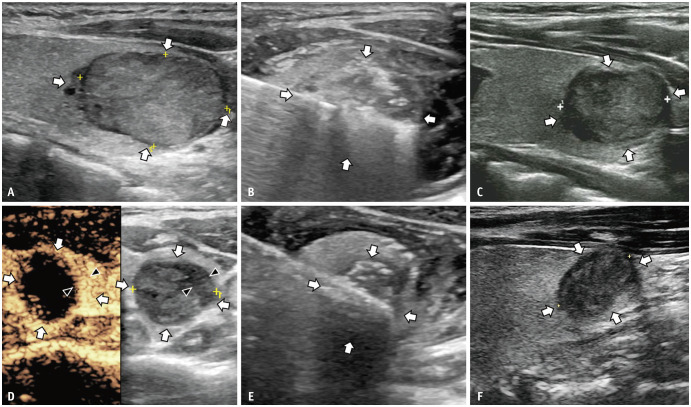
In the RFA group, there was one case of local progression located at the lower pole of the ablation zone, with a maximum diameter of 0.6 cm, noted 12 months post-ablation (Fig. 4). Conversely, in the MWA group, one patient developed a new 0.4-cm tumor in the contralateral lobe 10 months post-ablation (Fig. 5). Both cases were validated using FNA and subsequently treated with additional MWA. Notably, no further disease progression was noted up to the final follow-ups at 24 and 42 months after the initial treatment, avoiding the need for delayed surgery.
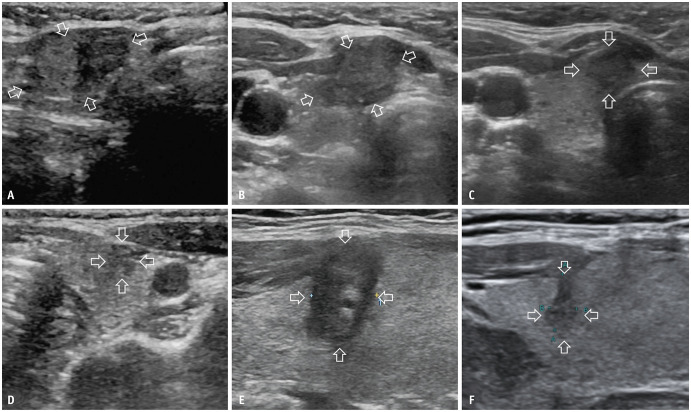
Fig. 4. A 21-year-old female with a T2N0M0 PTC in the right lobe treated with RFA and additional ablation of local tumor progression with microwave ablation. A: A 3-cm T2N0M0 PTC located 0.3 mm below the thyroid’s anterior capsule is shown on routine US (longitudinal view, arrows). B: RFA was performed on the nodule (transverse view, arrows). C: Routine US of the ablation zone of the nodule 3 months after the initial ablation (longitudinal view, arrows) showed no abnormality. D: The hypoechoic part (arrowheads) with hyperenhancement on contrast-enhanced US besides the ablation zone (transverse view, arrows) is identified 12 months after the initial ablation (transverse view, arrowheads). E: Additional microwave ablation is performed on the nodule (transverse view, arrows). F: Twelve months after additional ablation, the size of the ablation zone is 1.6 cm × 1.4 cm on routine US (longitudinal view, arrows), and no tumor progression is encountered. PTC = papillary thyroid carcinoma, RFA = radiofrequency ablation, US = ultrasound.
Fig. 5. A 38-year-old female with a T2N0M0 papillary thyroid carcinoma in the right lobe and a new tumor in the left lobe treated with MWA. A: The initial 2.2-cm T2N0M0 tumor (transverse view, arrows) in the right lobe is shown on routine US. B: Three months after the initial MWA, the size of the ablation zone (transverse view, arrows) is 1.8 cm × 1.4 cm on routine US. C: Ten months after the initial ablation, the size of the ablation zone (transverse view, arrows) is 1.1 cm × 1.1 cm on routine US. D: A new tumor (transverse view, arrows) is identified in the left lobe 10 months after the initial treatment on routine US, and MWA is performed on the new tumor. E: One day after ablation for the new tumor, the size of the new ablation zone (longitudinal view, arrows) is 1.2 cm × 0.8 cm. F: Fourteen months after ablation for the new tumor, the initial T2N0M0 tumor is absorbed on the US, and the size of new ablation zone (longitudinal view, arrows) is 0.8 cm × 0.3 cm and no tumor progression is encountered. MWA = microwave ablation, US = ultrasound.
Tumor Size Changes
Table 2 presents the changes in the maximum diameter and volume of the lesions according to time after ablation. Owing to the safety margins included in the ablation procedure, the VRR displayed negative values in the first month post-ablation; however, this later transitioned to positive values. The VRR increased to 23.8% (IQR: 4.4%–48.0%), 56.7% (IQR: 43.1%–71.1%), and 74.2% (53.7%–86.0%) at 3, 6, and 12 months, respectively. There was a significant reduction in the maximum diameter from the third month post-ablation, compared with pre-ablation measurements (P < 0.05 for all).
Table 2. Changes in the maximum diameter and volume of papillary thyroid carcinomas before and after ablation.
| Follow-up time | Maximum diameter, cm* | P † | Volume, mL* | P † | VRR, %* |
|---|---|---|---|---|---|
| Baseline (n = 34) | 2.5 (2.1, 2.8) | 3.3 (2.5, 4.9) | |||
| Immediately (n = 34) | 2.9 (2.4, 3.4) | <0.001 | 6.7 (4.5, 9.2) | <0.001 | -79.8 (-117.2, -32.1) |
| 1-month (n = 34) | 2.5 (2.3, 3.0) | 0.497 | 4.6 (3.3, 6.6) | 0.001 | -27.7 (-50.8, -3.0) |
| 3-months (n = 33) | 2.2 (2.1, 2.6) | 0.003 | 2.7 (1.9, 3.8) | 0.004 | 23.8 (4.4, 48.0) |
| 6-months (n = 34) | 1.9 (0.6, 2.2) | <0.001 | 1.4 (0.9, 2.3) | <0.001 | 56.7 (43.1, 71.1) |
| 12-months (n = 24) | 1.5 (1.2, 1.8) | <0.001 | 0.8 (0.4,1.4) | <0.001 | 74.2 (53.7, 86.0) |
| 18-months (n = 16) | 1.4 (1.1, 1.8) | <0.001 | 0.6 (0.4, 0.9) | <0.001 | 81.3 (67.7, 92.9) |
| 24-months (n = 14) | 1.4 (1.0, 1.7) | <0.001 | 0.7 (0.2, 1.1) | <0.001 | 83.6 (70.0, 96.4) |
| 36-months (n = 11) | 1.3 (0.2, 1.6) | <0.001 | 0.4 (0, 0.6) | 0.002 | 87.7 (80.5, 100.0) |
| 48-months (n = 8) | 0.6 (0, 1.2) | <0.001 | 0.1 (0, 0.3) | 0.005 | 97.3 (90.8, 100.0) |
| 60-months (n = 5) | 0 (0, 1.1) | 0.011 | 0 (0, 0.3) | 0.058 | 97.3 (93.6, 100.0) |
*Data are presented as medians (interquartile range), †For comparison with baseline value.
VRR = volume reduction ratio ([baseline volume - final volume]/baseline volume) × 100%
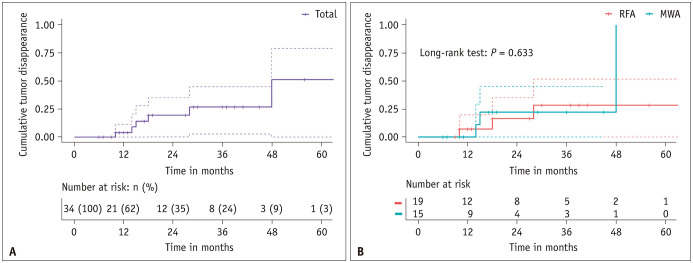
Complete tumor disappearance was observed in six patients, with three in each treatment group. The cumulative complete disappearance rates at 1, 3, and 5 years were 4.0% (95% CI: 0%–11.4%), 26.8% (95% CI: 2.7%–44.9%), and 51.2% (95% CI: 0%–79.1%), respectively (Fig. 6A), with no significant differences between the two groups (P = 0.633) (Fig. 6B).
Fig. 6. Kaplan–Meier curves for tumor disappearance. A: Graph illustrating Kaplan–Meier curves for cumulative tumor disappearance in 34 patients with T2N0M0 papillary thyroid cancer. B: The graph shows the Kaplan–Meier curves for cumulative tumor disappearance in RFA and MWA groups. RFA = radiofrequency ablation, MWA = microwave ablation.
Complications
The overall complication rate in this study was 14.7% (5/34), with all reported cases of transient hoarseness classified as significant complications. Regarding group-specific rates, complications occurred in 21.1% (4/19) and 6.7% (1/15) of patients in the RFA and MWA groups, respectively. However, the statistical analysis revealed no significant difference in the complication rates between the two groups (P = 0.355). Among the patients experiencing hoarseness, the primary tumor had a mean maximum diameter of 2.2 ± 0.2 cm (range: 2.1–2.7 cm) and an average distance from the tracheoesophageal groove of 0.8 ± 0.2 mm (range: 0.6–1.2 mm). Extensive calcification within the tumors was observed in two patients treated with RFA.
Notably, all instances of hoarseness resolved spontaneously within 3 months. Although 50% (17/34) of the patients experienced mild pain during the ablation procedure, all tolerated the procedure without significant issues. No life-threatening complications were encountered during the ablation or follow-up.
DISCUSSION
Our previous research showed favorable outcomes of thermal ablation for treating T1N0M0 PTC, consistent with the results of other studies [13,14,15,31]. A notable study in 2021 utilized RFA in a modest group of 12 patients diagnosed with T2N0M0 PTC and showed that, over an average monitoring period of 24 months, two patients experienced complete resolution of their tumors, with no further disease progression or complications [16]. However, the limited number of participants in that study poses a challenge to the broad applicability of its findings. To date, there have been no reports on the use of MWA for treating T2N0M0 PTC. To address this gap, the present study aimed to evaluate the efficacy and safety of thermal ablation for T2N0M0 PTC in a larger cohort and to compare the outcomes between MWA and RFA treatments.
In this study, thermal ablation successfully treated all T2N0M0 PTC cases, achieving a 100% technical success rate. Disease progression occurred in two patients over a median follow-up period of 18.0 months. The 1-, 3-, and 5-year disease progression rates remained at 8.8%, comprising 4.8% local tumor progression and 4.0% new tumor occurrences, with no LNM or distant metastases detected. In comparison, surgical resection has a 10-year progression rate of 1.3% for tumor recurrence in the remnant thyroid, 4.6% for LNM, and 1.6% for distant metastases [32]. Thus, thermal ablation provides short-term efficacy in treating T2N0M0 PTC, particularly as an alternative treatment for patients who are not candidates for surgery.
We noted one case of local progression post-RFA at the lower pole of the ablation zone, which may have occurred because of the large size of the initial tumor (up to 3.0 cm in diameter), leading to incomplete ablation at the extensive margins of the tumor. Despite the initial tumor’s proximity of only 0.3 mm to the anterior capsule, no progression was detected anterior to the ablation zone owing to effective capsular ablation. In contrast, a new tumor that developed post-MWA in one patient emerged in the contralateral thyroid lobe 10 months post-treatment. This could be attributed to microcarcinomas that were initially too small to be detected by the US but grew to detectable sizes during the follow-up [33]. Both patients with disease progression underwent additional ablations, after which no further progression was observed. These outcomes indicate that thermal ablation is an effective and repeatable intervention for managing disease progression in patients with PTC.
By the end of the follow-up, six ablation zones had disappeared entirely, with three each in the RFA and MWA groups. Compared with T1N0M0 PTC, the 12-month VRR in this study was lower (79.6% for T2N0M0 vs. 93.0% for T1N0M0) [34]. However, our findings are similar to those of studies on large benign thyroid nodules, particularly one study focusing on nodules averaging 4.6 cm in diameter (79.6% in this study vs. 76.1% in the previous study) [35]. The lower VRR in this study may be attributed to the larger size of the ablation zones, potentially hindering complete absorption [36]. Additionally, this study revealed increasing tumor disappearance rates of 4.0%, 26.8%, and 51.2% at 1, 3, and 5 years, respectively, suggesting that a short follow-up period may not be sufficient to observe the entire process of the lesion response. In contrast, a recent study involving patients with T1 PTC with a follow-up of >5 years demonstrated a high VRR of up to 100% ± 0.3% and a high complete disappearance rate of 96.6% [37], highlighting the importance of extended follow-up for thoroughly evaluating lesion absorption.
The 14.7% rate of transient hoarseness in our study involving T2N0M0 PTC was notably higher than 5.4% reported in another study involving T1N0M0 PTC [13]. This increase in the incidence of hoarseness was attributed to the larger size of the nodules and their proximity (within 4 mm) to the tracheoesophageal groove in all five cases, posing challenges in entirely eradicating tumors while preserving RLN integrity [38]. Additionally, mass calcification was observed in the tumor in two cases treated with RFA, potentially obscuring the vision of the ablation zone and intensifying heat transmission, thereby risking damage to the RLN [39]. However, all hoarseness cases resolved spontaneously within 3 months, and no instances of permanent RLN palsy were observed. This differed from the results of a thyroidectomy study, which showed a lower transient hoarseness rate of 2.8% but a higher rate of permanent RLN paralysis, at 1.6% [40].
To protect the RLN during thermal ablation, continuous hydro-dissection was employed to prevent damage to the surrounding structures, and the moving shot technique was used to minimize ablation time and thermal injury [41]. Due to its non-conductive properties, sterilized water has been employed in RFA, as an alternative to 5% glucose water [21,42]. Despite its hypoosmolar nature, owing to the small volume used in thyroid ablation, sterilized water cannot cause systemic fluid shifts. Despite these preventive measures, the complication rate tended to be higher in the RFA group than in the MWA group (21.1% vs. 6.7%); however, the difference was not significant (P = 0.355). The limited cohort size in this study may have provided insufficient power to detect a significant difference, indicating the need for further research with a larger sample size to assess the safety of these ablation modalities.
This study has a few limitations. First, the duration of the follow-up regarding the long-term outcomes was relatively short. Second, the disease progression rate was higher than that typically reported for T1N0M0 PTC, possibly because of the limited cohort size [12]. Third, the retrospective nature of this study may have led to an inherent selection bias. Fourth, accurate identification of aggressive pathological subtypes using US-guided biopsy is challenging [43]. Consequently, further in-depth studies with extended follow-up periods and larger sample sizes are required.
In conclusion, our initial findings suggest that both MWA and RFA are effective and safe short-term treatments for solitary low-risk T2N0M0 PTC with no significant differences. Therefore, thermal ablation may be a potential alternative for patients with T2N0M0 who are unwilling or unable to undergo surgery.
Acknowledgments
We would like to thank all participants for their support in this study.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yu-Lin Fei, Ying Wei, Zhen-Long Zhao, Ming-An Yu.
- Data curation: all authors.
- Formal analysis: Yu-Lin Fei, Ying Wei.
- Methodology: Yu-Lin Fei, Ying Wei.
- Investigation: all authors.
- Resources: all authors.
- Writing—original draft: Yu-Lin Fei.
- Writing—review & editing: Ying Wei, Zhen-Long Zhao, Ming-An Yu.
Funding Statement: This work was funded by National High Level Hospital Clinical Research Funding (Grant No. 2023-NHLHCRF-YYPP-TS-01) and National Natural Science Foundation of China (Grant No. 62176268).
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available to protect participant confidentiality but are available from the corresponding author on reasonable request.
References
- 1.Gimm O. Thyroid cancer. Cancer Lett. 2001;163:143–156. doi: 10.1016/s0304-3835(00)00697-2. [DOI] [PubMed] [Google Scholar]
- 2.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317:1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2018;68:55–63. doi: 10.3322/caac.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol. 2018;14:670–683. doi: 10.1038/s41574-018-0080-7. [DOI] [PubMed] [Google Scholar]
- 6.Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:925–951. doi: 10.6004/jnccn.2022.0040. [DOI] [PubMed] [Google Scholar]
- 7.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 8.Pace-Asciak P, Russell JO, Tufano RP. Review: improving quality of life in patients with differentiated thyroid cancer. Front Oncol. 2023;13:1032581. doi: 10.3389/fonc.2023.1032581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan Y, Luo Y, Zhang M, Jin Z, Xiao J, Yan L, et al. Quality of life in papillary thyroid microcarcinoma patients undergoing radiofrequency ablation or surgery: a comparative study. Front Endocrinol (Lausanne) 2020;11:249. doi: 10.3389/fendo.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Huo SN, Lu NC, Peng LL, Wei Y, Zhao ZL, et al. A comparative study of quality of life in patients with papillary thyroid carcinoma undergoing microwave ablation vs. total thyroidectomy. Int J Hyperthermia. 2023;40:2250935. doi: 10.1080/02656736.2023.2250935. [DOI] [PubMed] [Google Scholar]
- 11.Cho SJ, Baek JH, Chung SR, Choi YJ, Lee JH. Thermal ablation for small papillary thyroid cancer: a systematic review. Thyroid. 2019;29:1774–1783. doi: 10.1089/thy.2019.0377. [DOI] [PubMed] [Google Scholar]
- 12.Zhao GZ, Zhang MB. Ultrasound-guided radiofrequency ablation for the treatment of papillary thyroid carcinoma: a review of the current state and future perspectives. Ultrasonography. 2024;43:79–87. doi: 10.14366/usg.23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y, Niu WQ, Zhao ZL, Wu J, Peng LL, Li Y, et al. Microwave ablation versus surgical resection for solitary T1N0M0 papillary thyroid carcinoma. Radiology. 2022;304:704–713. doi: 10.1148/radiol.212313. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Zhang M, Song Q, Luo Y. Ultrasound-guided radiofrequency ablation versus thyroid lobectomy for low-risk papillary thyroid microcarcinoma: a propensity-matched cohort study of 884 patients. Thyroid. 2021;31:1662–1672. doi: 10.1089/thy.2021.0100. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Cho SJ, Baek JH. Comparison of thermal ablation and surgery for low-risk papillary thyroid microcarcinoma: a systematic review and meta-analysis. Korean J Radiol. 2021;22:1730–1741. doi: 10.3348/kjr.2020.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao J, Zhang Y, Zhang M, Xie F, Yan L, Luo Y, et al. Ultrasonography-guided radiofrequency ablation for the treatment of T2N0M0 papillary thyroid carcinoma: a preliminary study. Int J Hyperthermia. 2021;38:402–408. doi: 10.1080/02656736.2021.1895332. [DOI] [PubMed] [Google Scholar]
- 17.Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, et al. Sonographic assessment of the extent of extrathyroidal extension in thyroid cancer. Korean J Radiol. 2020;21:1187–1195. doi: 10.3348/kjr.2019.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon YH, Lee JY, Yoo RE, Rhim JH, Lee KH, Choi KS, et al. Validation of ultrasound and computed tomography-based risk stratification system and biopsy criteria for cervical lymph nodes in preoperative patients with thyroid cancer. Korean J Radiol. 2023;24:912–923. doi: 10.3348/kjr.2023.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roh YH, Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, et al. Validation of CT-based risk stratification system for lymph node metastasis in patients with thyroid cancer. Korean J Radiol. 2023;24:1028–1037. doi: 10.3348/kjr.2023.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong SY, Chung SR, Baek JH, Choi YJ, Kim S, Sung TY, et al. Impact of additional preoperative computed tomography imaging on staging, surgery, and postsurgical survival in patients with papillary thyroid carcinoma. Korean J Radiol. 2023;24:1284–1292. doi: 10.3348/kjr.2023.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655. doi: 10.3348/kjr.2018.19.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan J, Xu X, Cai Y, Zeng H, Luo M, Zhang W, et al. A practical CEUS thyroid reporting system for thyroid nodules. Radiology. 2022;305:149–159. doi: 10.1148/radiol.212319. [DOI] [PubMed] [Google Scholar]
- 23.Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021;22:2094–2123. doi: 10.3348/kjr.2021.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauri G, Pacella CM, Papini E, Solbiati L, Goldberg SN, Ahmed M, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29:611–618. doi: 10.1089/thy.2018.0604. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18:615–623. doi: 10.3348/kjr.2017.18.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim HK, Cho SJ, Baek JH, Lee KD, Son CW, Son JM, et al. US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2019;20:1653–1661. doi: 10.3348/kjr.2019.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gambardella C, Offi C, Romano RM, De Palma M, Ruggiero R, Candela G, et al. Transcutaneous laryngeal ultrasonography: a reliable, non-invasive and inexpensive preoperative method in the evaluation of vocal cords motility-a prospective multicentric analysis on a large series and a literature review. Updates Surg. 2020;72:885–892. doi: 10.1007/s13304-020-00728-3. [DOI] [PubMed] [Google Scholar]
- 28.Mauri G, Cova L, Tondolo T, Ierace T, Baroli A, Di Mauro E, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab. 2013;98:E1203–E1207. doi: 10.1210/jc.2013-1140. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 31.Wang MH, Liu X, Wang Q, Zhang HW. Safety and efficacy of ultrasound-guided thermal ablation in treating T1aN0M0 and T1bN0M0 papillary thyroid carcinoma: a meta-analysis. Front Endocrinol (Lausanne) 2022;13:952113. doi: 10.3389/fendo.2022.952113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito Y, Kudo T, Kihara M, Takamura Y, Kobayashi K, Miya A, et al. Prognosis of low-risk papillary thyroid carcinoma patients: its relationship with the size of primary tumors. Endocr J. 2012;59:119–125. doi: 10.1507/endocrj.ej11-0288. [DOI] [PubMed] [Google Scholar]
- 33.Jovanovic L, Delahunt B, McIver B, Eberhardt NL, Grebe SK. Most multifocal papillary thyroid carcinomas acquire genetic and morphotype diversity through subclonal evolution following the intra-glandular spread of the initial neoplastic clone. J Pathol. 2008;215:145–154. doi: 10.1002/path.2342. [DOI] [PubMed] [Google Scholar]
- 34.Zhou G, Zhang X, Xu K, Zhang B, Su R, Cai T, et al. Retrospective analysis of the efficacy and safety of ultrasound-guided radiofrequency ablation in the treatment of papillary thyroid microcarcinoma: a follow-up study of continuous postoperative surveillance and large-sample data. Int J Endocrinol. 2024;2024:2704087. doi: 10.1155/2024/2704087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cang YC, Fan FY, Liu Y, Li JM, Pang C, Xu D, et al. Efficacy of microwave ablation in the treatment of large benign thyroid nodules: a multi-center study. Eur Radiol. 2024 Mar 28; doi: 10.1007/s00330-024-10614-w. [Epub] [DOI] [PubMed] [Google Scholar]
- 36.Cao S, Wang L, Wei Y, Zhao Z, Wu J, Yu M. Influence factors and nomogram for volume reduction rate in benign thyroid nodule after thermal ablation. Int J Hyperthermia. 2023;40:2220562. doi: 10.1080/02656736.2023.2220562. [DOI] [PubMed] [Google Scholar]
- 37.Yan L, Li Y, Li XY, Xiao J, Tang J, Luo Y. Clinical outcomes of ultrasound-guided radiofrequency ablation for solitary T1N0M0 papillary thyroid carcinoma: a retrospective study with more than 5 years of follow-up. Cancer. 2023;129:2469–2478. doi: 10.1002/cncr.34802. [DOI] [PubMed] [Google Scholar]
- 38.Zhao ZL, Wei Y, Peng LL, Li Y, Lu NC, Yu MA. Recurrent laryngeal nerve injury in thermal ablation of thyroid nodules—risk factors and cause analysis. J Clin Endocrinol Metab. 2022;107:e2930–e2937. doi: 10.1210/clinem/dgac177. [DOI] [PubMed] [Google Scholar]
- 39.Baek JH, Lee JH, Valcavi R, Pacella CM, Rhim H, Na DG. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540. doi: 10.3348/kjr.2011.12.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Slycke S, Van Den Heede K, Bruggeman N, Vermeersch H, Brusselaers N. Risk factors for postoperative morbidity after thyroid surgery in a PROSPECTIVE cohort of 1500 patients. Int J Surg. 2021;88:105922. doi: 10.1016/j.ijsu.2021.105922. [DOI] [PubMed] [Google Scholar]
- 41.Zhao ZL, Wei Y, Peng LL, Li Y, Lu NC, Wu J, et al. Upgraded hydrodissection and its safety enhancement in microwave ablation of papillary thyroid cancer: a comparative study. Int J Hyperthermia. 2023;40:2202373. doi: 10.1080/02656736.2023.2202373. [DOI] [PubMed] [Google Scholar]
- 42.Xiaoyin T, Ping L, Dan C, Min D, Jiachang C, Tao W, et al. Risk assessment and hydrodissection technique for radiofrequency ablation of thyroid benign nodules. J Cancer. 2018;9:3058–3066. doi: 10.7150/jca.26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi ED, Faquin WC, Pantanowitz L. Cytologic features of aggressive variants of follicular-derived thyroid carcinoma. Cancer Cytopathol. 2019;127:432–446. doi: 10.1002/cncy.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are not publicly available to protect participant confidentiality but are available from the corresponding author on reasonable request.