Abstract
The recent surge in the incidence of small papillary thyroid cancers (PTCs) has been linked to the widespread use of ultrasonography, thereby prompting concerns regarding overdiagnosis. Active surveillance (AS) has emerged as a less invasive alternative management strategy for low-risk PTCs, especially for PTCs measuring ≤1 cm in maximal diameter. Recent studies report low disease progression rates of low-risk PTCs ≤1 cm under AS. Ongoing research is currently exploring the feasibility of AS for larger PTCs (<20 mm). AS protocols include meticulous ultrasound assessment, emphasis on standardized techniques, and a multidisciplinary approach; they involve monitoring the nodules for size, growth, potential extrathyroidal extension, proximity to the trachea and recurrent laryngeal nerve, and potential cervical nodal metastases. The criteria for progression, often defined as an increase in the maximum diameter of the PTC, warrant a review of precision and ongoing examinations. Challenges exist regarding the reliability of volume measurements for defining PTC disease progression. Although ultrasonography plays a pivotal role, challenges in assessing progression and minor extrathyroidal extension underscore the importance of a multidisciplinary approach in disease management. This comprehensive overview highlights the evolving landscape of AS for PTCs, emphasizing the need for standardized protocols, meticulous assessments, and ongoing research to inform decision-making.
Keywords: Thyroid, Papillary thyroid cancer, Active surveillance, Ultrasound, Outcomes
INTRODUCTION
The recent rapid increase in the incidence of small papillary thyroid cancers (PTCs) has been attributed to the worldwide increased use of diagnostic technologies (including ultrasonography), which have lead to the overdiagnosis of indolent PTCs. North American clinical practice guidelines generally do not recommend biopsy of thyroid nodules <1 cm and instead recommend ultrasound monitoring, regardless of the presence of suspicious sonographic features [1,2]. Patients with suspicious subcentimeter nodules may undergo active surveillance (AS). A recent study by Kim et al. [3] showed that highly suspicious nodules on ultrasound measuring ≤10 mm in maximal diameter without fine needle aspiration biopsy, had a low disease progression rate over a median follow up period of 4.8 years. The rationale for the use of AS is to avoid unnecessary interventions and their potentially associated risks in nodules that are less likely to pose a significant health threat. Given the low risk of disease progression in many incidentally discovered small PTCs, there has been increasing interest in less invasive alternatives such as AS and minimally invasive ablative interventions for small, low-risk thyroid cancers. Indirect evidence on the value of AS for low-risk malignancies may also be inferred from prostate cancer literature.
AS has become an increasingly attractive disease management option for small low-risk PTC. Miyauchi & Ito [4] from Kuma Hospital in Japan were the first to demonstrate that the majority of low-risk PTCs <1 cm on aspiration biopsy remain unchanged on serial ultrasound surveillance. In patients in whom low-risk PTC displayed growth or lymph node metastases, complete excision of the PTC and any metastatic nodes was routinely achieved. In 2015, the American Thyroid Association Management Guidelines discussed AS as an alternative to immediate surgery for low-risk PTC [2]. Since then, AS protocols have been adopted in several academic centers. For example, a Toronto-area prospective observational study initiated in 2016 was the first Canadian study to include low-risk PTCs up to 2 cm in maximum diameter [5]. Researchers at the Cedars-Sinai Medical Center, Los Angeles, have recently published their experience with the AS of small, low-risk PTC <2 cm in diameter [6]. A meta-analysis published in 2019 showed that AS is a good option for small, low-risk PTC management with 5.3% nodules depicting size enlargement of ≥3 mm at 5 years and that the pooled proportion of lymph node metastases was only 1.6% at 5 years [7]. Ultrasonography of the neck (thyroid and associated lymph nodes) is the gold standard imaging modality for evaluating the disease progression in AS.
A multidisciplinary team approach should be employed for the evaluation of patients with PTC whose disease may be suitable for AS [8,9]. It is also important to assess the patient and sonographic tumor characteristics, the feasibility of AS, and the willingness of the patient to consider such an approach. Meticulous ultrasound assessment is crucial for appropriate patient selection and accurate follow-up evaluation of disease progression. Thus, the implementation of standardized ultrasound scanning techniques and interpretation with strict attention to detail (with an understanding of associated limitations) in the context of multidisciplinary care settings is essential [9]. A coordinated response is needed should surgery be necessary due to disease progression or should the patient opt for surgery voluntarily.
Baseline Evaluation
The baseline scan evaluates the entire gland, focusing on the primary PTC and the absence of suspicious cervical lymph nodes for inclusion. It is important to record the size of the PTC in all three dimensions, to assess its relationship with the recurrent laryngeal nerve (RLN) and trachea and to examine any evidence of extrathyroidal extension (ETE).
PTC Size Inclusion Criteria for Consideration of Active Surveillance
While most of the initial AS studies examined PTCs ≤1 cm [4,10,11], several ongoing studies now include PTCs over 10 mm [12,13,14] with 2 studies including T1 stage PTCs measuring ≤20 mm [5,6]. Machens et al. [15] reported that the risk of distant metastases from PTC increased at a size of 20 mm, as measured in resected specimens. However, some discrepancies have been reported between sonographic and pathological size measurements, with a tendency for nodules to be larger on ultrasound. Bachar et al. [16] reported that for PTCs >1.5 cm, the mean diameter on ultrasound was 2.65 ± 1.07 cm, compared with 1.97 ± 1.17 cm on pathology [1,16]. Thus, it may be reasonable to include nodules up to 20 mm on ultrasonography in AS protocols. Since North American clinical practice guidelines do not recommend sampling of thyroid nodules <1 cm [2], in order to assess the incidence of progression in AS protocols, it is important that future studies include low-risk PTCs measuring 10–20 mm in maximal diameter. Roman et al. [17] estimated that between 2020 and 2024, approximately 92365 to 113395 patients in the United States are likely to be diagnosed with T1 PTCs (≤20 mm), of which 50578 to 61925 patients may be diagnosed with papillary microcarcinomas (<10 mm) and be eligible for AS. This implies that a substantial number of PTCs measuring between 10–20 mm in maximum diameter are likely to meet the AS criteria.
Assessment for Extra-Thyroidal Extension of PTC and Cervical Lymphadenopathy
Anterolateral Thyroid Capsule Assessment for ETE
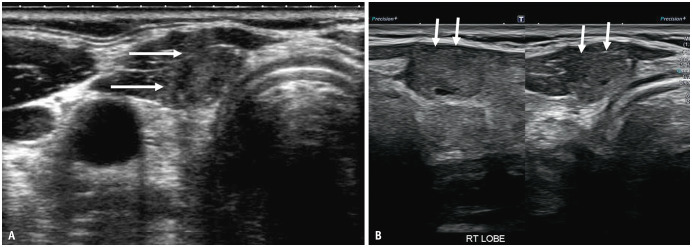
Although minor ETE is not considered an independent prognostic indicator for relapse-free survival in PTC [18], patients with these PTCs are not considered ideal for inclusion in AS protocols. Unlike major ETE, minor ETE in PTC is difficult to identify on ultrasonography and can be definitively ascertained on histological examination. To assess ETE, ultrasound images should be scrutinized for 1) nodule abutment with the thyroid margin, 2) disruption of the perithyroidal echogenic line at the site of contact with the PTC, and 3) bulging of the thyroid contour by the nodule [9]. Loss of the perithyroidal echogenic line has been noted to have the highest diagnostic accuracy with a positive predictive value of 58.5% [19]. However, we have observed that occasionally, patients with coexistent autoimmune thyroid disease may present with a discontinuity of the perithyroidal echogenic line on ultrasound and indistinct nodule margins, potentially leading to misinterpretation as an ETE (Fig. 1). Solymosi et al. [20] reported that ETE was correctly identified using ultrasound in only 45.8% of cases. There is a knowledge gap in the accurate and correct identification of minor ETE by ultrasound, and it is crucial for radiologists and the study team to be aware of this. Assessments over two consecutive ultrasound scans within a few months of each other may also help reassess ETE and/or the stability of appearance prior to making a decision on surgery. Of note, Ito & Miyauchi [21] recently suggested the inclusion of PTCs with suspected minor ETE in AS protocols, particularly in the context of “possible invasion of the anterior or lateral capsule of the thyroid.” Further research is required to optimize the identification and management of ETEs in the context of potential AS.
Fig. 1. Female patient, age in the 40’s with concurrent autoimmune disease and heterogenous background of thyroid parenchyma. A: Transverse image at enrollment into active surveillance shows a 9 mm PTC in right isthmus of the gland. The peri-thyroid echogenic line (arrows) is faintly visualised along the anterior margin of the PTC. B: Sagittal and transverse image after follow-up of two years. There is complete disruption of the anterior echogenic line, increase in bulge of the anterior thyroid margin at the tumor site and suspicion of extra-thyroid extension (arrows). Active surveillance was discontinued, and surgery was offered because of suspected extrathyroid extension and disease progression. However, the postoperative histopathological examination did not reveal any evidence of extrathyroidal extension. PTC = papillary thyroid cancer.
Relation of PTC to Trachea to Assess Tracheal Involvement
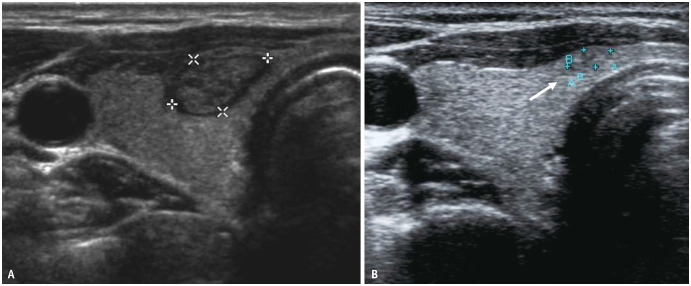
AS is not indicated if there is a suspicion of potential invasion of the trachea or RLN by PTC on ultrasonography. The risk of tracheal invasion is assessed based on the angle formed using PTC and tracheal cartilage [22]. In a retrospective study, Ito et al. [23] reported that PTCs <7 mm did not invade the trachea, irrespective of the contact angle between the PTC and the trachea. For PTCs measuring ≥7 mm, only 2% nodules with acute angle contact with the trachea showed extension to peritracheal connective tissue but none to the tracheal cartilage, while 24% and 17% of PTCs with obtuse angle or unclear/right angle contact with the trachea respectively invaded the tracheal cartilage on surgery [23]. While this study included microPTCs <1 cm, Chung et al. [19] similarly reported that 67% (12/18) of nodules with obtuse-angle contact with the trachea on ultrasound were confirmed to show tracheal invasion during surgery, whereas none of 265 nodules with acute-angle contact and only 1 of 134 nodules with right-angle contact with the trachea had tracheal invasion at surgery. The median nodule size in their study was 1.3 cm in maximum diameter (range 0.2–11.0 cm). Therefore, the angle of contact between the PTC and trachea appears to be a sensitive marker, and PTCs >7 mm with acute angle contact with the trachea can be considered for AS. However, an unexplored criterion for tracheal invasion is the length of contact between the PTC nodule and trachea. In prostate cancer, the length of capsular contact is considered the most robust MRI factor associated with minor extracapsular extension [24]. As study protocols increase the inclusion of larger PTCs (≤20 mm) for AS, evaluation of the length of contact between the PTC and trachea on ultrasound may help to further predict the risk of tracheal invasion, apart from the angle of contact (Fig. 2).
Fig. 2. Female patient, age early 50’s with 11 mm PTC in right thyroid lobe and isthmus. Transverse image shows the nodule to have an acute angle of contact with the trachea (long arrows) on both sides. The nodule has a broad contact (short arrows) with the trachea of approximately 10 mm. For larger nodules >10 mm in size, evaluation of the length of contact between the PTC and the trachea on ultrasound may help to further prognosticate the risk of tracheal invasion apart from the angle of contact. PTC = papillary thyroid cancer.
Assessment of PTC Relation to Recurrent Laryngeal Nerve
Evaluating the relationship between PTC and RLN along with its anticipated course is crucial for determining the eligibility for AS [8]. A significant indicator of RLN invasion risk is the loss of normal thyroid parenchyma between the PTC and the tracheoesophageal groove (TEG), particularly when the nodule protrudes into the TEG or posteriorly [22]. Ito et al. [23] reported that, similar to the evaluation of tracheal invasion, PTCs <7 mm did not demonstrate RLN invasion regardless of the aforementioned criteria.
Evaluation of Cervical Lymphadenopathy
The assessment of cervical lymphadenopathy is crucial during the follow-up of PTC nodules and is a definite marker of progression. Sonographic characteristics such as microcalcification, cystic changes, rounded morphology, irregular margins, hyperechogenicity (resembling thyroid tissue), abnormal vascularity (peripheral or diffuse) and loss of fatty hilum are indicative of nodal disease [2,25,26,27,28] and should be subjected to biopsy. Miyauchi et al. [29] reported nodal metastases in 1.6% of 3222 patients with micropapillary PTCs after 20 years of surveillance. In another study, the 10- and 20-year rates of nodal metastases for micropapillary PTCs were 1.4% and 3.9%, respectively [30]. Ongoing studies evaluating PTCs up to 2 cm in size are needed to assess the incidence of nodal metastases in larger PTCs.
Assessment of PTC Growth during Active Surveillance
Unlike the Gleason grade progression in prostate cancer, specific criteria for disease progression in patients undergoing AS management for PTC are not well established. As prospective studies on AS in PTCs have only commenced relatively recently, experts have adopted rigorous criteria for disease progression in the interest of vigilance and safety. Typical study protocols define disease progression as an increase in largest dimension of PTC by ≥3 mm [5,10,11,12,31] or a change in nodule volume of >50% [32]. However, a recently published study allowed for a size increase of up to 5 mm in the largest dimensions [6]. Intuitively, the 3 mm growth for a 20 mm PTC may not represent an equally significant change as it would for a <10 mm PTC. While absolute tumor size and growth are important considerations, the decision to intervene may be influenced not only by the magnitude of the size increase but also by whether the PTC exhibits rapid growth in the first or second year of initiating AS, as it may potentially suggest a more aggressive nature. Therefore, most AS study protocols require 6-month interval scans for the first 2 years, which may thereafter be reduced to yearly [9]. While the 30-year long-term oncological outcomes from Kuma Hospital for ≤10 mm PTCs are very positive and did not differ between those undergoing AS or immediate surgery [29], results of ongoing studies are crucial for evaluating feasibility and success of AS in the management of thyroid PTCs that measure 10–20 mm.
Tumor volume has also been studied to assess the progression of PTC. Tumor volume doubling time is considered a marker of progression in solid tumors and has also been studied in PTCs [33]. Oh et al. [33] reported that the tumor volume doubling time was a good indicator of tumor growth velocity and that younger patients <50 years and PTCs with macrocalcification showed a rapid doubling time (<5 years). Similarly, Yamamoto et al. [34] reported that the tumor volume doubling rate was negatively associated with patient age. While numerous studies have highlighted significant interobserver variability in ultrasound measurements of thyroid nodules [35,36], Ito et al. [37] reported that volume measurement is particularly susceptible to increased interobserver variation compared to assessing the maximum tumor diameter because of the necessity of 3-dimensional measurements in volume calculations. Chung et al. [38] observed interobserver differences of up to 24% in maximum diameter and 72% in PTC volume. Hence, the evaluation of progression using nodule volume as a measure may lack accuracy and reliability. Furthermore, the process of calculating nodule volume at each follow-up may not be practical.
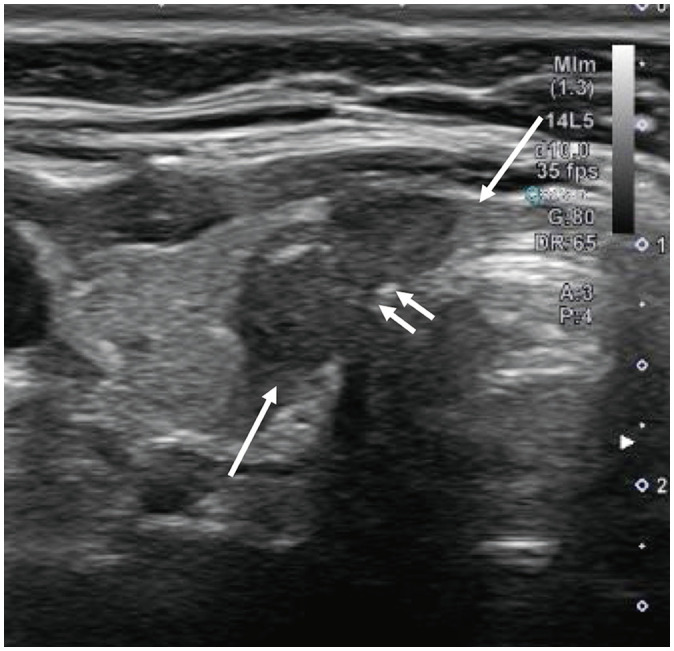
Since nodules may show growth or regression at different time points during AS, several studies have confirmed tumor growth through two consecutive ultrasound scans, generally performed within a few months of each other, to minimize the impact of measurement errors. Some nodules may regress after initial growth. Notably, Ito et al. [37] found that 47.4% showed a subsequent reduction in size during continued observation after an initial growth of ≥3 mm and therefore immediate surgery following a nodule enlargement of ≥3 mm might be premature for a PTC that is not associated with other adverse features such as suspected nodal metastases (Fig. 3). Yamamoto et al. [34] similarly reported that 56.4% (1200) of PTC nodules measuring ≤1 cm regressed (tumor volume doubling rate <0/year) over the course of the study. More recently, Tuttle et al. [39] proposed six different tumor volume kinetic patterns during AS of PTC nodules for a comprehensive description of the expected size changes. PTC nodules with coarse or rim calcification have been shown to be less likely to progress and are thus more likely to behave in an indolent manner [40]. Furthermore, Lee et al. [41] recently reported that the progression of PTCs measuring ≤1 cm was associated with diffuse thyroid disease and increased intratumoral vascularity.
Fig. 3. Transverse image at baseline in a female patient in her mid 40’s reveals (A) 10 mm papillary thyroid cancer in right thyroid (calipers). B: Transverse image 3 years after enrollment shows the nodule to have decrease in size, now measuring 4 mm in maximum transverse dimension (arrow).
CONCLUSION
In summary, ultrasound assessment is crucial for AS of PTCs; however, the currently available technology and its interpretation are subject to some limitations. Inter-observer variability in the measurement of thyroid nodules can affect the continuation of patients on AS protocols. Given the larger discrepancy in volume measurements compared to the single largest dimension in assessing progression, we recommend that the single largest dimension be used to assess progression. The 3 mm size cut off for defining progress is arbitrary, and ongoing studies should be conducted to determine if this cut-off may be too strict. Furthermore, studies focusing on PTCs with a maximum diameter of 2 cm will offer valuable insights into the progression rates of larger nodules (10–20 mm). It is important to acknowledge that ultrasonography has limited accuracy in detecting minor ETE. For PTC nodules that are equivocal or suspicious but not conclusively indicative of minor ETE, a multi-disciplinary approach is necessary to advise on disease management. In PTCs >10 mm, measuring the length of contact between the PTC and the trachea, along with the angle of contact, may provide information regarding tracheal invasion; however, further research is required to confirm this hypothesis. AS of small, low-risk fields is emerging as an important disease management option for small, low-risk PTC, and ultrasound remains the mainstay of evaluation for AS eligibility and monitoring of disease progression.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sangeet Ghai, Anna M Sawka.
- Validation: all authors.
- Writing—original draft: Sangeet Ghai.
- Writing—review & editing: Anna M Sawka, David P Goldstein.
Funding Statement: The authors have received the following grant funding to support research on active surveillance or surgery for management of small, low risk papillary thyroid cancer: Ontario Academic Health Sciences Centres Alternate Funding Plan Innovation Grant (Ontario Ministry of Health), the Canadian Cancer Society (Lotte and John Hecht Memorial Foundation Innovation Grant, #703948 and the Innovation to Impact Grant, #706302), as well as the Canadian Institutes of Health Research (Project Grant, #PJT-162314). Anna M Sawka is supported, in part, by a Scientific Merit award from the University of Toronto Department of Medicine.
References
- 1.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CA, Yoo J, Oh HS, Jeon MJ, Chung SR, Baek JH, et al. Undercover active surveillance of small highly suspicious thyroid nodules without fine needle aspiration. Endocrine. 2024;84:615–624. doi: 10.1007/s12020-023-03601-6. [DOI] [PubMed] [Google Scholar]
- 4.Miyauchi A, Ito Y. Conservative surveillance management of low-risk papillary thyroid microcarcinoma. Endocrinol Metab Clin North Am. 2019;48:215–226. doi: 10.1016/j.ecl.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Sawka AM, Ghai S, Tomlinson G, Rotstein L, Gilbert R, Gullane P, et al. A protocol for a Canadian prospective observational study of decision-making on active surveillance or surgery for low-risk papillary thyroid cancer. BMJ Open. 2018;8:e020298. doi: 10.1136/bmjopen-2017-020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho AS, Kim S, Zalt C, Melany ML, Chen IE, Vasquez J, et al. Expanded parameters in active surveillance for low-risk papillary thyroid carcinoma: a nonrandomized controlled trial. JAMA Oncol. 2022;8:1588–1596. doi: 10.1001/jamaoncol.2022.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Chung KW, et al. Active surveillance for small papillary thyroid cancer: a systematic review and meta-analysis. Thyroid. 2019;29:1399–1408. doi: 10.1089/thy.2019.0159. [DOI] [PubMed] [Google Scholar]
- 8.Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery Task Force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31:183–192. doi: 10.1089/thy.2020.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghai S, O’Brien C, Goldstein DP, Sawka AM Canadian Thyroid Cancer Active Surveillance Study Group. Ultrasound in active surveillance for low-risk papillary thyroid cancer: imaging considerations in case selection and disease surveillance. Insights Imaging. 2021;12:130. doi: 10.1186/s13244-021-01072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 11.Moon JH, Kim JH, Lee EK, Lee KE, Kong SH, Kim YK, et al. Study protocol of multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma (MAeSTro) Endocrinol Metab (Seoul) 2018;33:278–286. doi: 10.3803/EnM.2018.33.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian center. J Clin Endocrinol Metab. 2020;105:e172–e180. doi: 10.1210/clinem/dgz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143:1015–1020. doi: 10.1001/jamaoto.2017.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawka AM, Ghai S, Tomlinson G, Baxter NN, Corsten M, Imran SA, et al. A Protocol for a Pan-Canadian prospective observational study on active surveillance or surgery for very low risk papillary thyroid cancer. Front Endocrinol (Lausanne) 2021;12:686996. doi: 10.3389/fendo.2021.686996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103:2269–2273. doi: 10.1002/cncr.21055. [DOI] [PubMed] [Google Scholar]
- 16.Bachar G, Buda I, Cohen M, Hadar T, Hilly O, Schwartz N, et al. Size discrepancy between sonographic and pathological evaluation of solitary papillary thyroid carcinoma. Eur J Radiol. 2013;82:1899–1903. doi: 10.1016/j.ejrad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Roman BR, Gupta P, Tuttle RM, Morris LGT, Lohia S. Assessing the number of candidates there are for active surveillance of low-risk papillary thyroid cancers in the US. JAMA Otolaryngol Head Neck Surg. 2020;146:585–586. doi: 10.1001/jamaoto.2020.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg. 2006;30:780–786. doi: 10.1007/s00268-005-0270-z. [DOI] [PubMed] [Google Scholar]
- 19.Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, et al. Sonographic assessment of the extent of extrathyroidal extension in thyroid cancer. Korean J Radiol. 2020;21:1187–1195. doi: 10.3348/kjr.2019.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solymosi T, Hegedűs L, Bonnema SJ, Frasoldati A, Jambor L, Karanyi Z, et al. Considerable interobserver variation calls for unambiguous definitions of thyroid nodule ultrasound characteristics. Eur Thyroid J. 2023;12:e220134. doi: 10.1530/ETJ-22-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Miyauchi A. Active surveillance of low-risk papillary thyroid microcarcinomas. Gland Surg. 2020;9:1663–1673. doi: 10.21037/gs-2019-catp-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyauchi A, Ito Y, Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid. 2018;28:23–31. doi: 10.1089/thy.2017.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg. 2016;40:523–528. doi: 10.1007/s00268-015-3184-4. [DOI] [PubMed] [Google Scholar]
- 24.Sanguedolce F, Tedde A, Granados L, Hernández J, Robalino J, Suquilanda E, et al. Defining the role of multiparametric MRI in predicting prostate cancer extracapsular extension. World J Urol. 2024;42:37. doi: 10.1007/s00345-023-04720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumbhar SS, O’Malley RB, Robinson TJ, Maximin S, Lalwani N, Byrd DR, et al. Why thyroid surgeons are frustrated with radiologists: lessons learned from pre- and postoperative US. Radiographics. 2016;36:2141–2153. doi: 10.1148/rg.2016150250. [DOI] [PubMed] [Google Scholar]
- 26.Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021;22:2094–2123. doi: 10.3348/kjr.2021.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2:147–159. doi: 10.1159/000354537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung SR, Baek JH, Rho YH, Choi YJ, Sung TY, Song DE, et al. Sonographic diagnosis of cervical lymph node metastasis in patients with thyroid cancer and comparison of European and Korean guidelines for stratifying the risk of malignant lymph node. Korean J Radiol. 2022;23:1102–1111. doi: 10.3348/kjr.2022.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyauchi A, Ito Y, Fujishima M, Miya A, Onoda N, Kihara M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023;33:817–825. doi: 10.1089/thy.2023.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaoka R, Ebina A, Toda K, Jikuzono T, Saitou M, Sen M, et al. Multifocality and progression of papillary thyroid microcarcinoma during active surveillance. World J Surg. 2021;45:2769–2776. doi: 10.1007/s00268-021-06185-2. [DOI] [PubMed] [Google Scholar]
- 31.Davies L, Chang CH, Sirovich B, Tuttle RM, Fukushima M, Ito Y, et al. Thyroid cancer active surveillance program retention and adherence in Japan. JAMA Otolaryngol Head Neck Surg. 2021;147:77–84. doi: 10.1001/jamaoto.2020.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center’s experience in Korea. J Clin Endocrinol Metab. 2017;102:1917–1925. doi: 10.1210/jc.2016-4026. [DOI] [PubMed] [Google Scholar]
- 33.Oh HS, Kwon H, Song E, Jeon MJ, Kim TY, Lee JH, et al. Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid. 2019;29:642–649. doi: 10.1089/thy.2018.0609. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto M, Miyauchi A, Ito Y, Fujishima M, Sasaki T, Kudo T. Tumor volume-doubling rate is negatively associated with patient age in papillary thyroid microcarcinomas under active surveillance. Surgery. 2024;175:1089–1094. doi: 10.1016/j.surg.2023.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Yoon DY, Seo YL, Kim JH, Baek S, Lim KJ, et al. Intraobserver and interobserver variability in ultrasound measurements of thyroid nodules. J Ultrasound Med. 2018;37:173–178. doi: 10.1002/jum.14316. [DOI] [PubMed] [Google Scholar]
- 36.Brauer VF, Eder P, Miehle K, Wiesner TD, Hasenclever H, Paschke R. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid. 2005;15:1169–1175. doi: 10.1089/thy.2005.15.1169. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y, Miyauchi A, Kudo T, Higashiyama T, Masuoka H, Kihara M, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid. 2019;29:1765–1773. doi: 10.1089/thy.2019.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung SR, Choi YJ, Lee SS, Kim SO, Lee SA, Jeon MJ, et al. Interobserver reproducibility in sonographic measurement of diameter and volume of papillary thyroid microcarcinoma. Thyroid. 2021;31:452–458. doi: 10.1089/thy.2020.0317. [DOI] [PubMed] [Google Scholar]
- 39.Tuttle RM, Fagin J, Minkowitz G, Wong R, Roman B, Patel S, et al. Active surveillance of papillary thyroid cancer: frequency and time course of the six most common tumor volume kinetic patterns. Thyroid. 2022;32:1337–1345. doi: 10.1089/thy.2022.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuoka O, Sugitani I, Ebina A, Toda K, Kawabata K, Yamada K. Natural history of asymptomatic papillary thyroid microcarcinoma: time-dependent changes in calcification and vascularity during active surveillance. World J Surg. 2016;40:529–537. doi: 10.1007/s00268-015-3349-1. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Kim JH, Kim YK, Lee CY, Lee EK, Moon JH, et al. US predictors of papillary thyroid microcarcinoma progression at active surveillance. Radiology. 2023;309:e230006. doi: 10.1148/radiol.230006. [DOI] [PubMed] [Google Scholar]