Abstract
Objective
To evaluate the outcomes of drug-eluting bead transarterial chemoembolization (DEB-TACE) according to the size of the beads for the treatment of small hepatocellular carcinoma (HCC).
Materials and Methods
This retrospective study included 212 patients with a single HCC ≤5 cm from five tertiary institutions. One hundred and nine patients were treated with 70–150-µm doxorubicin DEBs (group A), and 103 patients received 100–300-µm doxorubicin DEBs (group B). The initial tumor response (assessed between 3 weeks and 2 months after DEB-TACE), time to local tumor progression (TTLTP), restricted mean duration of complete response (RMDCR), rate of complications, incidence of post-embolization syndrome, and length of hospital stay were compared between the two groups. Logistic regression was used to analyze prognostic factors for initial tumor response.
Results
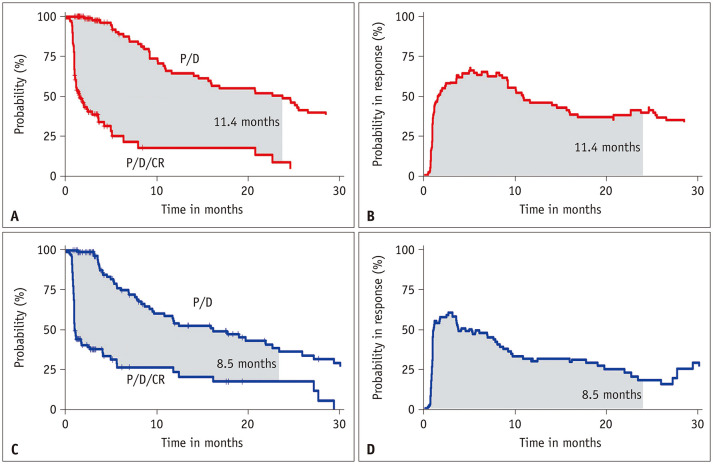
The initial objective response rates were 91.7% (100/109) and 84.5% (87/103) for groups A and B, respectively (P = 0.101). In the subgroup analysis of tumors ≤3 cm, the initial objective response rates were 94.6% (53/56) and 78.0% (39/50) for groups A and B, respectively (P = 0.012). There was no significant difference in the TTLTP (median, 23.7 months for group A vs. 19.0 months for group B; P = 0.278 [log-rank], 0.190 [multivariable Cox regression]) or RMDCR at 24 months (11.4 months vs. 8.5 months, respectively; P = 0.088). In the subgroup analysis of tumors >3-cm, the RMDCR at 24 months was significantly longer in group A than in group B (11.8 months vs. 5.7 months, P = 0.024). The incidence of mild bile duct dilatation after DEB-TACE was significantly higher in group B than in group A (5.5% [6/109] vs. 18.4% [19/103], P = 0.003).
Conclusion
DEB-TACE using 70–150-µm microspheres demonstrated a higher initial objective response rate in ≤3-cm HCCs and a longer RMDCR at 24 months in 3.1–5-cm HCCs compared to larger DEBs (100–300-µm).
Keywords: Chemoembolization, Drug-eluting bead, Hepatocellular carcinoma
INTRODUCTION
Transarterial chemoembolization (TACE) is recommended as a standard treatment for patients with intermediate-stage hepatocellular carcinoma (HCC) or those who are not suitable candidates for potentially curative treatments [1]. The use of drug-eluting beads (DEBs) loaded with anticancer drugs in TACE was once expected to provide higher local efficacy and lower systemic side effects compared with conventional TACE (cTACE) because DEBs slowly release high doses of anticancer agents in vivo [2]. However, randomized controlled trials (RCTs) comparing cTACE and DEB-TACE did not show significant differences in tumor response and survival [3,4].
DEBs can penetrate the tumor deeper as their size decreases, and larger DEBs are more likely to cause proximal embolization of tumor-feeding arteries, potentially leading to frequent tumor recurrence at the periphery of the tumor [5,6,7]. Retrospective studies comparing DEB sizes found that 100–300-µm DEBs had better outcomes and fewer complications than 300–500-µm or 500–700-µm DEBs [8,9]. In a prospective multicenter study on DEB efficacy, small HCCs had a lower objective response rate compared with medium-sized HCCs [10]. Furthermore, studies comparing DEB-TACE and cTACE have demonstrated a higher tumor response rate for cTACE in HCCs <3 cm [11,12]. These findings imply that in smaller HCCs, the tumor-feeding artery could be too small for 100–300-µm DEBs to effectively penetrate the tumor, resulting in lower tumor response rates compared to those of cTACE [13]. Studies comparing 70–150-µm DEBs with 100–300-µm DEBs have produced inconsistent results and were limited to small, single-center patient populations [14,15,16]. Thus, further investigation is needed regarding the outcomes of smaller DEBs. In addition, comparative studies evaluating the effect of DEB size on small HCCs are scarce. Therefore, this study aimed to compare the treatment outcomes and safety of 70–150-µm versus 100–300-µm DEBs for TACE in small-sized (≤5-cm) HCCs.
MATERIALS AND METHODS
Study Design and Patients
This retrospective, multicenter study analyzed data from consecutive patients who underwent DEB-TACE using 70–150-µm or 100–300-µm DEBs as an initial treatment for primary HCC at five tertiary medical centers in Korea between January 2011 and December 2020. The study was approved by the Institutional Review Board (IRB No. 4-2021-0817), which waived the requirement for informed consent due to the retrospective nature of the study. The primary objective was to evaluate the initial tumor response rate and time to local tumor progression (TTLTP) according to DEB size. The secondary objective was to evaluate the restricted mean duration of complete response (RMDCR) and the rate of complications.
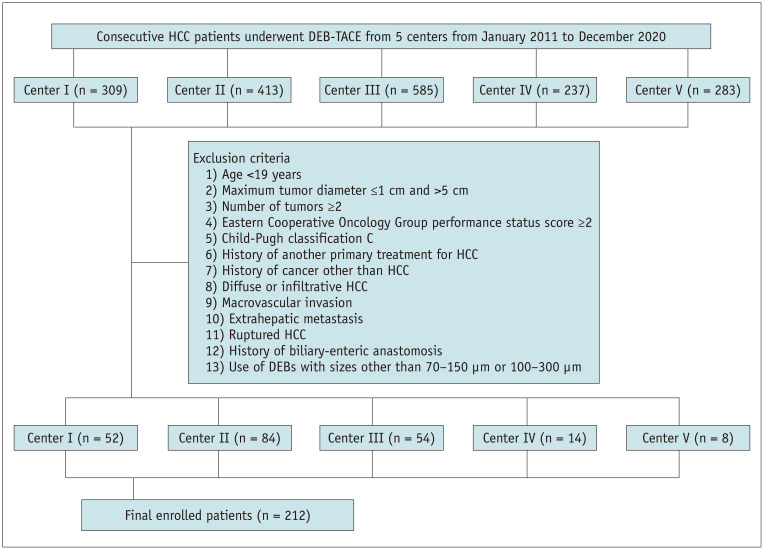
HCC was diagnosed according to the 2018 American Association for the Study of Liver Diseases guidelines using imaging findings (dynamic enhanced CT and/or MRI performed within 1 month before DEB-TACE) or histological examination [17]. The exclusion criteria were as follows: 1) age <19 years, 2) maximum tumor diameter >5 cm, 3) number of tumors ≥2, 4) Eastern Cooperative Oncology Group performance status score ≥2, 5) Child–Pugh class C, 6) history of receiving other primary treatments for HCC, 7) history of cancer other than HCC, 8) diffuse or infiltrative HCC, 9) HCC with macrovascular invasion, 10) HCC with extrahepatic metastasis, 11) ruptured HCC, 12) history of receiving biloenteric anastomosis, and 13) history of receiving DEBs other than 70–150 µm or 100–300 µm.
DEB-TACE Procedures
DEBs of 100–300 µm were used for all patients from January 2011 to September 2016, before the availability of 70–150-µm DEBs in Korea. From October 2016, the DEB size was determined by the intervention radiologists at each institution based on the patient and tumor characteristics. Doxorubicin (Adriamycin, Ildong, Seoul, Republic of Korea) at 50–75 mg was loaded for at least 1 hour into one vial (2 mL) of DEBs (DC Bead®, Boston Scientific, Marlborough, MA, USA). The doxorubicin-loaded DEBs were then suspended in a mixture of normal saline and non-ionic iodized contrast agent (ratio: 1:1 to 1:2, total volume: 20–50 mL).
The procedures were carried out under fluoroscopy and angiography guidance, with C-arm CT used based on the operator’s decision. Celiac and superior mesenteric artery angiographies were performed using a 4- or 5-Fr angiographic catheter, which was advanced to the common or proper hepatic artery for hepatic artery angiography. A microcatheter with a diameter of 1.7–2.4 Fr was used to select the tumor-feeding artery as distally as possible and at a level that would not block the antegrade flow or wedge the microcatheter. The DEB suspension was administered as slowly as possible. The intended endpoint of DEB-TACE was near or complete stasis of the feeding artery, and the DEB injection was stopped once the endpoint was achieved. When a vascular lake was observed, bland embolization was performed using a gelatin sponge or polyvinyl alcohol particles.
Data Collection
We collected data on the patients’ age, sex, laboratory findings (platelet count, prothrombin time, international normalized ratio, albumin, and bilirubin), tumor diameter, presence of ascites, varices, or portal hypertension, Child–Pugh classification, Barcelona Clinic Liver Cancer stage, and modified Union for International Cancer Control stage. Laboratory examinations were obtained before and within 1 week after TACE, and additional follow-up laboratory findings were investigated between 2 and 6 weeks after TACE. Portal hypertension was defined as the presence of gastroesophageal varices or other portosystemic collaterals or decompensated cirrhosis, including ascites, encephalopathy, or low platelet count (<100000/mm3) with splenomegaly (>12 cm on the largest dimension) [18]. Increased tumor marker was defined as alpha fetoprotein (AFP) (>100 ng/mL) or protein induced by vitamin K absence or antagonist-II (PIVKA-II) (100 mAU/mL) elevated from baseline [19].
Assessment of Oncologic Outcomes
Outcomes after DEB-TACE were evaluated using dynamic enhanced CT or MRI. Imaging analysis was independently performed at each institution, and the results were independently adjudicated and confirmed by two central reviewers (G.M.K. and J.W.C.). Follow-up imaging was scheduled 1, 3, and 6 months after DEB-TACE, followed by subsequent evaluations at 3–6-month intervals. The study endpoint was June 30, 2023. The evaluation of initial tumor response was conducted based on the initial CT scans, performed between 3 weeks and 2 months after DEB-TACE using modified Response Evaluation Criteria in Solid Tumors 1.1 [20]. The objective response was defined as complete response (CR) or partial response (PR). If residual or aggravated tumors were present, cTACE, DEB-TACE, radiofrequency ablation, percutaneous ethanol injection therapy, radiation therapy, hepatic resection, or liver transplantation was performed.
The TTLTP was defined as the interval from the initial DEB-TACE procedure to the first occurrence of local tumor recurrence or progression. Local tumor recurrence and progression were defined as the development of a new tumor around the target lesion for patients who achieved CR and a ≥20% increase in diameter of the viable target lesion compared to its diameter before treatment for patients who did not achieve CR. If a target lesion did not show progression but received additional treatment other than DEB-TACE, the patient was considered censored on the date of the other treatment.
The CR duration was measured from the date CR was first confirmed on imaging follow-up after the DEB-TACE until the progression of HCC or all-cause patient mortality [21]. Owing to significant differences in the follow-up period, the mean duration of CR data was restricted to 24 months, hereafter referred to as RMDCR [22,23]. For patients who underwent treatment conversion, the conversion date was used as the censoring date. When tumor progression or confirmation of death was not observed in the TTLTP and RMDCR, patients were censored at their last follow-up date.
Assessment of Safety
Major complications were defined as post-procedural events resulting in death, life-threatening conditions, significant morbidity and disability, or requiring an elevated level of care or longer hospitalization, including unscheduled re-admission after initial discharge [24]. Clinical records and follow-up imaging were analyzed to assess hepatobiliary complications, including bile duct dilatation, abscess, and biloma. Severe bile duct injury was defined as notable dilatation beyond the usual segmental distribution [12]. The occurrence of post-embolization syndrome during the hospitalization period after the procedure was evaluated [25]. Additionally, the length of hospitalization was assessed.
Statistical Analysis
Continuous variables were presented as means and ranges, and categorical variables were presented as counts and percentages. Comparative analysis between the 70–150-µm group (group A) and 100–300-µm group (group B) was performed using the chi-square test or Fisher’s exact test for categorical data and Student’s t-test for continuous data. Univariable and multivariable logistic regression analyses were performed to identify the factors influencing tumor response. TTLTP was estimated using Kaplan–Meier analysis and compared between the two groups with the log-rank test and multivariable Cox proportional hazard regression. Factors affecting initial tumor response and TTLTP were identified using univariable and multivariable Cox proportional hazard models. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated. The RMDCR was determined by calculating the area under the probability of response (PBIR) curve, which was plotted from the cumulative response rate defined as the proportion of patients who achieved and maintained CR [22,23]. Subgroup analyses were performed according to the tumor size. P-values <0.05 were considered significant for all analyses. Statistical analyses were performed using R-4.3.3 (http://www.r-project.org) and SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient and Tumor Baseline Characteristics
A total of 212 eligible patients were analyzed in this study (Fig. 1). Table 1 summarizes the baseline characteristics. Group A included 109 patients, and group B included 103 patients. There were no significant differences in the baseline characteristics between groups A and B.
Fig. 1. Diagram of the study population. HCC = hepatocellular carcinoma, DEB-TACE = drug-eluting bead transarterial chemoembolization.
Table 1. Patients’ baseline characteristics.
| Characteristics | Total (n = 212) | Group A: 70–150 μm (n = 109) | Group B: 100–300 μm (n = 103) | P | |
|---|---|---|---|---|---|
| Age, yr | |||||
| Mean (range) | 68.0 (34–91) | 68.3 (34–89) | 67.6 (40–91) | 0.626 | |
| Sex | |||||
| Male | 135 (63.7) | 67 (61.5) | 68 (66.0) | 0.491 | |
| Female | 77 (36.3) | 42 (38.5) | 35 (34.0) | ||
| Child–Pugh classification | |||||
| A | 177 (83.5) | 90 (82.6) | 87 (84.5) | 0.710 | |
| B | 35 (16.5) | 19 (17.4) | 16 (15.5) | ||
| Portal hypertension | |||||
| No | 107 (50.5) | 55 (50.5) | 52 (50.5) | 0.997 | |
| Yes | 105 (49.5) | 54 (19.5) | 51 (19.5) | ||
| BCLC stage | |||||
| 0 | 25 (11.8) | 13 (11.9) | 12 (11.7) | 0.950 | |
| A | 187 (88.2) | 96 (88.1) | 91 (88.3) | ||
| mUICC stage | |||||
| I | 25 (11.8) | 13 (11.9) | 12 (11.7) | 0.950 | |
| II | 187 (88.2) | 96 (88.1) | 91 (88.3) | ||
| Tumor marker | |||||
| Increased | 85 (41.7) | 47 (44.8) | 38 (38.4) | 0.356 | |
| Tumor size, cm | |||||
| Mean (range) | 31.1 (14–50) | 31.4 (14–50) | 30.8 (14–49) | 0.620 | |
| ≤3 | 106 (50.0) | 56 (51.4) | 50 (48.5) | 0.680 | |
| >3 | 106 (50.0) | 53 (48.6) | 53 (51.5) | ||
Data are number of patients with percentage in parentheses, unless specified otherwise.
BCLC = Barcelona Clinic Liver Cancer, mUICC = modified Union for International Cancer Control
Initial Tumor Response
Table 2 summarizes the initial tumor response. The small difference between groups A and B was not significant (P = 0.109). Multivariable logistic regression analysis identified tumor size as a significant predictor of CR rate (P = 0.047) (Table 3).
Table 2. Initial tumor response after drug-eluting bead transarterial chemoembolization.
| Tumor response | Group A: 70–150 μm | Group B: 100–300 μm | P | |
|---|---|---|---|---|
| All patients | n = 109 | n = 103 | 0.109* | |
| CR | 63 (57.8) | 63 (61.2) | ||
| PR | 37 (33.9) | 24 (23.3) | ||
| SD | 9 (8.3) | 16 (15.5) | ||
| PD | 0 (0.0) | 0 (0.0) | ||
| OR (CR + PR) | 100 (91.7) | 87 (84.5) | 0.101 | |
| ≤3 cm | n = 56 | n = 50 | 0.004* | |
| CR | 36 (64.3) | 34 (68.0) | ||
| PR | 17 (30.4) | 5 (10.0) | ||
| SD | 3 (5.4) | 11 (22.0) | ||
| PD | 0 (0.0) | 0 (0.0) | ||
| OR (CR + PR) | 53 (94.6) | 39 (78.0) | 0.012 | |
| >3 cm | n = 53 | n = 53 | 0.910* | |
| CR | 27 (50.9) | 29 (54.7) | ||
| PR | 20 (37.7) | 19 (35.8) | ||
| SD | 6 (11.3) | 5 (9.4) | ||
| PD | 0 (0.0) | 0 (0.0) | ||
| OR (CR + PR) | 47 (88.7) | 48 (90.6) | 0.750 | |
Data are number of patients with percentage in parentheses.
*For overall comparison of the distribution of responses (CR, PR, SD, and PD) between two groups.
CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease, OR = objective response
Table 3. Univariable and multivariable logistic regression analyses for initial tumor response.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |||
| Complete response | ||||||
| Age (for increase by 1 year) | 0.973 (0.949, 0.998) | 0.035 | 0.976 (0.951, 1.001) | 0.058 | ||
| Sex | ||||||
| Male (n = 135) | Reference | |||||
| Female (n = 77) | 1.318 (0.741, 2.345) | 0.347 | ||||
| Portal hypertension | ||||||
| No (n = 107) | Reference | |||||
| Yes (n = 105) | 1.434 (0.827, 2.489) | 0.199 | ||||
| Tumor size, cm | ||||||
| ≤3 (n = 106) | Reference | Reference | ||||
| >3 (n = 106) | 0.576 (0.331, 1.002) | 0.051 | 0.561 (0.317, 0.994) | 0.047 | ||
| Child-Pugh classification | ||||||
| A (n = 177) | Reference | |||||
| B (n = 35) | 1.029 (0.491, 2.155) | 0.941 | ||||
| Increased tumor marker | ||||||
| No (n = 117) | Reference | |||||
| Yes (n = 85) | 0.779 (0.443, 1.371) | 0.387 | ||||
| Bead size, μm | ||||||
| 70–150 (n = 109) | Reference | |||||
| 100–300 (n = 103) | 1.150 (0.664, 1.991) | 0.618 | ||||
| Objective response | ||||||
| Age (for increase by 1 year) | 0.984 (0.948, 1.022) | 0.404 | ||||
| Sex | ||||||
| Male (n = 135) | Reference | |||||
| Female (n = 77) | 0.577 (0.249, 1.337) | 0.200 | ||||
| Portal hypertension | ||||||
| No (n = 107) | Reference | |||||
| Yes (n = 105) | 0.745 (0.321, 1.725) | 0.492 | ||||
| Tumor size, cm | ||||||
| ≤3 (n = 106) | Reference | |||||
| >3 (n = 106) | 1.314 (0.567, 3.045) | 0.524 | ||||
| Child-Pugh classification | ||||||
| A (n = 177) | Reference | |||||
| B (n = 35) | 1.514 (0.427, 5.364) | 0.520 | ||||
| Increased tumor marker | ||||||
| No (n = 117) | Reference | |||||
| Yes (n = 85) | 1.312 (0.550, 3.127) | 0.540 | ||||
| Bead size, μm | ||||||
| 70–150 (n = 109) | Reference | Reference | ||||
| 100–300 (n = 103) | 0.489 (0.206, 1.163) | 0.106 | 0.486 (0.204, 1.158) | 0.104 | ||
Variable selection in the multivariable model was performed using backward elimination of all variables included in this study.
CI = confidence interval
Fifty-six patients in group A and 50 patients in group B had tumors ≤3-cm. There was a significant difference in tumor response between groups A and B (P = 0.004). The objective response rates were 94.6% and 78.0% in groups A and B, respectively (P = 0.012) (Table 2). In the analysis of lesions >3-cm, groups A and B showed no significant difference in tumor response. Supplementary Table 1 details the subgroup analyses of tumor response at 1-cm intervals of tumor size (Supplement).
TTLTP
The follow-up periods were 31–150 months (median, 80 months) for all patients, 31–82 months (median, 56 months) for group A, and 35–150 months (median, 130 months) for group B. Local tumor progression was observed in 92 patients (43.4%). Groups A (46/109, 42.2%) and B (46/103, 44.7%) had similar occurrence rates. In the multivariable Cox regression analysis, the bead size was not an influencing factor for TTLTP (adjusted HR = 1.331, 95% CI: 0.868–2.042, P = 0.190). Increased initial tumor markers were a risk factor for TTLTP (adjusted HR = 1.614, 95% CI: 1.058–2.464, P = 0.026). Table 4 summarizes the factors that influenced the TTLTP. The median TTLTPs for groups A and B were 23.7 and 19.0 months, respectively (P = 0.278 [log-rank]) (Fig. 2A). In the subgroup analysis of tumors ≤3-cm and tumors >3-cm, the TTLTP was not significant different between the two groups (P = 0.553 and 0.191, respectively [log-rank]) (Fig. 2B, C).
Table 4. Univariable and multivariable Cox proportional hazard regression analysis analyses for time to local tumor progression.
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Age (for increase by 1 year) | 1.004 (0.985, 1.023) | 0.709 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.924 (0.606, 1.408) | 0.712 | |||
| Portal hypertension | |||||
| No | Reference | ||||
| Yes | 0.826 (0.546, 1.250) | 0.366 | |||
| Tumor size, cm | |||||
| ≤3 | Reference | Reference | |||
| >3 | 1.303 (0.862, 1.969) | 0.210 | 1.310 (0.855, 2.009) | 0.215 | |
| Child-Pugh classification | |||||
| A | Reference | ||||
| B | 0.854 (0.465, 1.569) | 0.612 | |||
| Increased tumor marker | |||||
| No | Reference | Reference | |||
| Yes | 1.612 (1.056, 2.459) | 0.027 | 1.614 (1.058, 2.464) | 0.026 | |
| Bead size, μm | |||||
| 70–150 | Reference | Reference | |||
| 100–300 | 1.257 (0.831, 1.902) | 0.279 | 1.331 (0.868, 2.042) | 0.190 | |
Variable selection in the multivariable model was performed using backward elimination of all variables included in this study.
CI = confidence interval
Fig. 2. Kaplan–Meier curve showing the TTLTP of 70–150-µm and 100–300-µm drug-eluting bead groups. A: TTLTP of all participants. B: Subgroup analysis of ≤3-cm HCCs. C: Subgroup analysis of >3-cm HCCs. The P-values are from the log-rank test. TTLTP = time to local tumor progression, HCC = hepatocellular carcinoma, CI = confidence interval.
RMDCR
The 24-month RMDCR was slightly longer in group A than in group B, but the differences were not significant (11.4 vs. 8.5 months, P = 0.088) (Figs. 3, 4A). In the subgroup analysis of tumors ≤3-cm, groups A and B demonstrated comparable RMDCR (11.7 vs. 11.1 months, P = 0.760) (Fig. 4B). In the subgroup analysis for tumors >3-cm, the RMDCR was significantly longer in group A than in group B (11.8 vs. 5.7 months, P = 0.024) (Fig. 4C).
Fig. 3. RMDCR for the 70–150-µm and 100–300-µm DEB groups. A: RMDCR as the area between two Kaplan–Meier curves for P/D and the P/D/CR in 70–150-µm. B: RMDCR as the area under the PBIR curve in 70–150-µm DEBs. C: RMDCR as the area between two Kaplan–Meier curves for P/D and the P/D/CR in 100–300-µm DEBs. D: PBIR curve in 100–300-µm DEBs. RMDCR = restricted mean duration of complete response, DEB = drug-eluting bead, P = progression, D = death, CR = complete response, PBIR = probability-of-being-in-response.
Fig. 4. Comparison of PBIR curves for the 70–150-µm and 100–300-µm DEB groups. A: Difference in the PBIR of all participants. B: Subgroup analysis of ≤3-cm HCCs. C: Subgroup analysis of >3-cm HCCs. PBIR = probability being in response, DEB = drug-eluting bead, HCC = hepatocellular carcinoma, RMDCR = restricted mean duration of complete response, CI = confidence interval.
Safety
Table 5 summarizes the complications and hospital stays after DEB-TACE. There were no deaths due to adverse events following DEB-TACE. Major complications were observed in three patients (2.8%) in group A and two patients (1.9%) in group B, with no significant difference (P = 0.698). All major complications were cases of liver abscesses, and symptoms improved after percutaneous drainage. Mild duct dilatation was present in 25 patients and was significantly more prevalent in group B (18.4%) than in group A (5.5%) (P = 0.003). The hospital stay was slightly shorter in group A (mean, 2.1 days; range, 1–7) than in group B (mean, 2.3 days; range, 1–15), but the difference was not significant (P = 0.267).
Table 5. Post-procedural complications and hospital stays of all participants.
| Parameter | Group A: 70–150 μm (n = 109) | Group B: 100–300 μm (n = 103) | P |
|---|---|---|---|
| Mild bile duct dilatation | 6 (5.5) | 19 (18.4) | 0.003 |
| Severe bile duct injury | 3 (2.8) | 3 (2.9) | 1.000* |
| Biloma | 5 (4.6) | 2 (1.9) | 0.447* |
| Abscess | 4 (3.7) | 2 (1.9) | 0.684* |
| Infarction | 1 (0.9) | 2 (1.9) | 0.613* |
| Post-embolization syndrome | 54 (49.5) | 55 (53.4) | 0.574 |
| Hospital stay, days | 2.1 (1–7) | 2.3 (1–15) | 0.267 |
Data are number of patients with percentage in parentheses, except for hospital stay which is the mean (range).
*Fisher’s exact test
DISCUSSION
This study found no overall difference in tumor response based on the bead size used in DEB-TACE for single tumors single tumors ≤5-cm. However, the group that received small (70–150-µm) DEBs for HCCs ≤3-cm showed a better objective response rate. Although the TTLTP was slightly longer in the group that received smaller DEBs, the difference was not significant. Patients who received smaller DEBs showed a longer RMDCR, with a significant difference observed in HCCs >3-cm. These findings demonstrate that the use of small-sized DEBs can be beneficial for initial tumor response and tumor control in early- to intermediate-stage HCC.
In previous animal studies, smaller microspheres achieved deeper tissue penetration, suggesting the potential benefits of small DEBs over large ones in HCC treatment [5,6]. Studies comparing the efficacy of 100–300-µm DEBs with larger DEBs (300–500-µm or 500–700-µm) revealed that smaller DEBs were associated with a better tumor response [9,26]. However, the efficacy comparisons between 70–150-µm and 100–300-µm DEBs are controversial: one study reported a better 1-month tumor response for 70–150-µm DEBs (objective response rate: 96.2% vs. 61.9%, P = 0.027) [15], whereas other studies showed no significant difference [14,16]. However, the use of small DEBs improved the initial tumor response in smaller tumors (<3 cm) in the subsequent studies.
A study on DEB-TACE with 100–300-µm DEBs found inferior tumor response in tumors <2-cm compared to medium-sized HCCs [27]. Similarly, in our study, the use of 100–300-µm DEBs demonstrated a lower objective response rate in HCCs measuring ≤3-cm compared with those >3-cm. Previous research has demonstrated a positive correlation between tumor size and tumor-feeding artery diameter, frequently ≤200 µm in HCCs <3-cm [13]. In early-stage HCC, the underdeveloped tumor-feeding artery may receive blood supply from the portal vein [28]. The relationship between DEB size and tumor response is complex, involving factors such as tumor size and feeding vessel characteristics.
In this study, the TTLTP and RMDCR were longer in the 70–150-µm DEB group; however, the difference was not significant. Analysis of tumors ≤3-cm revealed little differences in the TTLTP and RMDCR between both groups but longer TTLTP and CR duration with smaller DEBs in larger tumors. Transarterial therapy for HCC may not achieve pathological CR owing to insufficient drug accumulation, even if CR is observed on imaging, suggesting a risk of recurrence through the small collateral feeding arteries [29,30]. The sufficient penetration of DEBs into the smaller feeding arteries will likely affect the duration of response. However, in tumors ≤3 cm, even 70–150-µm DEBs may not sufficiently penetrate the fine-feeding arteries to achieve pathologic CR and prolonged CR duration. This hypothesis may be partially consistent with the results of a recent RCT that showed better outcomes following cTACE compared with DEB-TACE in HCCs ≤3-cm [11].
The overall adverse event rates of 70–150-µm DEBs range from 58% to 100% [14,31,32]. Moreover, the safety profiles of 70–150-µm and 100–300-µm DEBs have not shown significant differences [15,16]. In our study, mild bile duct dilatation following DEB-TACE was more frequent in the 100–300-µm DEB group, although no significant differences were observed in severe hepatobiliary injury between both groups. Theoretically, biliary toxicity due to ischemic injury of the peribiliary plexus after DEB-TACE may be more intense when small DEBs are used [33]. In a comparative study of 70–150-µm and 100–300-µm DEBs in which lobar treatment was performed in most cases, hepatobiliary adverse effects were reported more frequently in the group that received smaller DEBs [14]. These conflicting results may be explained as follows: particles larger than the feeding artery diameter may not effectively reach the tumor, potentially resulting in the release of the chemo-agent from stagnancy into the surrounding areas, which may damage the nearby hepatic parenchyma and biliary tract. In our study, we strived to catheterize as distally as possible, suggesting that superselective catheterization may be a more important factor than particle size in severe hepatobiliary injury.
Our study has some limitations. As a retrospective study, it faced challenges in clarifying treatment decisions for DEB-TACE. The quantity of DEBs loaded with chemotherapy for small HCC may correlate with tumor response; however, this data was unavailable. DEB-TACE was performed by multiple radiologists, and the differences in their expertise were not considered. Variations in DEB-TACE methods by country or institution suggest the need for larger prospective studies. Follow-up periods differed owing to the late availability of 70–150-µm DEBs, possibly affecting outcomes with new treatments. To overcome this limitation, we used the restricted mean survival time, which helps to compare survival curves when proportional hazard assumptions are unmet. The difference in the restricted mean survival time between groups provides an absolute measure of effect size and can be used to quantify the treatment effect [34].
In conclusion, the use of smaller DEBs (70–150-µm) demonstrated a higher initial objective response rate in ≤3-cm HCCs and increased RMDCR at 24 months in 3.1–5-cm HCCs compared to larger DEBs (100–300-µm). Owing to differences in the treatment period and follow-up duration between the two groups, the results should be interpreted cautiously.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Gyoung Min Kim, Jin Wook Chung.
- Data curation: Byung Chan Lee, Gyoung Min Kim, Jung Ho Yang.
- Formal analysis: Byung Chan Lee, Gyoung Min Kim, Jin Woo Choi, Jung Ho Yang.
- Funding acquisition: Gyoung Min Kim.
- Investigation: Byung Chan Lee, Gyoung Min Kim, Juil Park, Jin Wook Chung, Ho Jong Chun, Jung Suk Oh, Dong Ho Hyun.
- Methodology: Byung Chan Lee, Gyoung Min Kim, Jin Woo Choi.
- Validation: Byung Chan Lee, Gyoung Min Kim, Jin Wook Chung, Ho Jong Chun, Dong Ho Hyun.
- Writing—original draft: Byung Chan Lee.
- Writing—review & editing: Byung Chan Lee, Gyoung Min Kim, Jin Wook Chung, Jin Woo Choi, Ho Jong Chun, Dong Ho Hyun.
Funding Statement: The Terumo Research Fund (2021) through the Korean Society of Interventional Radiology.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2024.0231.
References
- 1.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KH, Liapi E, Vossen JA, Buijs M, Ventura VP, Georgiades C, et al. Distribution of iron oxide-containing Embosphere particles after transcatheter arterial embolization in an animal model of liver cancer: evaluation with MR imaging and implication for therapy. J Vasc Interv Radiol. 2008;19:1490–1496. doi: 10.1016/j.jvir.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreher MR, Sharma KV, Woods DL, Reddy G, Tang Y, Pritchard WF, et al. Radiopaque drug-eluting beads for transcatheter embolotherapy: experimental study of drug penetration and coverage in swine. J Vasc Interv Radiol. 2012;23:257–264.e4. doi: 10.1016/j.jvir.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malagari K. Drug-eluting particles in the treatment of HCC: chemoembolization with doxorubicin-loaded DC Bead. Expert Rev Anticancer Ther. 2008;8:1643–1650. doi: 10.1586/14737140.8.10.1643. [DOI] [PubMed] [Google Scholar]
- 8.Padia SA, Shivaram G, Bastawrous S, Bhargava P, Vo NJ, Vaidya S, et al. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol. 2013;24:301–306. doi: 10.1016/j.jvir.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Prajapati HJ, Xing M, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, et al. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203:W706–W714. doi: 10.2214/AJR.13.12308. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017;28:502–512. doi: 10.1016/j.jvir.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Arai Y, Inaba Y, Tanaka T, Sugawara S, Kodama Y, et al. Conventional or drug-eluting beads? Randomized controlled study of chemoembolization for hepatocellular carcinoma: JIVROSG-1302. Liver Cancer. 2022;11:440–450. doi: 10.1159/000525500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee IJ, Lee JH, Lee YB, Kim YJ, Yoon JH, Yin YH, et al. Effectiveness of drug-eluting bead transarterial chemoembolization versus conventional transarterial chemoembolization for small hepatocellular carcinoma in Child-Pugh class A patients. Ther Adv Med Oncol. 2019;11:1758835919866072. doi: 10.1177/1758835919866072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irie T, Kuramochi M, Takahashi N. Diameter of main tumor feeding artery of a hepatocellular carcinoma: measurement at the entry site into the nodule. Hepatol Res. 2016;46:E100–E104. doi: 10.1111/hepr.12534. [DOI] [PubMed] [Google Scholar]
- 14.Deipolyi AR, Oklu R, Al-Ansari S, Zhu AX, Goyal L, Ganguli S. Safety and efficacy of 70-150 µm and 100-300 µm drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2015;26:516–522. doi: 10.1016/j.jvir.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Huo YR, Xiang H, Chan MV, Chan C. Survival, tumour response and safety of 70-150 µm versus 100-300 µm doxorubicin drug-eluting beads in transarterial chemoembolisation for hepatocellular carcinoma. J Med Imaging Radiat Oncol. 2019;63:802–811. doi: 10.1111/1754-9485.12971. [DOI] [PubMed] [Google Scholar]
- 16.Yi JW, Hong HP, Kim MS, Shin BS, Kwon HJ, Kim BI, et al. Comparison of clinical efficacy and safety between 70-150 µm and 100-300 µm doxorubicin drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma. Life (Basel) 2022;12:297. doi: 10.3390/life12020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 19.Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2022;23:1126–1240. doi: 10.3348/kjr.2022.0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. Am J Cancer Res. 2021;11:1121–1131. [PMC free article] [PubMed] [Google Scholar]
- 22.Huang B, Tian L. Utilizing restricted mean duration of response for efficacy evaluation of cancer treatments. Pharm Stat. 2022;21:865–878. doi: 10.1002/pst.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, Tian L, McCaw ZR, Luo X, Talukder E, Rothenberg M, et al. Analysis of response data for assessing treatment effects in comparative clinical studies. Ann Intern Med. 2020;173:368–374. doi: 10.7326/M20-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaba RC, Lewandowski RJ, Hickey R, Baerlocher MO, Cohen EI, Dariushnia SR, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2016;27:457–473. doi: 10.1016/j.jvir.2015.12.752. [DOI] [PubMed] [Google Scholar]
- 25.Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Ou HY, Yu CY, Huang TL, Tsang LL, Cheng YF. Drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma: does size really matter? Diagn Interv Radiol. 2020;26:230–235. doi: 10.5152/dir.2019.19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Prospective multi-center Korean registry of transcatheter arterial chemoembolization with drug-eluting embolics for nodular hepatocellular carcinoma: a two-year outcome analysis. Korean J Radiol. 2021;22:1658–1670. doi: 10.3348/kjr.2020.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K, et al. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36:264–272. doi: 10.1007/s00261-011-9685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bargellini I, Bozzi E, Campani D, Carrai P, De Simone P, Pollina L, et al. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol. 2013;82:e212–e218. doi: 10.1016/j.ejrad.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Dioguardi Burgio M, Ronot M, Bruno O, Francoz C, Paradis V, Castera L, et al. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl. 2016;22:1491–1500. doi: 10.1002/lt.24615. [DOI] [PubMed] [Google Scholar]
- 31.Aliberti C, Carandina R, Lonardi S, Dadduzio V, Vitale A, Gringeri E, et al. Transarterial chemoembolization with small drug-eluting beads in patients with hepatocellular carcinoma: experience from a cohort of 421 patients at an Italian center. J Vasc Interv Radiol. 2017;28:1495–1502. doi: 10.1016/j.jvir.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Odisio BC, Ashton A, Yan Y, Wei W, Kaseb A, Wallace MJ, et al. Transarterial hepatic chemoembolization with 70-150 µm drug-eluting beads: assessment of clinical safety and liver toxicity profile. J Vasc Interv Radiol. 2015;26:965–971. doi: 10.1016/j.jvir.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malagari K, Iezzi R, Goldberg SN, Bilbao JI, Sami A, Akhan O, et al. The ten commandments of chemoembolization: expert discussion and report from Mediterranean Interventional Oncology (MIOLive) congress 2017. Eur Rev Med Pharmacol Sci. 2018;22:372–381. doi: 10.26355/eurrev_201801_14184. [DOI] [PubMed] [Google Scholar]
- 34.Han K, Jung I. Restricted mean survival time for survival analysis: a quick guide for clinical researchers. Korean J Radiol. 2022;23:495–499. doi: 10.3348/kjr.2022.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.