Abstract
Objective
This study aimed to evaluate the diagnostic performance and procedural characteristics of fluoroscopy-guided percutaneous transthoracic pleural forceps biopsy (PTPFB) in patients with exudative pleural effusion.
Materials and Methods
Patients with exudative pleural effusion who underwent PTPFB between May 1, 2014, and February 28, 2023, were included in this retrospective study. The interval between percutaneous catheter drainage (PCD) and PTPFB, number of biopsies, procedural time, and procedure-related complications were evaluated. The sensitivity, specificity, and accuracy of diagnosing malignancy were computed for pleural cytology using PCD drainage, PTPFB, and combined PTPFB and pleural cytology.
Results
Seventy-one patients, comprising 50 male and 21 female (mean age, 69.5 ± 15.3 years), were included in this study. The final diagnoses were benign lesions in 48 patients (67.6%) and malignant in 23 patients (32.4%). The overall interval between PCD and biopsy was 2.4 ± 3.7 days. The interval between PCD and biopsy in the group that underwent delayed PTPFB was 5.2 ± 3.9 days. The mean number of biopsies was 4.5 ± 1.3. The mean procedural time was 4.4 ± 2.1 minutes. Minor bleeding complications were reported in one patient (1.4%). The sensitivity, specificity, and accuracy for pleural cytology, PTPFB, and combined PTPFB and pleural cytology were 47.8% (11/23), 100% (48/48), and 83.1% (59/71), respectively; 65.2% (15/23), 100% (48/48), and 88.7% (63/71), respectively; and 78.3% (18/23), 100% (48/48), and 93.0% (66/71), respectively. The sensitivity and accuracy of cytology combined with PTPFB were significantly higher than those of cytological testing alone (P = 0.008 and 0.001, respectively).
Conclusion
Fluoroscopy-guided PTPFB is an accurate and safe diagnostic technique for patients with exudative pleural effusion, with acceptable diagnostic performance, low complication rates, and reasonable procedural times.
Keywords: Exudate, Fluoroscopy, Percutaneous, Pleura, Forceps, Biopsy
INTRODUCTION
Pleural effusion is defined as an abnormal accumulation of fluid in the pleural space. The causes of pleural effusion can vary from benign conditions to diseases with poor prognoses such as cancer [1,2,3,4,5]. Among the pleural lesions characterized by diffuse or nodular thickening, malignancies are more common than benign lesions [6]. Thoracentesis or percutaneous catheter drainage (PCD) is indicated in patients for whom the cause of effusion is unclear or when symptoms such as dyspnea are present [7]. Analysis of pleural fluid can help determine whether the effusion is transudative or exudative, which can aid in establishing a treatment strategy [8]. In patients with exudative pleural effusion, further evaluations, such as CT with contrast enhancement, tuberculosis testing, or cytology, are often required [8]. Moreover, invasive diagnostic tests such as bronchoscopy or pleural biopsy should be performed if the results of imaging studies and pleural fluid analyses are inconclusive [9,10]. However, bronchoscopy, thoracoscopy, and video-assisted thoracic surgery (VATS) are invasive, expensive, not readily available at all facilities, and may be associated with potential complications [9,10]. Image-guided biopsy has several advantages. It can be performed in patients with or without pleural effusion under real-time guidance. It is less invasive, does not require general anesthesia, is more cost-effective, and has a shorter procedure time. Consequently, ultrasound (US)- and CT-guided pleural biopsies are widely used, exhibiting a sensitivity of 76%–90.9% for diagnosing malignancy [11,12,13,14,15,16,17,18,19,20,21].
To the best of our knowledge, fluoroscopy-guided pleural biopsy using biopsy forceps has not been previously reported. This study aimed to evaluate the diagnostic performance and procedural characteristics of fluoroscopy-guided percutaneous transthoracic pleural forceps biopsy (PTPFB) in patients with exudative pleural effusion.
MATERIALS AND METHODS
Study Population
This study was approved by our Institutional Review Board (IRB No. JEJUNUH 2023-05-029). The requirement for informed consent was waived due to the retrospective nature of the study.
At the authors’ institution, the PTPFB was used in patients with exudative pleural effusion according to the following principles: If a visible lung parenchymal lesion of adequate size for biopsy [22] or an easily accessible extrapulmonary lesion (e.g., suspected metastasis to the supraclavicular lymph node or liver) was present, biopsy was attempted first.
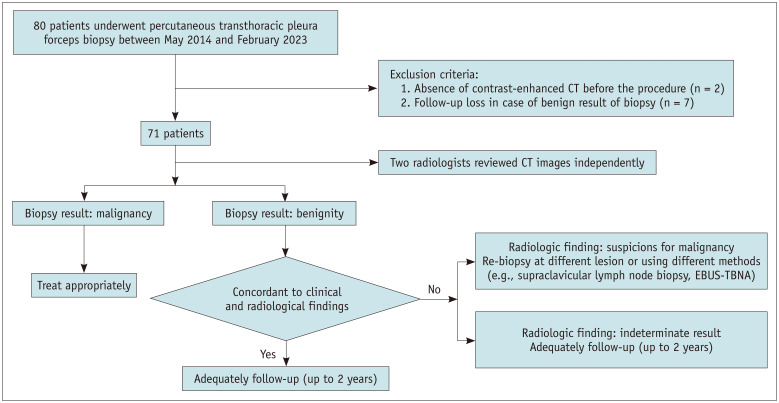
A retrospective review of our institutional database from May 1, 2014, to February 28, 2023, yielded 80 patients with exudative pleural effusion who underwent fluoroscopy-guided PTPFB. We excluded two patients who did not undergo contrast-enhanced CT prior to PTPFB and seven patients who were not adequately followed up. Therefore, 71 patients were included in this study. Figure 1 summarizes the patient flow.
Fig. 1. Study flowchart of the patients. EBUS-TBNA = endobronchial ultrasound guided transbronchial needle aspiration.
Fluoroscopy-Guided PTPFB Technique
All procedures were performed in one of the two angiosuites (AlluraClarity FD20, Philips Healthcare, Best, Netherlands; Artis Zee Ceiling, Siemens Healthcare, Erlangen, Germany) by one of four interventional radiologists with 3, 5, 11, and 19 years of clinical experience. All operators reviewed the patients’ chest CT images to approximate the location of the lesions before PTPFB.
According to the Cardiovascular and Interventional Radiological Society of Europe guidelines for procedures with a significant risk of bleeding, the coagulation status was routinely monitored before the procedure [23]. Patients receiving clopidogrel or aspirin were instructed to discontinue the medication for 5 days before the procedure. Transfusions were performed if the platelet count was <50000, and the international normalized ratio was corrected if it was >1.5.
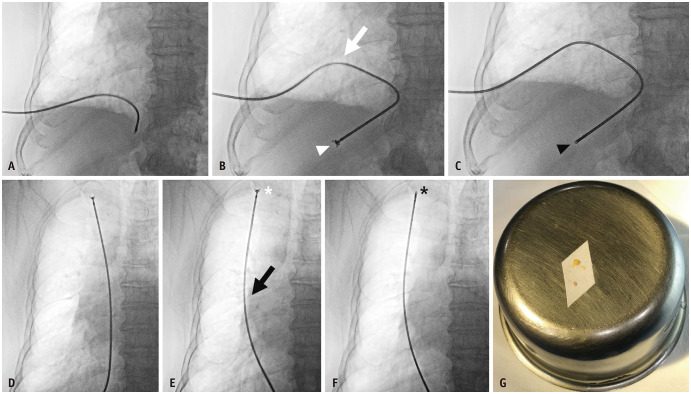
Prior to the procedure, the operator performed US on all patients to evaluate the assessment site. Subsequently, local anesthesia (10–15 mL of 2% lidocaine) was injected along the entrance point, followed by a 22G Chiba needle (Cook Medical, Bloomington, IN, USA) approach under fluoroscopic guidance, and the needle tip was positioned inside the pleural space. Then, the core needle was removed, and a 0.018″ nitinol micro-guidewire (Cook Medical) was advanced under fluoroscopic guidance, followed by the insertion of a 4Fr introducer sheath (Neff Percutaneous Access Set, Cook Medical). A 0.035″ hydrophilic guidewire was inserted into the pleural space. An 8Fr sheath was inserted if a biopsy was performed immediately. For pleural biopsy, we used the biopsy forceps (Optimos® biopsy forceps, Taewoong Medical, Gimpo, South Korea), which have a sufficient long and flexible shaft. As the tip of the biopsy forceps approached the pleura, it was inserted with minimal resistance until it made contact with the pleural tissue. Once contact was made, the tip ceased to advance. Further pressure applied by the operator resulted in the shaft bending while the tip remained stationary. With the tip was fixed and shaft was bending, the operator ensured that the tip maintained contact with the pleura, allowing for an accurate biopsy. The biopsy forceps were then retracted to obtain the tissue after grasping the pleura without targeting any specific lesions, three to six times in each patient, ensuring that at least one specimen of at least 1 mm in diameter, which was required for pathological evaluation, was included. The position of the biopsy tip could be distinguished as anterior or posterior in the oblique view. Figure 2 shows the fluoroscopy-guided PTPFB procedure.
Fig. 2. An 82-years-old male presenting with dyspnea and a history of end-stage renal disease, diabetes mellitus, and hypertension admitted to the emergency department. A: The right lower hemithorax is punctured, followed by the insertion of an 8Fr sheath. Subsequently, biopsy forceps are inserted. B: When the tip of the biopsy forceps is in contact with the pleura and resistance is felt, pushing the biopsy forceps slightly further allows for the observation of forceps shaft bending (arrow). The tip of the forceps is open while being fixed to the pleura (arrowhead). C: With the tip anchoring and shaft bending, the biopsy forceps are manipulated to grasp the pleura and retract the pleural tissue. The tip of the forceps is grasping the pleura while being fixed to it (arrowhead). D: The biopsy forceps are gently pushed until resistance is felt while performing the pleural biopsy in the upper hemithorax. E: Once the tip contacts and fixes to the pleura, it is pushed slightly further to bend the shaft (arrow). The tip of the forceps is open while being fixed to the pleura (asterisk). F: In this position, biopsy forceps are manipulated to grasp and retract the pleural tissue to obtain pleural tissue. Note that the tip of the forceps grasping the pleura is fixed to it (asterisk). G: The photograph shows four pieces of pleural tissue obtained.
After the PTPFB, a drainage catheter (8.5Fr or 10.2Fr) was inserted. In cases of delayed biopsy, a drainage catheter was initially inserted and a biopsy was performed several days later in the same manner. Cytological testing was performed on the pleural effusion obtained after PCD insertion. Immediately after the procedure and from the next day onwards, a chest radiograph was acquired daily to identify complications such as pneumothorax and observe the progress of changes in pleural effusion.
Qualitative Analysis of CT Images
Given that PTPFB is a nontargeting biopsy technique, we conducted a qualitative analysis of CT images obtained before PTPFB to address and mitigate the impact of potential false-negative outcomes. Three multidetector CTs scanners were used to perform the chest CT scan: two SOMATOM Force dual-source CT scanners with single energy (Siemens Healthcare) and one SOMATOM Definition Edge single-source CT scanner with single energy (Siemens Healthcare). All patients were placed in a supine position and scanned from the upper neck to the mid-portion of the kidney. Pre- and postcontrast scans were performed. CT scanning was initiated 40 seconds after contrast injection. All images were independently reviewed by one of two radiologists with 12 and 19 years of clinical experience, who determined whether the pleural lesions were benign, malignant, or indeterminate. Both radiologists were blinded to all clinical information except that the effusions were exudative. Each reader recorded their score according to a 5-point confidence scoring system, with each score indicating the following: 1, compatible with benignity; 2, possibly benign; 3, indeterminate; 4, possibly malignant; and 5, compatible with malignancy. Scores of 1 or 2 were considered benign and scores of 4 or 5 were considered malignant. In cases where the conclusions of the two readers were discordant, another radiologist with 11 years of experience in chest imaging, who was blinded to the patient’s clinical information and results from each reader, independently scored the confidence level. A consensus was reached based on majority opinion.
Data Collection
Data regarding the age, sex, medical history, and laboratory results were also collected. Procedural data, including the interval between PCD and PTPFB, number of biopsies, procedural time, and procedure-related complications were evaluated. In cases where both PCD insertion and PTPFB were performed in a single session, only the biopsy component of the procedural time was measured separately. Complications were categorized according to the classification system established by the Cardiovascular and Interventional Radiological Society of Europe [24]. Complications graded >3 on this scale were considered clinically significant.
Statistical Analysis
We reviewed the pathological results of cytological testing of pleural effusion and biopsy specimens. The final diagnosis of pleural lesions was established based on the following criteria: 1) If the biopsy results revealed malignancy, the pathologic report determined the final diagnosis (Supplementary Fig. 1), 2) If the biopsy results were benign but the radiological findings suggested malignancy, patients underwent a re-biopsy of another lesion using different methods if accessible, such as supraclavicular lymph node biopsy, bronchoscopy-assisted biopsy, or endobronchial US guided transbronchial needle aspiration (EBUS-TBNA) (Supplementary Fig. 2), 3) Patients with benign biopsy results and indeterminate radiological findings underwent adequate follow-up for a maximum of 2 years [25] to ascertain their benign status, and 4) If both the biopsy results and radiological findings indicated benign conditions, the patients were followed up conservatively (Supplementary Fig. 3). Finally, we categorized the text results as true positives, true negatives, false positives, or false negatives for malignancies.
The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of PTPFB for diagnosing malignancy were assessed, and Wilson’s method was used to calculate confidence intervals. McNemar’s test was used to compare pairwise sensitivity, accuracy, and NPV among the three different diagnostic methods: PTPFB, cytological testing, and combined PTPFB with cytological testing. Differences were considered statistically significant if the P value was <0.05. Continuous variables are expressed as mean ± standard deviation. Interobserver agreement between the two readers was calculated using weighted kappa statistics. All statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA) and R software (version 4.3.1; DTComPair package; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
Seventy-one PTPFB procedures were performed on 71 patients, comprising 50 (70.4%) male and 21 (29.6%) female (age range, 26–95 years; mean age, 69.5 ± 15.3 years). The final diagnoses were benign lesions in 48 patients (67.6%) and malignant lesions in 23 patients (32.4%). Table 1 summarizes the diseases associated with these effusions.
Table 1. Characteristics of the 71 patients and the diseases associated with exudative pleural effusion.
| Age, yr | 69.5 ± 15.3 (range, 26–95) | ||
| Sex | |||
| Male | 50 (70.4) | ||
| Female | 21 (29.6) | ||
| Final diagnosis | |||
| Malignant effusion (n = 23)* | |||
| Lung cancer | 17 (73.9) | ||
| Breast cancer | 1 (4.3) | ||
| Renal cell carcinoma | 1 (4.3) | ||
| Esophageal cancer | 1 (4.3) | ||
| Primary mesothelioma | 1 (4.3) | ||
| Thyroid cancer | 1 (4.3) | ||
| Unknown origin of malignancy | 1 (4.3) | ||
| Benign effusion (n = 48)* | |||
| Pneumonia | 22 (45.8) | ||
| Tuberculosis | 16 (33.3) | ||
| Empyema | 4 (8.3) | ||
| Idiopathic | 4 (8.3) | ||
| Bilothorax | 1 (2.1) | ||
| Radiation-induced effusion | 1 (2.1) | ||
Data are mean ± standard deviation (range) or patient number (%).
*The percentage values are calculated in the malignant and benign subgroups, respectively
Procedural Data
We obtained three to six specimens, including at least one specimen of at least 1 mm diameter, from each patient. Of the 71 patients, 38 underwent PTPFB and PCD immediately after the procedure. A PCD was initially inserted in the remaining 33 patients and delayed PTPFB was performed. The overall interval between PCD and biopsy was 2.4 ± 3.7 days, whereas the interval was 5.2 ± 3.9 days in the delayed PTPFB group. The mean number of biopsies was 4.5 ± 1.3. The mean procedural time was 4.4 ± 2.1 minutes. Minor bleeding was reported in one patient (1.4%). A small amount of blood-tinged pleural effusion was drained and spontaneously resolved after one day. No medication or extra management was necessary, except absolute bed rest, to control minor bleeding. This patient underwent a delayed biopsy 6 days after PCD, and the number of biopsies was six. The patient was receiving aspirin, which was discontinued only one day before the procedure. No cases of pneumothorax occurred.
Diagnostic Performance
The PTPFB results showed malignant lesions in 15 patients and benign in 56 patients. Among the 56 benign results, eight results were ultimately diagnosed as malignancies through additional evaluations, which were confirmed by cytological testing in three, neck lymph node biopsy in two, EBUS-TBNA in two, and VATS in one patient. Supplementary Table 1 summarizes the final diagnoses of the eight patients with false-negative PTPFB results. Cytological testing showed malignant and benign lesions in 11 and 60 patients, respectively, with 12 benign results ultimately being diagnosed as malignancies. The combined PTPFB and cytological results showed malignant and benign lesions in 18 and 53 patients, respectively. Among the patients with benign results, five had false-negative results and were ultimately diagnosed with malignancies. The performance of PTPFB, cytological testing, and combined PTPFB and cytological testing for diagnosing malignancy and their comparisons are shown in Table 2. Overall, the combined interpretation of PTPFB with cytological testing showed significantly higher sensitivity, accuracy, and NPV than cytological testing alone (P = 0.008, 0.001, and 0.008, respectively).
Table 2. Performance of PTPFB, cytologic testing, and the combined PTPFB and cytologic testing for diagnosing malignancy.
| PTPFB | Cytologic testing | P | ||
|---|---|---|---|---|
| PTPFB vs. cytologic testing | ||||
| Sensitivity | 65.2 (15/23) [42.7–83.6] | 47.8 (11/23) [26.8–69.4] | 0.206 | |
| Specificity | 100 (48/48) | 100 (48/48) | NA | |
| Accuracy | 88.7 (63/71) [81.4–96.1] | 83.1 (59/71) [74.4–91.8] | 0.013 | |
| PPV | 100 (16/16) | 100 (11/11) | NA | |
| NPV | 85.7 (48/56) [77.4–91.3] | 80.0 (48/60) [73.0–85.5] | 0.206 | |
| PTPFB | PTPFB + cytologic testing | P | ||
| PTPFB vs. PTPFB + cytologic testing | ||||
| Sensitivity | 65.2 (15/23) [42.7–83.6] | 78.3 (18/23) [56.3–92.5] | 0.083 | |
| Specificity | 100 (48/48) | 100 (48/48) | NA | |
| Accuracy | 88.7 (63/71) [81.4–96.1] | 93.0 (66/71) [87.0–98.9] | 0.013 | |
| PPV | 100 (16/16) | 100 (19/19) | NA | |
| NPV | 85.7 (48/56) [77.4–91.3] | 90.6 (48/53) [81.6–95.4] | 0.083 | |
| Cytologic testing | PTPFB + cytologic testing | P | ||
| Cytologic testing vs. PTPFB + cytologic testing | ||||
| Sensitivity | 47.8 (11/23) [26.8–69.4] | 78.3 (18/23) [56.3–92.5] | 0.008 | |
| Specificity | 100 (48/48) | 100 (48/48) | NA | |
| Accuracy | 83.1 (59/71) [74.4–91.8] | 93.0 (66/71) [87.0–98.9] | 0.001 | |
| PPV | 100 (11/11) | 100 (19/19) | NA | |
| NPV | 80.0 (48/60) [73.0–85.5] | 90.6 (48/53) [81.6–95.4] | 0.008 | |
Data are percentage with numerator/denominator in parentheses and 95% confidence interval in brackets.
PTPFB = percutaneous transthoracic pleural forceps biopsy, NA = not applicable, PPV = positive predictive value, NPV = negative predictive value
Qualitative Analysis of CT Images
The inter-observer agreement between the two readers was 0.81, demonstrating almost perfect agreement. Table 3 shows the cross-tabulation of the consensus CT findings, PTPFB results, and final diagnosis. All 10 patients with benign lesions on CT had benign lesions according to PTPFB and were confirmed to have benign lesions. Of the 40 patients with indeterminate CT results, 39 and 37 showed benign lesions with PTPFB and at final diagnosis, respectively. Among the remaining 21 patients with malignant findings on CT, the PTPFB results were benign in seven patients, and the final diagnosis was benign in one patient and malignant in 20 patients.
Table 3. PTPFB results and final diagnosis according to the CT findings.
| CT findings | ||||
|---|---|---|---|---|
| Benign (1–2) | Indeterminate (3) | Malignancy (4–5) | ||
| PTPFB result | ||||
| Benign | 10 | 39 | 7 | |
| Malignant | 0 | 1 | 14 | |
| Final diagnosis | ||||
| Benign | 10 | 37 | 1 | |
| Malignant | 0 | 3 | 20 | |
PTPFB = percutaneous transthoracic pleural forceps biopsy
DISCUSSION
Exudative pleural effusion frequently requires additional evaluations such as contrast-enhanced CT scans, tuberculosis testing, cytological testing, and pleural biopsy. Several studies have utilized US- or CT-guided pleural biopsy for diagnosing malignancy [11,12,13,14,15,16,17,18,19,20,21]. However, to the best of our knowledge, this is the first study to assess the diagnostic performance and procedural characteristics of fluoroscopy-guided pleural biopsy.
CT- or US-guided pleural biopsy requires targeting of an actively moving pleura during respiration, which requires a highly skilled expert. In contrast, the PTPFB is a simple technique that can be performed by interventional radiologists or trainees, irrespective of the patient’s breathing. This is evidenced by the fact that the procedure time was short, with an average of 4.4 ± 2.1 minutes. Lim et al. [16] recently implemented a new method for pleural biopsy using cone-beam CT virtual navigation guidance, with a total procedure time of 11.0 ± 4.6 minutes. Although the methods used for the procedures differed, the total procedure time in our study was significantly shorter, and obtaining sufficient pleural tissue in a short time without significant complications can be considered a strength of PTPFB.
Complications occurred in only one patient, who experienced minor bleeding that resolved spontaneously. The patient received aspirin until the day before the procedure, suggesting that potential complications could have been avoided. Benamore et al. [14] reported that pneumothorax occurred in four of 85 patients (5%) who underwent CT- or US-guided pleural biopsy. Cao et al. [15] reported that pneumothorax occurred in six of 92 patients (7%) who underwent CT-guided pleural biopsy. However, pneumothorax did not occur in our study, possibly due to the fact that CT- or US-guided biopsies are performed via the centripetal approach, i.e., piercing the needle towards the lung, whereas PTPFB is performed from the pleura towards the chest wall (centrifugal approach). Furthermore, PTPFB is considered less invasive as it is performed through the existing PCD site, eliminating the need for additional access sites, which are required for other methods of pleural biopsy.
In terms of diagnostic performance, PTPFB alone showed a sensitivity, specificity, accuracy, PPV, and NPV of 65.2%, 100%, 88.7%, 100%, and 85.7%, respectively, for diagnosing malignancy. These results are acceptable when compared with those of existing studies [11,12,13,14,15,16,17,18,19], with the exception that the sensitivity is lower. This could be attributed to the fact that biopsy was performed blindly. Nevertheless, the operators obtained pleural effusion during the PTPFB procedure, which facilitated the concurrent execution of PTPFB and cytological tests, consequently improving the overall diagnostic performance and increasing the sensitivity, accuracy, and NPV to 78.3%, 93.0%, and 90.6%, respectively. This level of sensitivity was comparable to that reported in previous studies [11,12,13,14,15,16,17,18,19], indicating a satisfactory outcome. Future investigations should explore the use of cone-beam CT in conjunction with PTPFB to improve diagnostic performance.
Because PTPFB is a new technique, a mechanism is required to reduce the false-negative rate and enhance its diagnostic performance. For this purpose, we used qualitative CT analysis. A qualitative analysis of the CT grading system is noteworthy. If indeterminate CT findings were considered benign, the sensitivity, specificity, accuracy, PPV, and NPV of visual CT grading were 87.0%, 97.9%, 94.4%, 95.2%, and 94.1%, respectively. Based on these outcomes, performing a contrast-enhanced CT before conducting PTPFB can lower the false-negative rate and complement the diagnostic performance of PTPFB. In addition, further evaluation is warranted even if the biopsy results are benign when there are malignant findings on CT. Piacibello et al. [26] analyzed the sensitivity and specificity of malignancy according to various CT findings of pleural lesions and reported the overall range of sensitivity as 0.41–0.89, and that of specificity as 0.53–0.91. In this study, we did not define the sensitivity or specificity of each CT scan. Further investigations, such as a quantitative analysis of the CT visual grading system, are necessary in the future.
Eight patients (34.8%) had discordant PTPFB results and final diagnoses. Three patients (13.0%), two with indeterminate CT findings and one with suspected malignant CT findings, were ultimately confirmed to have malignancy in the cytologic test. The remaining five patients (21.7%) had negative results for PTPFB and cytology but suspected malignant CT findings, two underwent neck lymph node biopsy, two underwent EBUS-TBNA, and one underwent VATS, which ultimately confirmed malignancy. This suggests that when tissue biopsy is necessary for diagnosis, it may be more appropriate to initially perform less invasive procedures, such as PTPFB with cytological testing. If the PTPFB and cytology results are negative, further investigation of alternative target lesions for biopsy or more invasive diagnostic procedures should be considered. This approach minimizes patient discomfort, potential complications, costs, and hospitalization associated with invasive procedures.
Our study has a few limitations. First, the sample size was relatively small because pleural biopsies are performed less frequently than lung biopsies in routine practice. Second, information regarding radiation doses was unavailable. Considering that PTPFB is guided by fluoroscopy, it is crucial to assess and minimize radiation exposure in patients and operators. Further research focusing on radiation dose optimization and its potential impact on patient outcomes is warranted. Lastly, for malignant pleural mesothelioma, there can be instances in which diagnosis is difficult, even with a core needle biopsy. Only one patient of malignant pleural mesothelioma was included in the present study; therefore, the evaluation of this aspect was insufficient.
In conclusion, fluoroscopy-guided PTPFB is an accurate and safe diagnostic technique for patients with exudative pleural effusion, with acceptable diagnostic performance, low complication rates, and reasonable procedural times.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Doo Ri Kim, In Chul Nam.
- Data curation: Doo Ri Kim, In Chul Nam, Jeong Jae Kim.
- Formal analysis: In Chul Nam, Duk Ju Kim, Hye Jin Baek, Sung Wook Song.
- Funding acquisition: In Chul Nam.
- Investigation: Doo Ri Kim, In Chul Nam, Jeong Jae Kim, Im Kyung Hwang, Jeong Sub Lee.
- Methodology: In Chul Nam.
- Supervision: In Chul Nam.
- Validation: Hye Jin Baek, Sung Wook Song.
- Visualization: In Chul Nam, Duk Ju Kim, Hye Jin Baek.
- Writing—original draft: Doo Ri Kim, In Chul Nam.
- Writing—review & editing: all authors.
Funding Statement: This work was supported by a research grant from Jeju National University Hospital in 2023 (2023-20).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2023.0960.
References
- 1.Walker SP, Morley AJ, Stadon L, De Fonseka D, Arnold DT, Medford ARL, et al. Nonmalignant pleural effusions: a prospective study of 356 consecutive unselected patients. Chest. 2017;151:1099–1105. doi: 10.1016/j.chest.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Anevlavis S, Tzouvelekis A, Bouros D. Mechanisms of pleural involvement in orphan diseases. Respiration. 2012;83:5–12. doi: 10.1159/000335128. [DOI] [PubMed] [Google Scholar]
- 3.Choi SH, Cha SI, Shin KM, Lim JK, Yoo SS, Lee SY, et al. Clinical relevance of pleural effusion in patients with pulmonary embolism. Respiration. 2017;93:271–278. doi: 10.1159/000457132. [DOI] [PubMed] [Google Scholar]
- 4.Bintcliffe OJ, Hooper CE, Rider IJ, Finn RS, Morley AJ, Zahan-Evans N, et al. Unilateral pleural effusions with more than one apparent etiology. A prospective observational study. Ann Am Thorac Soc. 2016;13:1050–1056. doi: 10.1513/AnnalsATS.201601-082OC. [DOI] [PubMed] [Google Scholar]
- 5.Evans AL, Gleeson FV. Radiology in pleural disease: state of the art. Respirology. 2004;9:300–312. doi: 10.1111/j.1440-1843.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- 6.Hussein-Jelen T, Bankier AA, Eisenberg RL. Solid pleural lesions. AJR Am J Roentgenol. 2012;198:W512–W520. doi: 10.2214/AJR.11.7626. [DOI] [PubMed] [Google Scholar]
- 7.Thomas R, Jenkins S, Eastwood PR, Lee YC, Singh B. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med. 2015;21:338–345. doi: 10.1097/MCP.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jany B, Welte T. Pleural effusion in adults—etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116:377–386. doi: 10.3238/arztebl.2019.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii4–ii17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Hsu A, Lo C, Colt HG. Prospective evaluation of flex-rigid pleuroscopy for indeterminate pleural effusion: accuracy, safety and outcome. Respirology. 2007;12:881–886. doi: 10.1111/j.1440-1843.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- 11.Scott EM, Marshall TJ, Flower CD, Stewart S. Diffuse pleural thickening: percutaneous CT-guided cutting needle biopsy. Radiology. 1995;194:867–870. doi: 10.1148/radiology.194.3.7862993. [DOI] [PubMed] [Google Scholar]
- 12.Adams RF, Gray W, Davies RJ, Gleeson FV. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest. 2001;120:1798–1802. doi: 10.1378/chest.120.6.1798. [DOI] [PubMed] [Google Scholar]
- 13.Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326–1330. doi: 10.1016/s0140-6736(03)13079-6. [DOI] [PubMed] [Google Scholar]
- 14.Benamore RE, Scott K, Richards CJ, Entwisle JJ. Image-guided pleural biopsy: diagnostic yield and complications. Clin Radiol. 2006;61:700–705. doi: 10.1016/j.crad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Cao YY, Fan N, Xing F, Xu LY, Qu YJ, Liao MY. Computed tomography-guided cutting needle pleural biopsy: accuracy and complications. Exp Ther Med. 2015;9:262–266. doi: 10.3892/etm.2014.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim HJ, Park CM, Yoon SH, Bae JS, Goo JM. Cone-beam CT virtual navigation-guided percutaneous needle biopsy of suspicious pleural metastasis: a pilot study. Korean J Radiol. 2018;19:872–879. doi: 10.3348/kjr.2018.19.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, Park B, Lim JK, Shin KM, Lee J, Kim CH, et al. Ultrasound-guided percutaneous needle biopsy for small pleural lesions: diagnostic yield and impact of CT and ultrasound characteristics. AJR Am J Roentgenol. 2021;217:699–706. doi: 10.2214/AJR.20.24120. [DOI] [PubMed] [Google Scholar]
- 18.Lin Z, Wu D, Wang J, Wang C, Huang M. Diagnostic value of ultrasound-guided needle biopsy in undiagnosed pleural effusions: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21076. doi: 10.1097/MD.0000000000021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Tang J, Zhou X, Zhou D, Wang J, Tang Q. Ultrasound-guided pleural cutting needle biopsy: accuracy and factors influencing diagnostic yield. J Thorac Dis. 2018;10:3244–3252. doi: 10.21037/jtd.2018.05.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun W, Zhou Y, Yang C, Dong Z, Zhang Z, Wang Y, et al. Contrast-enhanced ultrasound guided pleural biopsy improves diagnostic confidence for pleural based lesions: a 3-year prospective study. BMC Pulm Med. 2021;21:224. doi: 10.1186/s12890-021-01583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, Bruwer JW, Batubara EM, Diacon AH. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax. 2015;70:995–997. doi: 10.1136/thoraxjnl-2014-206567. [DOI] [PubMed] [Google Scholar]
- 22.Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, et al. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920–936. doi: 10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146. doi: 10.1007/s00270-017-1703-4. [DOI] [PubMed] [Google Scholar]
- 25.Janssen JP, Ramlal S, Mravunac M. The long-term follow up of exudative pleural effusion after nondiagnostic thoracoscopy. J Bronchol. 2004;11:169–174. [Google Scholar]
- 26.Piacibello E, Cardinale L, Righi L, Sverzellati N, Ardissone F, Veltri A. Correlation between CT findings and thoracoscopic diagnosis in diffuse pleural disease. Acta Biomed. 2020;91:e2020058. doi: 10.23750/abm.v91i3.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.