Abstract
Background.
The 2022–2023 United States influenza season had unusually early influenza activity with high hospitalization rates. Vaccine-matched A(H3N2) viruses predominated, with lower levels of A(H1N1)pdm09 activity also observed.
Methods.
Using the test-negative design, we evaluated influenza vaccine effectiveness (VE) during the 2022–2023 season against influenza A–associated emergency department/urgent care (ED/UC) visits and hospitalizations from October 2022 to March 2023 among adults (aged ≥18 years) with acute respiratory illness (ARI). VE was estimated by comparing odds of seasonal influenza vaccination among case-patients (influenza A test positive by molecular assay) and controls (influenza test negative), applying inverse-propensity-to-be-vaccinated weights.
Results.
The analysis included 85 389 ED/UC ARI encounters (17.0% influenza A positive; 37.8% vaccinated overall) and 19 751 hospitalizations (9.5% influenza A positive; 52.8% vaccinated overall). VE against influenza A–associated ED/UC encounters was 44% (95% confidence interval [CI], 40%–47%) overall and 45% and 41% among adults aged 18–64 and ≥65 years, respectively. VE against influenza A–associated hospitalizations was 35% (95% CI, 27%–43%) overall and 23% and 41% among adults aged 18–64 and ≥65 years, respectively.
Conclusions.
VE was moderate during the 2022–2023 influenza season, a season characterized with increased burden of influenza and co-circulation with other respiratory viruses. Vaccination is likely to substantially reduce morbidity, mortality, and strain on healthcare resources.
Keywords: influenza, H3N2, vaccine effectiveness, test-negative design, clinical outcomes
After historically low influenza activity during the first year of the coronavirus disease 2019 (COVID-19) pandemic (ie, the 2020–2021 influenza season), increased and prolonged circulation of vaccine-mismatched influenza A(H3N2) viruses was observed during the 2021–2022 United States (US) influenza season, although the overall burden of illness was low [1, 2]. The 2022–2023 season was characterized by early and intense influenza activity and co-circulation of other respiratory viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and respiratory syncytial virus (RSV) [3, 4]. A majority of influenza infections were caused by influenza A(H3N2) viruses belonging to the 3C.2a1b.2a.2 clade that were antigenically similar to the vaccine strain, with lower circulation of A(H1N1) pdm09 viruses and minimal B virus circulation [4].
Seasonal influenza vaccination is recommended annually in the US to protect against influenza-associated illness [5]. Vaccination remains the most effective way of preventing influenza illness and associated complications [5]. Prevention of influenza and other viral diseases through vaccination may substantially reduce the impact of serious respiratory infections on the healthcare system.
Using data from a multistate vaccine effectiveness (VE) network, we assessed 2022–2023 influenza VE against influenza-associated emergency department or urgent care (ED/UC) visits and hospitalizations in adults. In addition, we describe characteristics and outcomes of vaccinated and unvaccinated patients hospitalized with influenza.
METHODS
Population, Settings, and Study Design
This study from the VISION Network included healthcare encounters between 16 October 2022 and 31 March 2023 in 3 large health systems with integrated clinical, laboratory, and vaccination data in 4 states (Kaiser Permanente Northern California [KPNC], Intermountain Healthcare [IH] in Utah, and HealthPartners [HP] in Minnesota and Wisconsin). VISION’s study sites and methods used to evaluate influenza VE have been previously described [2]. In summary, using a test-negative case-control study design, among adults ≥18 years of age with an acute respiratory illness (ARI)–associated ED/UC encounter or hospitalization who underwent molecular testing for influenza (eg, reverse-transcription polymerase chain reaction), we estimated VE by comparing the odds of current-season influenza vaccination among those who tested positive for influenza A (case-patients) with those who tested negative for influenza A and B (controls). All influenza testing was clinician-initiated. Encounters were eligible if the patient received influenza testing within 10 days before or up to 72 hours after an ED/UC encounter date or date of hospital admission and also received SARS-CoV-2 molecular testing (in order to exclude controls with COVID-19, given the confounding bias due to the association between influenza and COVID-19 vaccination behaviors [2, 6, 7]). SARS-CoV-2-positive encounters (including coinfections with influenza), as well as a small number of influenza B–positive encounters, were excluded from the analysis. In addition, patients with an influenza or COVID-19 discharge diagnosis without a positive test result were excluded. We defined ARI as having ≥1 International Classification of Diseases, Tenth Revision (ICD-10) discharge code during the index encounter, including acute respiratory clinical diagnoses (eg, influenza, pneumonia) or acute respiratory signs or symptoms (eg, shortness of breath, cough), as previously defined [2]. A patient could contribute >1 encounter if ED/UC encounters occurred >24 hours apart or hospitalizations >30 days apart; repeat ED/UC visits within 24 hours or readmissions that occurred within 30 days of discharge were combined into a single encounter.
Demographic characteristics (eg, age, sex, race, and ethnicity) and underlying medical conditions were extracted from the electronic health records (EHRs). Current-season influenza vaccination status was classified based on EHR (HP, IH, KPNC), immunization information systems (HP, IH), or claims record (KPNC) of ≥1 dose of influenza vaccine since 1 August 2022. Events among patients with documented vaccination <14 days before the index date were excluded. Index date was defined as the earlier of the associated influenza test or the ED/UC visit or admission date. We collected data on the date of vaccination and the type of influenza vaccine product received, including standard-dose egg- or cell culture–based, recombinant, adjuvanted, or high-dose inactivated vaccine (Supplementary Table 1). For hospitalizations, additional clinical outcome measures were collected, including length of stay, intensive care unit (ICU) admission, receipt of invasive mechanical ventilation (IMV), and death during hospitalization or within 28 days from the admission date.
ED/UC encounters and hospitalizations were restricted to the period when influenza began circulating across sites (starting 16 October 2022) until no influenza cases were identified for 2 or more consecutive weeks at a study site and care setting or 31 March 2023, whichever came first. Exclusion criteria for ED/UC encounters and hospitalizations are detailed in Supplementary Figures 1 and 2, respectively.
Statistical Analysis
Demographic and clinical characteristics by case and vaccination status were described using counts and percentages and standardized mean difference (SMD) across groups (by case/control status or by vaccination status). An SMD ≥0.20 was considered a meaningful difference indicating imbalance of means or proportions between comparison groups. VE was calculated as (1 – adjusted odds ratio) × 100% using multivariable logistic regression by comparing the odds of influenza vaccination between case-patients and controls. Models were adjusted for prespecified confounders including age, study site, and calendar time, as well as any covariate with an SMD >0.20. Age and calendar time were modeled as natural cubic spline variables. Also, inverse-propensity-to-be-vaccinated weights (IPVWs) were estimated via generalized boosted regression trees and used in logistic regression models to account for additional imbalances between vaccinated and unvaccinated groups [8].
VE against influenza A–associated ED/UC encounters and hospitalizations were estimated by care setting, by age group (18–64, ≥65 years), presence of 1 or more likely immunocompromising conditions (any, none) [9], and presence of medical conditions by number of organ system categories (0–2 or ≥3 categories). Medical condition categories included pulmonary, cardiovascular, cerebrovascular, neurological or musculoskeletal, endocrine or metabolic, hematologic, renal, hepatic, and immune conditions (a list of ICD-10 codes is provided in Supplementary Table 2). Sensitivity analyses included restricting to influenza A–positive case-patients with influenza-associated ICD-10 discharge code (eg, influenza pneumonia) and restricting to the period from 16 October 2022 to 31 January 2023, when influenza circulation was observed across all study sites and healthcare settings.
As a secondary objective, to explore whether patient characteristics and in-hospital outcomes were different between vaccinated and unvaccinated influenza-positive cases, we compared proportions of patients with more severe clinical outcomes by influenza vaccination status stratified by age (18–64, ≥65 years) using counts and percentages and SMDs.
Analyses were conducted in SAS version 9.4 (SAS Institute) or R version 4.1.0 (R Foundation for Statistical Computing) software. The study was approved by institutional review boards at Westat and participating sites or under a reliance agreement with Westat and the Centers for Disease Control and Prevention.
RESULTS
Patient Characteristics
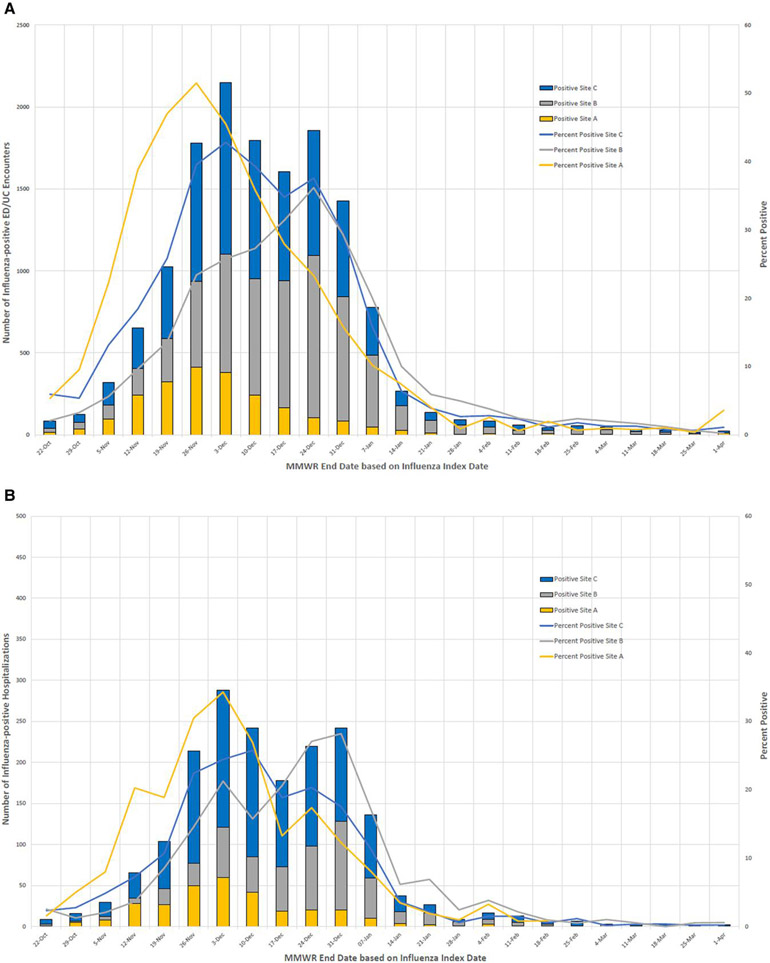
Overall, 85 389 ED/UC encounters and 19 751 ARI hospitalizations were included in analyses. Exclusions are shown in Supplementary Figures 1 and 2. Among ED/UC encounters that met inclusion criteria, 14 484 (17.0%) of patients tested positive for influenza A, with influenza cases peaking in November–December 2022 (Table 1 and Figure 1A). The median time between the ED/UC encounter date and most recent influenza test was 0 days (interquartile range [IQR], 0–0 days). ED/UC patients who tested positive for influenza were more likely to be 18–64 compared to ≥65 years of age (19.7% vs 11.9% positive, respectively; SMD, 0.275), and less likely to have a respiratory chronic condition or nonrespiratory chronic condition (all SMD >0.20); 37.8% of ED/UC patients were vaccinated for influenza (24.1% of 14 484 case-patients and 40.6% of 70 905 controls), with the overall percentage vaccinated increasing from 19.2% in October 2022 to 47.0% in March 2023 and ranging from 31.6% to 44.6% across sites (Table 1).
Table 1.
Characteristics of Acute Respiratory Illness–Associated Emergency Department/Urgent Care Encounters by Influenza Vaccination and Test Status, VISION Network
| Characteristic | Total, No. (Column %) | Influenza Vaccination Status | Influenza Test Result | ||||
|---|---|---|---|---|---|---|---|
| Unvaccinated, No. (Row %) |
Vaccinated, No. (Row %) |
SMDa | Negative, No. (Row %) |
Positive, No. (Row %) |
SMDa | ||
| All ED/UC events | 85 389 (100.0) | 53 137 (62.2) | 32 252 (37.8) | … | 70 905 (83.0) | 14 484 (17.0) | … |
| Influenza vaccination status | |||||||
| Unvaccinated | 53 137 (62.2) | 53 137 (100.0) | 0 (0.0) | … | 42 145 (79.3) | 10 992 (20.7) | 0.357 |
| Vaccinated | 32 252 (37.8) | 0 (0.0) | 32 252 (100.0) | … | 28 760 (89.2) | 3492 (10.8) | … |
| Month of encounter | |||||||
| October 2022 | 5488 (6.4) | 4436 (80.8) | 1052 (19.2) | 0.357 | 5214 (95.0) | 274 (5.0) | 1.129 |
| November 2022 | 19 094 (22.4) | 13 487 (70.6) | 5607 (29.4) | … | 13 992 (73.3) | 5102 (26.7) | … |
| December 2022 | 23 075 (27.0) | 14 687 (63.6) | 8388 (36.4) | … | 15 634 (67.8) | 7441 (32.2) | … |
| January 2023 | 14 336 (16.8) | 8007 (55.9) | 6329 (44.1) | … | 13 027 (90.9) | 1309 (9.1) | … |
| February 2023 | 11 559 (13.5) | 6244 (54.0) | 5315 (46.0) | … | 11 326 (98.0) | 233 (2.0) | … |
| March 2023 | 11 837 (13.9) | 6276 (53.0) | 5561 (47.0) | … | 11 712 (98.9) | 125 (1.1) | … |
| Site | |||||||
| Site A | 10 315 (12.1) | 6361 (61.7) | 3954 (38.3) | 0.261 | 8092 (78.4) | 2223 (21.6) | 0.148 |
| Site B | 39 687 (46.5) | 27 163 (68.4) | 12 524 (31.6) | … | 33 712 (84.9) | 5975 (15.1) | … |
| Site C | 35 387 (41.4) | 19 613 (55.4) | 15 774 (44.6) | … | 29 101 (82.2) | 6286 (17.8) | … |
| Age, y, median (IQR) | 53 (33–71) | 43 (29–62) | 68 (50–79) | … | 55 (35–72) | 43 (28–64) | … |
| Age group, y | |||||||
| 18–64 | 55 423 (64.9) | 41 406 (74.7) | 14 017 (25.3) | 0.754 | 44 506 (80.3) | 10 917 (19.7) | 0.275 |
| ≥65 | 29 966 (35.1) | 11 731 (39.1) | 18 235 (60.9) | … | 26 399 (88.1) | 3567 (11.9) | … |
| Sex | |||||||
| Female | 50 409 (59.0) | 31 129 (61.8) | 19 280 (38.2) | 0.024 | 41 896 (83.1) | 8513 (16.9) | 0.006 |
| Male | 34 980 (41.0) | 22 008 (62.9) | 12 972 (37.1) | … | 29 009 (82.9) | 5971 (17.1) | … |
| Race | |||||||
| White | 58 323 (68.3) | 35 215 (60.4) | 23 108 (39.6) | 0.203 | 49 286 (84.5) | 9037 (15.5) | 0.161 |
| Black | 6936 (8.1) | 5037 (72.6) | 1899 (27.4) | … | 5536 (79.8) | 1400 (20.2) | … |
| Otherb | 9761 (11.4) | 5592 (57.3) | 4169 (42.7) | … | 7980 (81.8) | 1781 (18.2) | … |
| Unknown | 10 369 (12.1) | 7293 (70.3) | 3076 (29.7) | … | 8103 (78.1) | 2266 (21.9) | … |
| Ethnicity | |||||||
| Hispanic | 15 376 (18.0) | 10 762 (70.0) | 4614 (30.0) | 0.158 | 12 209 (79.4) | 3167 (20.6) | 0.117 |
| Non-Hispanic | 70 013 (82.0) | 42 375 (60.5) | 27 638 (39.5) | … | 58 696 (83.8) | 11 317 (16.2) | … |
| Any medical condition | |||||||
| Yes | 28 482 (33.4) | 15 839 (55.6) | 12 643 (44.4) | 0.199 | 25 418 (89.2) | 3064 (10.8) | 0.330 |
| No | 56 907 (66.6) | 37 298 (65.5) | 19 609 (34.5) | … | 45 487 (79.9) | 11 420 (20.1) | … |
| Respiratory condition | |||||||
| Yes | 20 049 (23.5) | 11 141 (55.6) | 8908 (44.4) | 0.156 | 17 915 (89.4) | 2134 (10.6) | 0.266 |
| No | 65 340 (76.5) | 41 996 (64.3) | 23 344 (35.7) | … | 52 990 (81.1) | 12 350 (18.9) | … |
| Nonrespiratory condition | |||||||
| Yes | 16 762 (19.6) | 9220 (55.0) | 7542 (45.0) | 0.150 | 15 110 (90.1) | 1652 (9.9) | 0.270 |
| No | 68 627 (80.4) | 43 917 (64.0) | 24 710 (36.0) | … | 55 795 (81.3) | 12 832 (18.7) | … |
| Immunosuppressive condition | |||||||
| Yes | 2566 (3.0) | 1392 (54.2) | 1174 (45.8) | 0.059 | 2381 (92.8) | 185 (7.2) | 0.139 |
| No | 82 823 (97.0) | 51 745 (62.5) | 31 078 (37.5) | … | 68 524 (82.7) | 14 299 (17.3) | … |
Abbreviations: ED/UC, emergency department/urgent care; IQR, interquartile range; SMD, standardized mean difference.
An absolute SMD >0.20 indicates a nonnegligible difference in variable distributions between ED/UC encounters for vaccinated versus unvaccinated patients or for patients with positive influenza test results versus patients with negative influenza test results. For influenza vaccination status, SMD was calculated by comparing the vaccinated category versus unvaccinated.
Other race defined as any 1 of the following responses: Asian, Hawaiian or other Pacific Islander, American Indian or Alaska Native, other, multiple races.
Figure 1.
A, Influenza-positive emergency department/urgent care encounters and percent positivity by week; B, Influenza-positive hospitalizations and percent positivity by week. Abbreviations: EC/UC, emergency department/urgent care; MMWR, Morbidity and Mortality Weekly Report calendar week.
Among hospitalization episodes, 1874 (9.5%) patients tested positive for influenza A, with a similar peak in activity as seen in ED/UC settings (Table 2 and Figure 1B). The median time between the hospital admission date and most recent influenza test was 0 days (IQR, 0–1 days). Among 19 751 hospitalized patients, 52.8% were vaccinated for influenza (39.1% of 1874 case-patients and 54.3% of 17 877 controls), increasing from 29.8% in October 2022 to 62.3% in March 2023 and ranging from 40.5% to 58.6% across sites.
Table 2.
Characteristics of Acute Respiratory Illness–Associated Hospitalizations by Influenza Vaccination and Test Status, VISION Network
| Characteristic | Total, No. (Column %) | Influenza Vaccination Status | Influenza Test Result | ||||
|---|---|---|---|---|---|---|---|
| Unvaccinated, No. (Row %) |
Vaccinated, No. (Row %) |
SMDa | Negative, No. (Row %) |
Positive, No. (Row %) |
SMDa | ||
| All hospitalizations | 19 751 (100.0) | 9318 (47.2) | 10 433 (52.8) | … | 17 877 (90.5) | 1874 (9.5) | … |
| Influenza vaccination status | |||||||
| Unvaccinated | 9318 (47.2) | 9318 (100.0) | 0 (0.0) | … | 8177 (87.8) | 1141 (12.2) | 0.307 |
| Vaccinated | 10 433 (52.8) | 0 (0.0) | 10 433 (100.0) | … | 9700 (93.0) | 733 (7.0) | … |
| Month of encounter | |||||||
| October 2022 | 1165 (5.9) | 818 (70.2) | 347 (29.8) | 0.352 | 1131 (97.1) | 34 (2.9) | 1.168 |
| November 2022 | 3791 (19.2) | 2169 (57.2) | 1622 (42.8) | … | 3203 (84.5) | 588 (15.5) | … |
| December 2022 | 4654 (23.6) | 2215 (47.6) | 2439 (52.4) | … | 3667 (78.8) | 987 (21.2) | … |
| January 2023 | 3896 (19.7) | 1672 (42.9) | 2224 (57.1) | … | 3677 (94.4) | 219 (5.6) | … |
| February 2023 | 3169 (16.0) | 1283 (40.5) | 1886 (59.5) | … | 3135 (98.9) | 34 (1.1) | … |
| March 2023 | 3076 (15.6) | 1161 (37.7) | 1915 (62.3) | … | 3064 (99.6) | 12 (0.4) | … |
| Site | |||||||
| Site A | 2238 (11.3) | 1140 (50.9) | 1098 (49.1) | 0.318 | 1936 (86.5) | 302 (13.5) | 0.170 |
| Site B | 5098 (25.8) | 3034 (59.5) | 2064 (40.5) | … | 4592 (90.1) | 506 (9.9) | … |
| Site C | 12 415 (62.9) | 5144 (41.4) | 7271 (58.6) | … | 11 349 (91.4) | 1066 (8.6) | … |
| Age, y, median (IQR) | 74 (62–83) | 68 (55–79) | 77 (68–84) | … | 74 (62–83) | 72 (59–81) | … |
| Age group, y | |||||||
| 18–64 | 5794 (29.3) | 3877 (66.9) | 1917 (33.1) | 0.524 | 5133 (88.6) | 661 (11.4) | 0.141 |
| ≥65 | 13 957 (70.7) | 5441 (39.0) | 8516 (61.0) | … | 12 744 (91.3) | 1213 (8.7) | … |
| Sex | |||||||
| Female | 10 479 (53.1) | 4908 (46.8) | 5571 (53.2) | 0.015 | 9427 (90.0) | 1052 (10.0) | 0.068 |
| Male | 9272 (46.9) | 4410 (47.6) | 4862 (52.4) | … | 8450 (91.1) | 822 (8.9) | … |
| Race | |||||||
| White | 13 614 (68.9) | 6258 (46.0) | 7356 (54.0) | 0.190 | 12 344 (90.7) | 1270 (9.3) | 0.099 |
| Black | 1606 (8.1) | 976 (60.8) | 630 (39.2) | … | 1421 (88.5) | 185 (11.5) | … |
| Otherb | 2712 (13.7) | 1132 (41.7) | 1580 (58.3) | … | 2490 (91.8) | 222 (8.2) | … |
| Unknown | 1819 (9.2) | 952 (52.3) | 867 (47.7) | … | 1622 (89.2) | 197 (10.8) | … |
| Ethnicity | |||||||
| Hispanic | 2617 (13.2) | 1340 (51.2) | 1277 (48.8) | 0.063 | 2333 (89.1) | 284 (10.9) | 0.060 |
| Non-Hispanic | 17 134 (86.8) | 7978 (46.6) | 9156 (53.4) | … | 15 544 (90.7) | 1590 (9.3) | … |
| Any medical condition | |||||||
| Yes | 19 353 (98.0) | 9032 (46.7) | 10 321 (53.3) | 0.140 | 17 545 (90.7) | 1808 (9.3) | 0.103 |
| No | 398 (2.0) | 286 (71.9) | 112 (28.1) | … | 332 (83.4) | 66 (16.6) | … |
| Respiratory condition | … | … | … | … | … | … | … |
| Yes | 15 904 (80.5) | 7324 (46.1) | 8580 (53.9) | 0.092 | 14 566 (91.6) | 1338 (8.4) | 0.239 |
| No | 3847 (19.5) | 1994 (51.8) | 1853 (48.2) | … | 3311 (86.1) | 536 (13.9) | … |
| Nonrespiratory condition | |||||||
| Yes | 18 746 (94.9) | 8613 (45.9) | 10 133 (54.1) | 0.212 | 17 031 (90.9) | 1715 (9.1) | 0.151 |
| No | 1005 (5.1) | 705 (70.1) | 300 (29.9) | … | 846 (84.2) | 159 (15.8) | … |
| Immunosuppressive condition | |||||||
| Yes | 4728 (23.9) | 2025 (42.8) | 2703 (57.2) | 0.098 | 4414 (93.4) | 314 (6.6) | 0.197 |
| No | 15 023 (76.1) | 7293 (48.5) | 7730 (51.5) | … | 13 463 (89.6) | 1560 (10.4) | … |
| IMV | |||||||
| Yes | 1673 (8.5) | 919 (54.9) | 754 (45.1) | 0.094 | 1579 (94.4) | 94 (5.6) | 0.151 |
| No | 18 078 (91.5) | 8399 (46.5) | 9679 (53.5) | … | 16 298 (90.2) | 1780 (9.8) | … |
| ICU admission | |||||||
| Yes | 3504 (17.7) | 1663 (47.5) | 1841 (52.5) | 0.005 | 3276 (93.5) | 228 (6.5) | 0.172 |
| No | 16 247 (82.3) | 7655 (47.1) | 8592 (52.9) | … | 14 601 (89.9) | 1646 (10.1) | … |
| Death | |||||||
| Yes | 1172 (5.9) | 514 (43.9) | 658 (56.1) | 0.034 | 1114 (95.1) | 58 (4.9) | 0.149 |
| No | 18 579 (94.1) | 8804 (47.4) | 9775 (52.6) | … | 16 763 (90.2) | 1816 (9.8) | … |
Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range; SMD, standardized mean difference.
An absolute SMD >0.20 indicates a nonnegligible difference in variable distributions between hospitalizations for vaccinated versus unvaccinated patients or for patients with positive influenza test results versus patients with negative influenza test results.
Other race defined as any 1 of the following responses: Asian, Hawaiian or other Pacific Islander, American Indian or Alaska Native, other, multiple races.
In both ED/UC encounters and hospitalizations, vaccine product was documented for 32 065 (75.1%) vaccinated patients; 10 792 (33.7%) received egg-based standard-dose inactivated vaccine, 14 786 (46.1%) high-dose vaccine, 5894 (18.4%) adjuvanted vaccine, and 593 (1.1%) a different product (Supplementary Table 1). Of those with known product type, most adults aged 18–64 years (94.2%) received a standard-dose inactivated vaccine, whereas most adults aged ≥65 years (92.1%) per recommendations received high-dose inactivated, adjuvanted, or recombinant vaccine [5].
Vaccine Effectiveness
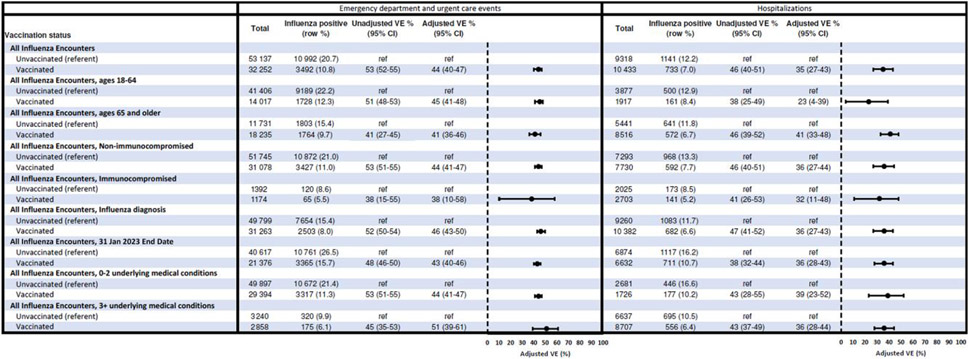
In the ED/UC setting, 20.7% of unvaccinated patients versus 10.8% of vaccinated patients tested positive for influenza A (Table 1 and Figure 2). VE against influenza A–associated ED/UC encounters was 44% (95% confidence interval [CI], 40%–47%) overall. VE was similar in adults aged 18–64 and ≥65 years (45% [95% CI, 41%–48%] and 41% [95% CI, 36%–46%], respectively) as well as those without and with likely immunocompromising conditions (44% [95% CI, 41%–47%] and 38% [95% CI, 10%–58%], respectively). Estimates were 44% (95% CI, 41%–47%) among patients with <3 underlying medical condition categories and 51% (95% CI, 39%–61%) among those with ≥3 categories.
Figure 2.
Vaccine effectiveness against influenza-associated emergency department/urgent care visits or hospitalizations. Abbreviations: CI, confidence interval; Ref, reference group; VE, vaccine effectiveness.
For ARI-associated hospitalizations, 12.2% of unvaccinated patients versus 7.0% of vaccinated patients tested positive for influenza (Table 2 and Figure 2). VE against influenza A–associated hospitalizations was 35% (95% CI, 27%–43%) overall. VE was 23% (95% CI, 4%–39%) in adults aged 18–64 years and 41% (95% CI, 33%–48%) in adults aged ≥65 years. Similar VE was observed among hospitalized adults without and with likely immunocompromising conditions. VE was 39% (95% CI, 23%–52%) and 36% (95% CI, 28%–44%) among adults with <3 and ≥3 categories of underlying medical condition, respectively.
In the first sensitivity analysis restricting case-patients to those with an influenza-related ICD-10 discharge code, 11 922 (72.9%) case-patients across settings had 1 or more influenza-related ICD-10 discharge codes. VE was 46% (95% CI, 43%–50%) against influenza A–associated ED/UC visits and 36% (95% CI, 27%–43%) against influenza A–associated hospitalizations; these estimates were similar (within 1%–2%) to the primary analyses (Figure 2). In the second sensitivity analysis using an earlier study end date of 31 January 2023, 29 641 (28%) patients were excluded who had encounter index dates during February–March 2023. VE was 43% (95% CI, 40%–46%) against influenza A–associated ED/UC visits and 36% (95% CI, 28%–43%) against influenza A–associated hospitalizations, similar to the primary analyses.
Baseline Characteristics and Outcomes of Vaccinated Versus Unvaccinated Hospitalized Influenza A Case-Patients
Of 1874 hospitalized influenza A cases, median age was 72 years; 733 (39.1%) had been vaccinated and 1141 (60.9%) were unvaccinated. Among 661 adults aged 18–64 years hospitalized with influenza A, 161 (24.4%) were vaccinated and 500 (75.6%) unvaccinated (Table 3). Within this age group, compared to unvaccinated, vaccinated case-patients were older and more likely to have underlying cardiovascular, endocrine or metabolic, renal, or immunocompromising conditions (all SMD >0.20). However, vaccinated case-patients were less likely to have an ICD-10 discharge code for influenza disease or influenza pneumonia (89.4% vs 95.6%; SMD, 0.24) or to receive IMV during hospitalization (2.5% vs 7.0%; SMD, 0.21) than unvaccinated case-patients. Other outcomes, including ICU admission and death, were similar between vaccinated and unvaccinated groups.
Table 3.
Baseline Characteristics and In-Hospital Outcomes Among Vaccinated Versus Unvaccinated Influenza-Positive Patients, by Age Group
| Characteristic | Age 18–64 y | Age ≥65 y | ||||
|---|---|---|---|---|---|---|
| Vaccinated, No. (Column %) |
Unvaccinated, No. (Column %) |
SMDa | Vaccinated, No. (Column %) |
Unvaccinated, No. (Column %) |
SMDa | |
| Total | 161 | 500 | … | 572 | 641 | … |
| Demographic characteristics | ||||||
| Age, y | ||||||
| 18–49 | 49 (30.4) | 243 (48.6) | 0.378 | … | … | … |
| 50–64 | 112 (69.6) | 257 (51.4) | … | … | … | … |
| 65–74 | … | … | … | 173 (30.2) | 252 (39.3) | 0.191 |
| 75–84 | … | … | … | 246 (43.0) | 241 (37.6) | … |
| ≥85 | … | … | … | 153 (26.7) | 148 (23.1) | … |
| Sex | ||||||
| Male | 52 (32.3) | 221 (44.2) | 0.247 | 262 (45.8) | 287 (44.8) | 0.021 |
| Female | 109 (67.7) | 279 (55.8) | … | 310 (54.2) | 354 (55.2) | … |
| Race/Ethnicity | ||||||
| Black, NH | 21 (13.0) | 76 (15.2) | 0.210 | 32 (5.6) | 56 (8.7) | 0.174 |
| Hispanic | 30 (18.6) | 96 (19.2) | 71 (12.4) | 87 (13.6) | … | |
| Other, NHb | 27 (16.8) | 50 (10.0) | … | 51 (8.9) | 69 (10.8) | … |
| White, NH | 82 (50.9) | 272 (54.4) | … | 416 (72.7) | 423 (66.0) | … |
| Unknown | 1 (0.6) | 6 (1.2) | … | 2 (0.3) | 6 (0.9) | … |
| Clinical characteristics | ||||||
| Influenza pneumonia or disease diagnosis | ||||||
| Yes | 144 (89.4) | 478 (95.6) | 0.236 | 538 (94.1) | 605 (94.4) | 0.014 |
| No | 17 (10.6) | 22 (4.4) | … | 34 (5.9) | 36 (5.6) | … |
| Respiratory condition | ||||||
| Yes | 112 (69.6) | 340 (68.0) | 0.034 | 424 (74.1) | 462 (72.1) | 0.046 |
| No | 49 (30.4) | 160 (32.0) | … | 148 (25.9) | 179 (27.9) | … |
| Cardiological condition | ||||||
| Yes | 95 (59.0) | 237 (47.4) | 0.234 | 481 (84.1) | 516 (80.5) | 0.094 |
| No | 66 (41.0) | 263 (52.6) | … | 91 (15.9) | 125 (19.5) | … |
| Cerebrovascular condition | ||||||
| Yes | 3 (1.9) | 19 (3.8) | 0.117 | 33 (5.8) | 31 (4.8) | 0.042 |
| No | 158 (98.1) | 481 (96.2) | … | 539 (94.2) | 610 (95.2) | … |
| Neurological and/or musculoskeletal condition | ||||||
| Yes | 37 (23.0) | 99 (19.8) | 0.078 | 215 (37.6) | 253 (39.5) | 0.039 |
| No | 124 (77.0) | 401 (80.2) | … | 357 (62.4) | 388 (60.5) | … |
| Hematologic condition | ||||||
| Yes | 14 (8.7) | 28 (5.6) | 0.120 | 41 (7.2) | 42 (6.6) | 0.024 |
| No | 147 (91.3) | 472 (94.4) | … | 531 (92.8) | 599 (93.4) | … |
| Endocrine condition | ||||||
| Yes | 109 (67.7) | 268 (53.6) | 0.292 | 455 (79.5) | 479 (74.7) | 0.115 |
| No | 52 (32.3) | 232 (46.4) | … | 117 (20.5) | 162 (25.3) | … |
| Renal condition | ||||||
| Yes | 38 (23.6) | 68 (13.6) | 0.259 | 240 (42.0) | 242 (37.8) | 0.086 |
| No | 123 (76.4) | 432 (86.4) | … | 332 (58.0) | 399 (62.2) | … |
| Gastrointestinal and hepatic condition | ||||||
| Yes | 10 (6.2) | 28 (5.6) | 0.026 | 36 (6.3) | 33 (5.1) | 0.049 |
| No | 151 (93.8) | 472 (94.4) | … | 536 (93.7) | 608 (94.9) | … |
| Any immunocompromising condition | ||||||
| Yes | 39 (24.2) | 65 (13.0) | 0.291 | 102 (17.8) | 108 (16.8) | 0.026 |
| No | 122 (75.8) | 435 (87.0) | … | 470 (82.2) | 533 (83.2) | … |
| In-hospital treatment and outcomes | ||||||
| Any influenza antiviral therapy received | ||||||
| Yes | 146 (90.7) | 441 (88.2) | 0.081 | 527 (92.1) | 592 (92.4) | 0.008 |
| No | 15 (9.3) | 59 (11.8) | … | 45 (7.9) | 49 (7.6) | … |
| ICU admission during encounter | ||||||
| Yes | 21 (13.0) | 77 (15.4) | 0.068 | 67 (11.7) | 63 (9.8) | 0.061 |
| No | 140 (87.0) | 423 (84.6) | … | 505 (88.3) | 578 (90.2) | … |
| IMV | ||||||
| Yes | 4 (2.5) | 35 (7.0) | 0.214 | 25 (4.4) | 30 (4.7) | 0.015 |
| No | 157 (97.5) | 465 (93.0) | … | 547 (95.6) | 611 (95.3) | … |
| Death | ||||||
| Yes | 6 (3.7) | 12 (2.4) | 0.077 | 23 (4.0) | 17 (2.7) | 0.076 |
| No | 155 (96.3) | 488 (97.6) | … | 549 (96.0) | 624 (97.3) | … |
| Length of stay ≥5 d | ||||||
| Yes | 43 (26.7) | 119 (23.8) | 0.067 | 161 (28.1) | 174 (27.1) | 0.022 |
| No | 118 (73.3) | 381 (76.2) | … | 411 (71.9) | 467 (72.9) | … |
Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; NH, non-Hispanic; SMD, standardized mean difference.
An absolute SMD >0.20 indicates a nonnegligible difference in variable distributions between hospitalizations for vaccinated versus unvaccinated patients.
Other race defined as any 1 of the following responses: Asian, Hawaiian or other Pacific Islander, American Indian or Alaska Native, other, multiple races.
Among 1213 adults aged ≥65 years hospitalized with influenza A, 572 (47.2%) were vaccinated and 641 (52.8%) were unvaccinated. Baseline demographic characteristics and underlying medical conditions were similar across vaccinated and unvaccinated groups within this age strata (SMD, <0.20). The percentage experiencing severe in-hospital clinical outcomes including ICU admission, receipt of IMV, or death, was similar across vaccination groups.
DISCUSSION
During the 2022–2023 US influenza season, we observed moderate VE against influenza A–associated ED/UC encounters and hospitalizations, ranging from 35% to 44% across care settings. Similar VE was also observed among groups at higher risk for severe complications from influenza, including older adults, those with immunocompromising conditions, and those with multiple underlying chronic medical conditions. These estimates were higher than those from the VISION Network during the 2021–2022 season (25% for both ED/UC encounters and hospitalizations [2]) when most infections were caused by viruses from an A(H3N2) subclade that differed antigenically from the vaccine component. The Northern Hemisphere vaccine was updated with a new A(H3N2) component that was antigenically similar to circulating A(H3N2) viruses during the 2022–2023 season [10, 11]. This may have contributed to higher VE during the 2022–2023 season, although co-circulation of A(H1N1)pdm09 viruses may have also impacted VE.
Findings from this analysis were consistent with other VE estimates from North America and Europe for the 2022–2023 influenza season. A test-negative design study from the US state of Wisconsin found VE against influenza A–associated outpatient illness of 54% (95% CI, 23%–73%) among persons aged 6 months to 64 years, similar to estimates we observed among adults aged 18–64 years in ED/UC settings (45%) [12]. Another study based in the US of hospitalized adults ≥18 years of age found VE against influenza A–associated hospitalizations of 37% (compared to 35% in our study), with VE against A(H3N2) and A(H1N1)pdm09 subtype viruses of 29% and 47%, respectively [13]. Using data from community-based sentinel surveillance practitioners, a study in Canada reported influenza VE against medically attended A(H3N2)-associated illness [14]. Influenza A(H3N2) VE estimates of 58% (95% CI, 33%–73%) for adults 20–64 years of age and 59% (95% CI, 15%–80%) for adults ≥65 years of age were higher than influenza A VE estimates we observed within ED/UC settings but with overlapping CIs. Studies in multiple European countries reported interim 2022–2023 influenza VE findings in primary care, ED, and hospital settings [15]. Across all ages and care settings, influenza A VE ranged from 27% to 44%. Primary care or ED setting VE estimates ranged from 29% to 44%, with estimates for VE against influenza A–associated hospitalization ranging from 27% to 33%.
Influenza VE can be interpreted as the incremental benefit from vaccination in the background of complex immune histories from prior influenza infection and vaccination. During the 2010–2011 through 2019–2020 influenza seasons, an estimated 9.3–41.0 million symptomatic influenza illnesses occurred annually in the US [16], suggesting that a large proportion of people are infected over multiple seasons of exposure. If infections differentially occur among unvaccinated individuals, these people may have stronger infection-induced immunity over time that impacts future VE [17]. Preexisting immunity from natural infection or influenza vaccination, coupled with timing of these immunizing exposures, may further impact the quality of immune responses and associated VE [18, 19]. Even in seasons with moderate VE like the 2022–2023 season, however, vaccination is likely to have a substantial public health impact by preventing millions of medical visits, tens of thousands of hospitalizations, and thousands of deaths [20-22]. This reduction in medically attended illness and associated morbidity and mortality is especially critical to reduce burden on healthcare resources should influenza, SARS-CoV-2, RSV, and other respiratory viruses continue to co-circulate in high numbers. Although we did not evaluate incidence of medically attended influenza in vaccinated and unvaccinated individuals, in the setting of high influenza activity, it is likely that only a modest number of individuals would need to receive influenza vaccination to prevent a medically attended influenza-related encounter [20].
Influenza vaccination does not produce long-lasting immunity to prevent infection, but there may be additional benefits related to immune responses that attenuate illness severity following infection [23]. Studies of COVID-19 have demonstrated greater vaccine protection against severe illness including hospitalization and ICU admission or death compared to mild-to-moderate illness, as well as lower severity of hospitalized COVID-19 among vaccinated patients [24-26]. However, influenza VE studies over the past decade have generally found similar VE against mild-to-moderate outpatient illness and influenza-associated hospitalization [27, 28]. These studies have frequently compared estimates across different VE networks or healthcare systems with differing enrollment criteria or ability to control for confounding, which limits interpretation of findings. In our study, we also found similar VE within the same health systems across ambulatory and inpatient settings. This lack of gradient in VE may be related to increased hospital-based testing, including multipathogen testing that recognizes any symptomatic influenza illness regardless of severity. In addition, ambulatory and hospitalized patients with ARI differed in important demographic and clinical characteristics, complicating direct comparison. However, among patients hospitalized with influenza, the proportion experiencing critical outcomes (ICU admission, IMV, and death) was generally similar between vaccine breakthrough and unvaccinated case-patients, especially in older adults. Future studies and population-based cohorts are warranted to improve understanding of the overall impact of vaccination.
This study was subject to several limitations. Due to a lack of subtype data for most influenza cases, we were unable to generate influenza A(H3N2)- and A(H1N1)pdm09-specific VE estimates. VE against all influenza A viruses may be impacted by the relative proportion of cases by subtype. Second, VE by age group cannot be directly compared as most young adults received standard-dose inactivated vaccines and most older adults received enhanced products such as high-dose inactivated or adjuvanted vaccines. Despite most vaccinated older adults receiving enhanced vaccine products, VE was similar compared to younger adults who mostly received standard-dose inactivated vaccines. Third, encounters and hospitalizations in 3 healthcare systems are not representative of all encounters among US adults. Fourth, unmeasured or residual confounding is possible after application of IPVWs and adjustment for measured confounders. For example, we did not capture preventive behaviors such as masking. Fifth, we did not account for influenza vaccination during prior seasons or prior infection history. Finally, VE estimates rely on clinical influenza testing rather than systematic testing in patients with ARI, and bias may be introduced if patients who receive influenza clinical testing differ from those who do not. This was likely to be minimal in this study, however, as >80% of patients with ARI encounters received influenza testing.
In summary, during the 2022–2023 US influenza season, we observed moderate VE against influenza-associated ED/UC encounters and hospitalizations. With high levels of circulation of influenza and other respiratory viruses, influenza vaccination is a critical tool to reduce serious illness and minimize strain on healthcare resources as well as individual and societal impacts of influenza.
Supplementary Material
Acknowledgments.
We would like to acknowledge the contributions of the VISION Network: Westat (Akintunde Akinseye, MSPH, MPH; Elizabeth Bassett, BA; Jewel Bernard-Hunte, MPH; Bria Berry, MPH; Rebecca Birch, MPH; Kevin Cheng, BS; Sumanthi Croos, BA; Jonathan Davis, PhD; Rebecca Fink, MPH; Carly Hallowell, MPH; Nina Hamburg, MBA; Jean Keller, MS; Salome Kiduko, MPH; Magdalene Kish, BS; Victoria Lazariu, PhD; Yong Lee, BSEE; Yessie Martinez, MPH; Vanessa Masick, MS; Thomas Mienk, MPA; Patrick Mitchell, ScD; Jean Opsomer, PhD; Weijia Ren, PhD; John Riddles, MS; Sarah Reese, PhD; Anna Rukhlya, MA; Kristin Schrader, MA; Patricia Shifflett, MS; Talia Spark, PhD; Brenda Sun, MS; Hansong Wang, PhD; Donald Warden, MPH; Steph Wraith, PhD; Yan Zhuang, PhD); HealthPartners Institute (Sunita Thapa, MPH; Linda Fletcher, MPH; Sheryl Kane, MPH); Intermountain Healthcare (Bert Lopansri, MD); Kaiser Permanente Center for Health Research (Padma Dandamudi, MPH; Brad Crane, MS); Baylor Scott & White Health (Deepika Konatham, BS; I-Chia Liao, MPH; Kempapura Murthy, MBBS, MPH; Deborah Hendricks, BS, Jason Ettlinger, MA; Joel Blais, BTh; Elisa Priest, DrPH; Michael Smith, BS; Spencer Rose, BS; Natalie Settele, PA; Jennifer Thomas, MS; Muralidhar Jatla, MD; Madhava Beeram, MD; Javed Butler, MD; Alejandro Arroliga, MD); University of Colorado (Health Data Compass: David Mayer, BS; Bryant Doyle; Briana Kille, PhD; Catia Chavez, MPH); Regenstrief Institute (Ashley Wiensch, MPH, Amy Hancock, MPA); Vanderbilt University Medical Center (Allison B. McCoy, PhD; Donald Sengstack, MS; Coda L. Davison, MPA).
Financial support.
This study was supported by the Centers for Disease Control and Prevention (contract numbers 75D30120C07986 to Westat and 75D30120C07765 to Kaiser Foundation Hospitals).
Footnotes
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Disclaimer. The findings and conclusions of this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention (CDC). Preliminary vaccine effectiveness estimates were presented at a CDC Advisory Committee on Immunization Practices meeting on 22 February 2023.
Potential conflicts of interest. During the conduct of the study, all Westat- and Kaiser Permanente Northern California Division of Research–affiliated authors reported receiving contractual support from the CDC via payments made to their respective institutions. Additionally, all authors affiliated with Baylor Scott & White Health, Children’s Minnesota, Columbia University Irving Medical Center, HealthPartners Institute, Intermountain Healthcare, Kaiser Permanente Center for Health Research, Regenstrief Institute, University of Colorado Anschutz Medical Campus, and Vanderbilt University Medical Center reported receiving contractual support from the CDC during the conduct of the study, via subcontracts from Westat, Inc, with payments made to their respective institutions. Unrelated to the submitted work, the following disclosures were reported from the past 36 months: A. L. N. received grants from Pfizer and Vir Biotechnology; M. N. received grants directly from CDC and from CDC via subcontracts from Abt Associates and Vanderbilt University Medical Center to her institution; C. E. M. received grants AstraZeneca; S. R. received grants from GSK; and N. P. K. received research support from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, and Seqirus. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Delahoy MJ, Mortenson L, Bauman L, et al. Influenza A(H3N2) outbreak on a university campus—Michigan, October–November 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1712–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenforde MW, Weber ZA, DeSilva MB, et al. Vaccine effectiveness against influenza-associated urgent care, emergency department, and hospital encounters during the 2021–2022 season, VISION Network. J Infect Dis 2023; 228:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Health Alert Network (HAN). Increased respiratory virus activity, especially among children, early in the 2022–2023 fall and winter. 2022. https://emergency.cdc.gov/han/2022/han00479.asp. Accessed 8 November 2022.
- 4.Centers for Disease Control and Prevention. Influenza activity in the United States during the 2022–23 season and composition of the 2023–24 influenza vaccine. 2023. https://www.cdc.gov/flu/spotlights/2023-2024/22-23-summary-technical-report.htm. Accessed 30 October 2023.
- 5.Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 influenza season. MMWR Recomm Rep 2022; 71:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll MK, Pettigrew SM, Ma J, Verma A. Effects of confounding bias in coronavirus disease 2019 (COVID-19) and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis 2022; 75:e564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenforde MW, Patel MM, Lewis NM, et al. Vaccine effectiveness against influenza A(H3N2)–associated hospitalized illness: United States, 2022. Clin Infect Dis 2023; 76:1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 2004; 9:403–25. [DOI] [PubMed] [Google Scholar]
- 9.Britton A, Embi PJ, Levy ME, et al. Effectiveness of COVID-19 mRNA vaccines against COVID-19-associated hospitalizations among immunocompromised adults during SARS-CoV-2 Omicron predominance—VISION Network, 10 states, December 2021–August 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2022–2023 Northern Hemisphere influenza season. 2022. https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2022-2023/202202_recommendation.pdf. Accessed 11 June 2023.
- 11.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2023–2024 Northern Hemisphere influenza season. 2023. https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2023-2024/202302_seasonal_recommendation_a.pdf?sfvrsn=42612ae5_3&download=true. Accessed 11 June 2023.
- 12.Mclean H, Petrie J, Hanson K, et al. Interim estimates of 2022–23 seasonal influenza vaccine effectiveness—Wisconsin, October 2022–February 2023. MMWR Morb Mortal Wkly Rep 2023; 72:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis NM, Zhu Y, Peltan ID, et al. Vaccine effectiveness against influenza A–associated hospitalization, organ failure, and death: United States, 2022–2023. Clin Infect Dis 2024; 78:1056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skowronski DM, Chuang ES, Sabaiduc S, et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Euro Surveill 2023; 28:2300043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissling E, Maurel M, Emborg H-D, et al. Interim 2022/23 influenza vaccine effectiveness: six European studies, October 2022 to January 2023. Euro Surveill 2023; 28:2300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Disease burden of flu. 2022. https://www.cdc.gov/flu/about/burden/index.html. Accessed 3 October 2022.
- 17.Thompson MG, Cowling BJ. How repeated influenza vaccination effects might apply to COVID-19 vaccines. Lancet Respir Med 2022; 10:636–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugan HL, Guthmiller JJ, Arevalo P, et al. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci Transl Med 2020; 12:eabd3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skowronski DM, Chambers C, De Serres G, et al. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infect Dis 2017; 215:1059–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung JR, Rolfes MA, Flannery B, et al. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis 2020; 71:e368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolfes MA, Flannery B, Chung J, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, and hospitalizations averted by vaccination. 2023. https://www.cdc.gov/flu/vaccines-work/burden-averted.htm. Accessed 16 June 2023.
- 23.Patel MM, York IA, Monto AS, Thompson MG, Fry AM. Immune-mediated attenuation of influenza illness after infection: opportunities and challenges. Lancet Microbe 2021; 2: e715–25. [DOI] [PubMed] [Google Scholar]
- 24.Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021; 326:2043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSilva MB, Mitchell PK, Klein NP, et al. Protection of two and three mRNA vaccine doses against severe outcomes among adults hospitalized with COVID-19—VISION Network, August 2021 to March 2022. J Infect Dis 2023; 227:961–9. [DOI] [PubMed] [Google Scholar]
- 26.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 2021; 385:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenforde MW, Chung J, Smith ER, et al. Influenza vaccine effectiveness in inpatient and outpatient settings in the United States, 2015–2018. Clin Infect Dis 2021; 73:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng S, Cowling BJ, Sullivan SG. Influenza vaccine effectiveness by test-negative design—comparison of inpatient and outpatient settings. Vaccine 2016; 34:1672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.