Abstract
Poliovirus has a single-stranded RNA genome of positive polarity that serves two essential functions at the start of the viral replication cycle in infected cells. First, it is translated to synthesize viral proteins and, second, it is copied by the viral polymerase to synthesize negative-strand RNA. We investigated these two reactions by using HeLa S10 in vitro translation-RNA replication reactions. Preinitiation RNA replication complexes were isolated from these reactions and then used to measure the sequential synthesis of negative- and positive-strand RNAs in the presence of different protein synthesis inhibitors. Puromycin was found to stimulate RNA replication overall. In contrast, RNA replication was inhibited by diphtheria toxin, cycloheximide, anisomycin, and ricin A chain. Dose-response experiments showed that precisely the same concentration of a specific drug was required to inhibit protein synthesis and to either stimulate or inhibit RNA replication. This suggested that the ability of these drugs to affect RNA replication was linked to their ability to alter the normal clearance of translating ribosomes from the input viral RNA. Consistent with this idea was the finding that the protein synthesis inhibitors had no measurable effect on positive-strand synthesis in normal RNA replication complexes. In marked contrast, negative-strand synthesis was stimulated by puromycin and was inhibited by cycloheximide. Puromycin causes polypeptide chain termination and induces the dissociation of polyribosomes from mRNA. Cycloheximide and other inhibitors of polypeptide chain elongation “freeze” ribosomes on mRNA and prevent the normal clearance of ribosomes from viral RNA templates. Therefore, it appears that the poliovirus polymerase was not able to dislodge translating ribosomes from viral RNA templates and mediate the switch from translation to negative-strand synthesis. Instead, the initiation of negative-strand synthesis appears to be coordinately regulated with the natural clearance of translating ribosomes to avoid the dilemma of ribosome-polymerase collisions.
Poliovirion RNA is released into the cytoplasm of infected cells and is translated by polyribosomes associated with the rough endoplasmic reticulum (10, 32). Viral proteins are synthesized after ribosomes bind in the 5′ nontranslated region at the internal ribosome entry site (IRES) and initiate translation of the single long open reading frame in poliovirion RNA (vRNA). After multiple copies of the viral polyprotein are synthesized, the input virion RNA must switch roles from serving as an mRNA and become a template for negative-strand RNA synthesis (3). The molecular mechanisms involved in switching from translation to RNA replication are unknown but are essential in regulating the overall replication cycle of poliovirus and other positive-strand RNA viruses.
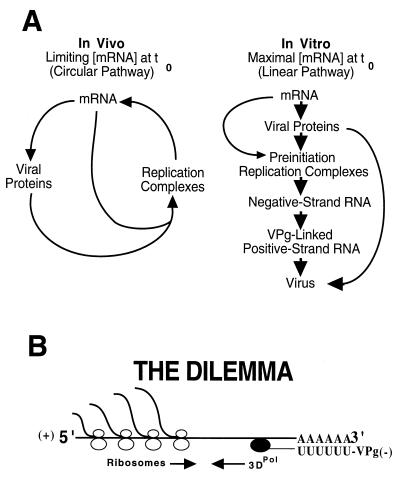
Cell culture systems capable of supporting poliovirus replication allow for the analysis of poliovirus RNA replication in one-step growth experiments (15, 18). Populations of cells can be synchronously infected, and these studies have resulted in our current understanding of the defined steps in the viral replication cycle (i.e., attachment → penetration → uncoating → translation → RNA replication → assembly → release) (31). These steps are temporally sequential in the order indicated. During the course of the replication cycle, however, a significant overlap develops between the translation and RNA replication steps because of the interdependence of viral protein synthesis and RNA replication (Fig. 1A, in vivo). Therefore, even in synchronously infected cells, amplification of the infecting viral RNA molecule occurs in an asynchronous circular pathway in which viral RNA translation and RNA replication occur simultaneously (Fig. 1A, in vivo). The interdependent, simultaneous translation and replication of viral RNA within this circular pathway precludes the direct experimental analysis of the regulatory mechanisms controlling the switch from translation to RNA replication.
FIG. 1.
Pathways of poliovirus replication and the dilemma of ribosome-polymerase collisions. (A) The replication of poliovirus RNA in infected cells and in cell-free replication reactions in vitro is fundamentally different. The initial concentration of viral RNA in the cytoplasm of infected cells is very low. In the infected cell, protein synthesis and RNA replication are codependent since protein synthesis requires RNA replication and vice versa. During the course of the infection, this results in the amplification of the input viral RNA and leads to a circular replication pathway. In contrast, a relatively high concentration of input virion RNA is used in the in vitro reactions to achieve maximum rates of protein synthesis. In this case, amplification of the input RNA is not required to achieve maximum rates of protein synthesis and RNA replication in vitro, which leads to a linear replication pathway. (B) Poliovirus RNA is translated before it replicates (30). Translating ribosomes move 5′ to 3′ on the viral mRNA, while the poliovirus polymerase must initiate negative-strand RNA synthesis at the 3′ terminus of the viral RNA and move in a 3′-to-5′ direction. The results of this study indicate that the poliovirus polymerase is not able to dislodge translating ribosomes from viral mRNA. This suggests that the virus has evolved a different mechanism to regulate the switch from translation to RNA replication to avoid this dilemma which would impede the efficient replication of the viral genome.
Poliovirus replication can now be studied in cell extracts of uninfected HeLa cells programmed with poliovirion RNA (4, 5, 29). The HeLa S10 extracts used in these experiments support translation, RNA replication, and the assembly of infectious virus in a temporally regulated fashion (Fig. 1A, in vitro). In the cell-free reactions, the translation and replication steps follow a linear pathway, since relatively high concentrations of virion RNA are added at the start of the reactions. Viral protein synthesis is driven by the input RNA and is not dependent to any measurable extent on newly synthesized progeny RNA. Cell-free replication reactions are programmed with optimal concentrations of viral RNA, and viral protein synthesis starts at maximum rates from time zero (5). By using guanidine HCl, a reversible inhibitor of poliovirus RNA replication, we are able to isolate preinitiation RNA replication complexes. Guanidine blocks the initiation of negative-strand RNA synthesis but has no effect on poliovirus protein synthesis or polypeptide processing (4). Negative-strand RNA synthesis initiates in a highly synchronous manner upon the removal of guanidine and is then followed by the synthesis of infectious positive-strand RNA (6). As expected, RNA replication in these reactions is highly asymmetric, with positive-strand RNA being made in large excess over negative-strand RNA (6). In this report we exploit the linear replication pathway of poliovirus in cell-free reactions to examine the transition between translation of viral mRNA and the synthesis of viral negative-strand RNA.
Previously, we demonstrated that ongoing viral protein synthesis is not required for RNA replication in reactions containing preinitiation RNA replication complexes resuspended in fresh HeLa S10 extracts (4). In these experiments, we added puromycin to block the synthesis of new viral proteins. Puromycin is an amino acyl tRNA analogue that causes polypeptide chain termination and the release of translating ribosomes from mRNA (8, 26). Surprisingly, puromycin was found to increase the amount of viral RNA synthesized in preinitiation RNA replication complexes severalfold (4). To investigate this observation further, we determined what effect other protein synthesis inhibitors had on viral RNA replication in preinitiation replication complexes. Cycloheximide (26), anisomycin (21), diphtheria toxin (23, 26), and the ricin A chain (20, 26) inhibit protein synthesis via different mechanisms. In contrast to puromycin, however, all of these drugs inhibit polypeptide chain elongation and consequently lead to polyribosome stabilization (20, 21). Therefore, these drugs essentially freeze the ribosomes on the mRNA they are translating.
In this study, we show that protein synthesis inhibitors that stabilized polyribosomes inhibited viral RNA replication. These results contrast with the stimulation in RNA replication that was observed with puromycin. Using assays specific for positive- and negative-strand synthesis, we showed that all of the inhibitors tested only affected negative-strand synthesis. These results suggest that the presence of translating ribosomes on the input viral RNA inhibit RNA replication by blocking negative-strand synthesis. Therefore, negative-strand RNA synthesis must be coordinately regulated with the clearance of translating ribosomes from the input RNA to avoid the dilemma of polymerase-ribosome collisions (Fig. 1B).
MATERIALS AND METHODS
Cells and virus.
Suspension cultures of HeLa S3 cells were maintained at 37°C by daily passage at 2 × 105 to 4 × 105 cells per ml in Joklik’s modified Eagle medium (Life Technologies, Gaithersburg, Md.) supplemented with 5% calf serum and 2% fetal calf serum. Cells were infected with poliovirus type 1 (Mahoney strain) as described earlier (38).
RNA preparation.
Poliovirion RNA was isolated and purified from CsCl banded virus by phenol-chloroform-isoamyl alcohol (25:24:1) extraction and ethanol precipitation as described earlier (7). Where indicated, RNA transcripts of an infectious poliovirus cDNA clone, pT7-PV1(A)80, were used in place of poliovirion RNA. The clone, pT7-PV1(A)80, was derived from an infectious cDNA clone of poliovirus type 1, pT7D-polio (33). We modified pT7D-polio to lengthen the 3′-terminal poly(A) sequence from 12 to 80 nucleotides in length. RNA transcripts of MluI-linearized pT7-PV1(A)80 DNA are predicted to contain two additional GMP nucleotides at the 5′ termini, the poliovirus genomic sequence and poly(A)80CGCG at the 3′ termini. The presence of the 3′-terminal CGCG nonviral sequence is derived from the MluI restriction site in the original plasmid DNA. RNA2(A)80 is a transcript of a subgenomic clone of pT7-PV1(A)80, which contains a single in-frame deletion of 1,782 nucleotides in the capsid coding region of the viral genome (see Fig. 6A). Preparations of T7-PV1(A)80 RNA and RNA2(A)80 were transcribed with bacteriophage T7 RNA polymerase, extracted with phenol-chloroform-isoamyl alcohol, ethanol precipitated, and purified by chromatography on a Sephedex G-50 gel filtration column (7). The concentrations of the final preparations of virion RNA and the transcript RNAs were determined by measuring the A260.
FIG. 6.
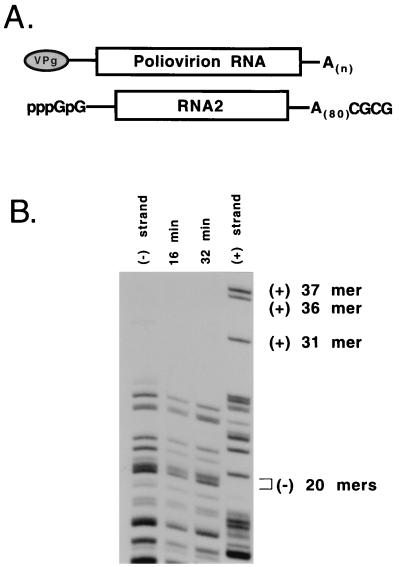
Two 5′-terminal GMP nucleotides in transcript RNA block positive-strand synthesis. (A) RNA transcripts of poliovirus cDNA clones used in this study contained two nonviral 5′-terminal GMP nucleotides that are required for efficient in vitro transcription by bacteriophage T7 RNA polymerase. The 3′-terminal CGCG sequence is derived from the MluI restriction site in the original plasmid DNA and has no significant inhibitory effect on negative-strand synthesis. RNA2(A)80 is a poliovirus subgenomic transcript RNA that contains a single in-frame deletion of 1,782 nucleotides in the capsid coding region. (B) Preinitiation RNA replication complexes were isolated from HeLa S10 translation-RNA replication reactions containing RNA2(A)80. The RNA2(A)80-preinitiation replication complexes were resuspended in reaction mixtures containing [α-32P]CTP and were incubated at 37°C for 16 or 32 min (method 4 in Materials and Methods). Labeled RNAs isolated from the 16 min (lane 2) and 32 min (lane 3) reactions were digested with RNase T1 and then characterized by using a one-dimensional gel electrophoresis procedure as described earlier (6). RNase T1 digests of [32P]CMP-labeled negative-strand (lane 1) and positive-strand (lane 4) transcripts were used as markers for the poliovirus strand-specific oligonucleotides.
HeLa S10 translation-RNA replication reaction.
HeLa S10 extracts and translation initiation factors were prepared as previously described (5, 7). HeLa S10 translation-RNA replication reactions (25 to 50 μl) contained 50% (by volume) HeLa S10 extract, 20% (by volume) HeLa cell translation initiation factors, 1 mM ATP, 0.25 mM GTP, 0.25 mM CTP, 0.25 mM UTP, 120 mM KCH3CO2, 2.75 mM Mg(CH3CO2)2, 3 mM dithiothreitol (DTT), 35 mM HEPES (pH 7.4), 25 mM creatine phosphate (Boehringer Mannheim, Indianapolis, Ind.), 400 μg of creatine phosphokinase (Boehringer Mannheim) per ml, and either 25 μg of poliovirion RNA or 50 μg of T7-PV1(A)80 RNA per ml as indicated. All reactions were incubated at 30°C for the times indicated.
Protein synthesis.
HeLa S10 translation-RNA replication reactions containing poliovirion RNA and 1.2 mCi of [35S]methionine (1,200 Ci/mmol; Amersham) per ml were incubated for the indicated times. Puromycin, cycloheximide, anisomycin, diphtheria toxin, and the ricin A chain (Sigma Chemical Co., St. Louis, Mo.) were added to the reactions as indicated. Diphtheria toxin (Sigma) was activated by trypsin treatment as previously described (16). Samples (1 μl) were removed from these reactions at the indicated times and were added to 100 μl of 0.1 M KOH–3% Casamino Acids. Labeled proteins were precipitated by adding 2 ml of 5% trichloroacetic acid to each sample. After being incubated for 10 min on ice, the samples were filtered, and the radioactivity associated with the labeled proteins was quantitated by liquid scintillation counting.
Isolation of poliovirus RNA replication complexes.
Preinitiation RNA replication complexes were isolated as previously described (4, 6). Briefly, HeLa S10 translation-RNA replication reactions containing 2 mM guanidine HCl and either poliovirion RNA or T7-PV1(A)80 RNA were incubated at 30°C for 5 h. The reactions were then centrifuged at 15,000 × g for 15 min at 4°C, and the supernatant (S15) containing soluble proteins and guanidine HCl was removed from the pellet (P15) that contained the preinitiation RNA replication complexes. Normal preformed RNA replication complexes containing replicative intermediate RNA were isolated by using the same procedure except that guanidine HCl was not added to the HeLa S10 translation-RNA replication reaction and only poliovirion RNA was used as the input RNA.
RNA replication assays.
RNA was measured by using procedures designated methods 3 to 6 to be consistent with previous publications from our laboratory.
(i) Method 3.
Viral RNA synthesis was assayed by using preinitiation RNA replication complexes formed in HeLa S10 translation-RNA replication reactions containing poliovirion RNA. The complexes were isolated as described above and were resuspended in 50-μl reaction mixtures containing HeLa S10 extract, translation initiation factors, and the indicated amounts of [α-32P]CTP as the labeled substrate (method 3 in references 4 and 6). Labeled RNA synthesis was assayed at 30°C for 90 min. This assay measures both the initiation and elongation of negative strands and the subsequent initiation and elongation of positive strands.
(ii) Method 4.
RNA replication was assayed by labeling viral RNA synthesized in preinitiation RNA replication complexes resuspended in reactions that did not contain added HeLa S10 extract or HeLa translation initiation factors. The pellet (P15) that contained the preinitiation RNA replication complexes was resuspended in 50-μl reactions containing 25 μl of S10 buffer [40 mM HEPES-KOH (pH 7.4), 120 mM KCH3CO2, 5.5 mM Mg(CH3CO2)2, 6 mM DTT, 10 mM KCl, 1 mM CaCl2, and 2 mM EGTA], 5 μl of 10× nucleotide reaction mix lacking CTP, and [α-32P]CTP at a final concentration of 5 μM (method 4 in reference 6). This method supports the synchronous and sequential synthesis of negative- and positive-strand RNA on poliovirion RNA templates as previously described (6). At 5 μM CTP, the elongation rate of RNA synthesis is approximately 500 nucleotides per min at 37°C.
(iii) Method 5.
Viral RNA synthesis was assayed by using normal preformed RNA replication complexes formed in HeLa S10 translation-RNA replication reactions containing poliovirion RNA. The complexes were isolated as described above and were resuspended in reaction mixtures identical to those used in method 3 except that 2 mM guanidine HCl was added to block negative-strand initiation. We have previously shown that guanidine HCl specifically blocks negative-strand initiation but has no effect on the initiation or elongation of positive strands (6). Therefore, this assay specifically measures the initiation and elongation of positive strands in preformed RNA replication complexes.
(iv) Method 6.
Viral RNA synthesis was assayed by procedures identical to method 3, with one significant difference. In this case, the preinitiation RNA replication complexes were isolated from HeLa S10 translation-RNA replication reactions that contained T7-PV1(A)80 transcript RNA instead of poliovirion RNA. The transcript RNA contains two 5′-terminal nonviral GMP nucleotides that block the initiation of positive-strand RNA synthesis. Because positive-strand synthesis is inhibited, this assay specifically measures negative-strand initiation and elongation.
The above reactions were incubated at 30°C for the indicated period of time, terminated by the addition of 0.5% sodium dodecyl sulfate (SDS) buffer (0.5% SDS, 10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 mM NaCl), extracted with phenol-chloroform-isoamyl alcohol, and ethanol precipitated. The reaction products were analyzed by electrophoresis in a CH3HgOH–1% agarose gel as previously described (42). An RNA molecular weight ladder (BRL) was used to determine the size of RNA products. The gels were stained with ethidium bromide and photographed under UV light to visualize the RNA in each lane. The gels were then dried, and the radiolabeled RNA was detected by autoradiography.
RESULTS
Effect of protein synthesis inhibitors on viral RNA replication.
Poliovirus RNA replication was measured in reactions that contained preinitiation RNA replication complexes resuspended in reaction mixtures containing fresh HeLa S10 extracts and translation initiation factors. These reaction conditions provided the soluble cellular factor(s) required to initiate RNA synthesis (4), as well as those required to translate viral RNAs. The initiation and synthesis of full-length negative strands, followed by the initiation and synthesis of full-length positive strands, occurs in these reactions (6). As a result of the asymmetric nature of poliovirus RNA replication, the majority of the RNA synthesized in a 90-min reaction is labeled positive-strand RNA (6). Positive-strand RNA synthesis, however, is dependent on the prior synthesis of a negative-strand RNA template. Therefore, drugs that are added to these reactions can stimulate or inhibit labeled RNA synthesis by affecting either one or both steps in the replication cycle.
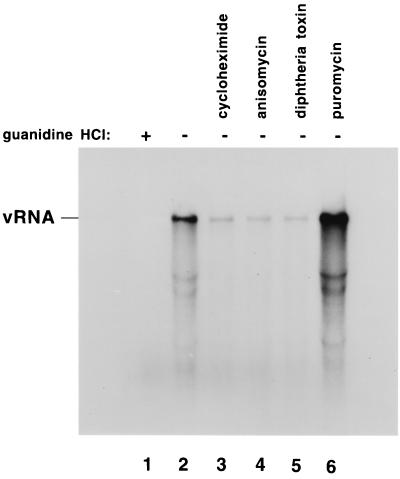
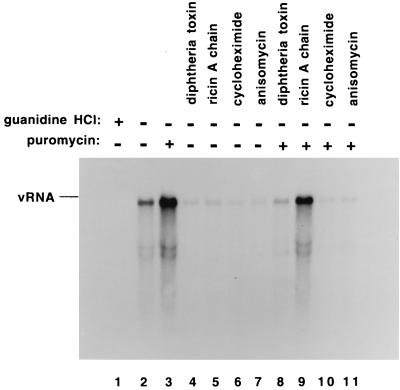
To determine whether continued viral protein synthesis was required in these reactions to support RNA replication, we measured RNA synthesis in the presence of added puromycin (4). We were surprised to find that adding puromycin to these reactions increased the amount of labeled RNA synthesized in a 90-min reaction (Fig. 2, compare lanes 2 and 6) (4). This demonstrated that RNA replication in the preinitiation replication complexes was not dependent on the continued synthesis of viral proteins. Because of the unexpected increase in RNA synthesis that was observed with puromycin, we determined how other protein synthesis inhibitors would affect RNA replication. In contrast to the results observed with puromycin, we found that cycloheximide, anisomycin, and diphtheria toxin all decreased the level of RNA synthesis observed in these reactions (Fig. 2, lanes 3 to 5). Ricin A chain, another inhibitor of protein synthesis, was also found to inhibit RNA replication (Fig. 3 and 4). Because puromycin completely inhibited viral protein synthesis but stimulated RNA replication, we concluded that the decrease in RNA synthesis observed with the other drugs was not a result of their ability to inhibit the synthesis of new viral proteins. Rather, it appeared more likely that these drugs affected RNA replication by a mechanism that was related to their ability to inhibit the continued elongation and/or release of translating ribosomes from viral mRNAs.
FIG. 2.
Effect of protein synthesis inhibitors on the poliovirus RNA replication cycle. Preinitiation RNA replication complexes were resuspended in reaction mixtures containing fresh HeLa S10 extract, translation initiation factors, and 15 μCi of [α-32P]CTP (method 3 in Materials and Methods). The individual reactions also contained 2 mM guanidine (lane 1), no additional drug (lane 2), 200 μg of cycloheximide per ml (lane 3), 8 μg of anisomycin per ml (lane 4), 40 μg of diphtheria toxin per ml plus 40 μg of NAD+ per ml (lane 5), or 100 μg of puromycin per ml (lane 6). The reactions were incubated at 30°C for 90 min, and the labeled RNA products were characterized by CH3HgOH-agarose gel electrophoresis and autoradiography.
FIG. 3.
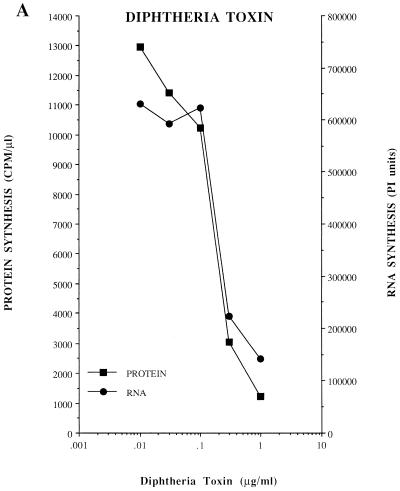
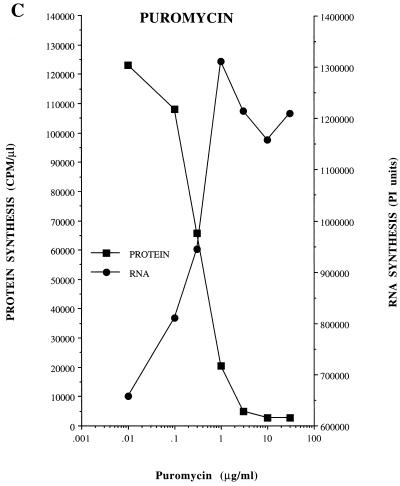
Dose-response effect of diphtheria toxin, ricin A chain, and puromycin on viral protein synthesis and RNA replication. Protein synthesis was measured in HeLa S10 translation-RNA replication reactions containing [35S]methionine and diphtheria toxin, ricin A chain, or puromycin at the indicated concentrations. The reactions were incubated at 30°C as indicated in Materials and Methods. The incorporation of [35S]methionine was determined after 30 min (A and B) or 5 h (C) of incubation and was plotted versus the concentration of each drug used. For RNA synthesis, preinitiation RNA replication complexes were resuspended in reaction mixtures containing fresh HeLa S10 extract (method 3; see Materials and Methods). The individual reactions also contained the indicated concentrations of diphtheria toxin, ricin A chain, or puromycin. The reactions were incubated at 30°C for 90 min, and the labeled RNA products were characterized by CH3HgOH-agarose gel electrophoresis and visualized by autoradiography. The amount of labeled genome-length viral RNA present in each lane was quantitated by using a Molecular Dynamics PhosphorImager and was plotted versus the concentration of each protein synthesis inhibitor.
FIG. 4.
Dominant effect of polypeptide chain elongation inhibitors over puromycin. Preinitiation RNA replication complexes were isolated from 50 μl of HeLa S10 translation-replication reactions that contained 2 mM guanidine and were incubated at 30°C for 5 h. The preinitiation replication complexes were resuspended in 50 μl of fresh HeLa S10 translation-replication reactions containing 10 μCi of [α-32P]CTP as described in Fig. 2 (method 3 in Materials and Methods). The reactions also contained 2 mM guanidine HCl (lane 1); no additional drug (lane 2); 50 μg of puromycin per ml (lane 3); 10 μg of diphtheria toxin per ml plus 40 μg of NAD+ per ml (lane 4); 0.2 μg of ricin A chain per ml (lane 5); 200 μg of cycloheximide per ml (lane 6); 8 μg of anisomycin per ml (lane 7); 10 μg of diphtheria toxin, 40 μg of NAD+, and 50 μg of puromycin per ml (lane 8); 0.2 μg of ricin A chain and 50 μg of puromycin per ml (lane 9); 200 μg of cycloheximide and 50 μg of puromycin per ml (lane 10); or 8 μg of anisomycin and 50 μg of puromycin per ml (lane 11) as indicated. The reactions were incubated at 30°C for 90 min, and the radiolabeled RNA products were phenol extracted, ethanol precipitated, separated by CH3HgOH-agarose gel electrophoresis, and visualized by autoradiography.
Dose-response curves for protein synthesis inhibitors.
If the effects of the protein synthesis inhibitors on RNA replication were linked to their effect on the translation of viral mRNA, then the threshold concentrations of drug required to affect protein synthesis and RNA replication should be the same. The inhibition of protein synthesis in HeLa S10 translation-replication reactions by diphtheria toxin and the ricin A chain occurred at the same concentrations that inhibited RNA replication in reactions containing preinitiation RNA replication complexes (Fig. 3A and B). The concentration of puromycin that inhibited protein synthesis was also identical to the concentration required to stimulate RNA replication (Fig. 3C). Therefore, the concentration of a drug that inhibited protein synthesis corresponded precisely with the drug concentration that either inhibited or stimulated RNA replication.
Polypeptide chain elongation inhibitors have dominant effect over puromycin.
Because puromycin is a substrate for ribosomes, it does not inhibit their catalytic activity and, in fact, requires the catalytic activities associated with ribosomes to inhibit protein synthesis via chain termination. Diphtheria toxin, ricin A chain, cycloheximide, and anisomycin inhibit different activities of ribosomes that are required for polypeptide chain elongation. Therefore, we expected these drugs to be the dominant inhibitors when used simultaneously with puromycin (Fig. 4). In the absence of puromycin, diphtheria toxin, ricin A chain, cycloheximide, and anisomycin inhibited RNA synthesis (Fig. 4, compare lane 2 with lanes 4 to 7). When added simultaneously with puromycin, diphtheria toxin, cycloheximide, and anisomycin continued to inhibit RNA synthesis (Fig. 4, lanes 8, 10, and 11 respectively). In contrast, RNA replication was stimulated in the reaction that contained both ricin A chain and puromycin (Fig. 4, lane 9). Ricin A chain is an enzyme which inhibits protein synthesis by depurinating A4324 in 28S rRNA (21). This modification of the ribosome prevents continued translation elongation. Puromycin, in addition to being an amino acyl tRNA analogue, is a dimethyl adenosine compound (26, 40). The dominant effect of puromycin over ricin A chain inhibition of RNA synthesis suggests that puromycin inhibited the ability of ricin A chain to depurinate A4324 in 28S rRNA. Based on these results, we concluded that all of the drugs that inhibited ribosome chain elongation, with the exception of the ricin A chain, were the dominant inhibitors over puromycin.
Protein synthesis inhibitors have no effect on positive-strand synthesis.
The same set of protein synthesis inhibitors were analyzed for their effect on RNA synthesis in “normal” preformed RNA replication complexes in reactions which also contained 2 mM guanidine HCl (Fig. 5). Because of the asymmetric nature of poliovirus RNA replication, normal RNA replication complexes contain replicative-intermediate RNA which consists of a negative-strand RNA template and multiple nascent chains of positive-strand RNA (3, 4). Because guanidine specifically blocks negative-strand initiation but has no effect on positive-strand initiation or chain elongation reactions (6), positive-strand synthesis can be specifically measured in reactions that contain normal RNA replication complexes and guanidine (method 5 in Materials and Methods). Adding 2 mM guanidine blocks negative-strand RNA synthesis (6) and consequently the formation of new RNA replication complexes, resulting in about a 50% inhibition in RNA synthesis overall (Fig. 5, compare lanes 1 and 2). Therefore, the synthesis of viral RNA in the presence of guanidine (Fig. 5, lanes 2 through 7) corresponds to the synthesis of nascent positive-strand RNA molecules on preexisting negative-strand templates. Using this assay, we showed that none of the protein synthesis inhibitors had any effect on positive-strand RNA synthesis (Fig. 5, lanes 3 to 7 versus lane 2).
FIG. 5.
Effect of protein synthesis inhibitors on positive-strand RNA synthesis. Normal RNA replication complexes were isolated from 50 μl of HeLa S10 translation-RNA replication reactions that were incubated at 30°C for 5 h in the absence of 2 mM guanidine HCl (method 5 in Materials and Methods). The RNA replication complexes were resuspended in reactions containing HeLa S10 extract and 15 μCi of [α-32P]CTP. The individual reactions also contained 2 mM guanidine HCl (lanes 2 to 7), 200 μg of cycloheximide per ml (lane 3), 8 μg of anisomycin per ml (lane 4), 20 μg of diphtheria toxin plus 20 μg of NAD+ per ml (lane 5), 100 μg of puromycin per ml (lane 6), or 2 μg of ricin A chain per ml (lane 7). The reactions were incubated at 30°C for 90 min, and the labeled RNAs were separated by CH3HgOH-agarose gel electrophoresis and visualized by autoradiography.
Positive-strand synthesis inhibited on viral transcript RNAs.
Finding that the protein synthesis inhibitors had no effect on positive-strand synthesis suggested that their effect on viral RNA replication resulted from a direct effect on negative-strand synthesis. Previously, we have shown that it is possible to specifically measure the synthesis of both negative- and positive-strand RNAs in preinitiation RNA replication complexes containing poliovirion RNA (6). In these reactions, RNA replication initiates with the synthesis of full-length negative-strand RNA. This is then rapidly followed by repeated rounds of positive-strand synthesis. The exclusive synthesis of negative-strand RNA is restricted to a short period at the beginning of the reaction. To overcome this time limitation, we used preinitiation replication complexes formed with poliovirus transcript RNA which contained two nonviral 5′-terminal GMP nucleotides and a 3′-terminal poly(A)80 tail (Fig. 6A) (7). With viral transcript RNAs containing a long poly(A) tail, protein synthesis and negative-strand synthesis are only slightly reduced from the levels observed with virion RNA (7). Positive-strand synthesis, however, appears to be strongly inhibited by the presence of the two nonviral 5′-terminal GMP nucleotides in the transcript RNA (7).
To verify that positive-strand synthesis was blocked in reactions containing viral transcript RNA, we used a one-dimensional RNase T1 fingerprint analysis (6). RNA2(A)80 transcript (Fig. 6B) was used in this experiment since it replicates more efficiently in vitro than full-length transcripts and results in the synthesis of increased amounts of labeled RNA that are then available for use in the fingerprint analysis (data not shown). RNA2(A)80 is identical to T7-PV1(A)80 RNA except for an in-frame deletion in the capsid-coding region (14). In previous experiments with poliovirion RNA, we showed that full-length negative-strands were synthesized in ca. 18 min and that positive-strand RNA was the predominant labeled RNA synthesized during the next 18 min (6). Using identical reaction conditions, we showed that only labeled negative-strand RNA was synthesized in RNA2(A)80-preinitiation replication complexes at 16 and 32 min (Fig. 6B). The reaction times were slightly reduced to account for the smaller size of RNA2(A)80. As expected, only negative-strand specific oligonucleotides were observed in the RNase T1 digest of the labeled RNA from the 16-min reaction (Fig. 6B, lane 2). In the 32-min reaction, however, only negative-strand specific oligonucleotides were observed (Fig. 6B, lane 3). This finding was in direct contrast to the previous results with poliovirion RNA where labeled positive-strands were the predominant labeled RNA synthesized during the second half of the reaction (6). Therefore, these results showed that the synthesis of labeled positive-strand RNA was inhibited below the limits of detection in the transcript RNA reactions.
Puromycin simulates and cycloheximide inhibits negative-strand synthesis.
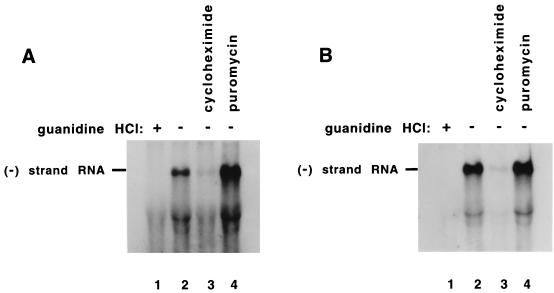
We next determined what effect puromycin and cycloheximide had on negative-strand synthesis in preinitiation replication complexes containing T7-PV1(A)80 RNA (method 6 in Materials and Methods). The replication complexes were resuspended in a reaction mixture containing HeLa S10 extracts and translation initiation factors. Therefore, negative-strand synthesis was measured under translationally competent reaction conditions. The results showed that adding puromycin increased the amount of negative-strand RNA synthesis by ca. 3.5-fold during the first 60 min of the reaction (Fig. 7A, lanes 2 and 4). In a 90-min reaction, however, the presence of puromycin had little effect on the total amount of negative-strand RNA synthesized (Fig. 7B, lanes 2 and 4). Therefore, given sufficient time the total amount of negative-strand RNA synthesized in the absence of puromycin appears to “catch up” with the amount synthesized in the presence of puromycin. This suggests that in the 90-min reactions the viral mRNAs were naturally cleared of translating ribosomes, which then allowed these RNAs to serve as templates for negative-strand RNA synthesis. The addition of cycloheximide to the negative-strand specific reactions decreased the amount of labeled RNA synthesized in the 60- and 90-min reactions by 4- and 10-fold, respectively (Fig. 7A, lanes 2 to 3, and Fig. 7B, lanes 2 to 3). In this case, the difference in the amount of RNA synthesized in the presence or absence of cycloheximide increased with time. This is consistent cycloheximide’s ability to inhibit ribosome elongation, which would also prevent the natural clearance of the ribosomes from the viral mRNA. The continued presence of the ribosomes would be expected to block negative-strand synthesis throughout the course of these reactions. Therefore, the difference in the quantity of RNA synthesized in the presence or absence of cycloheximide should increase with time. The results of these experiments confirmed that the protein synthesis inhibitors affected viral RNA replication overall by having a direct effect on negative-strand RNA synthesis.
FIG. 7.
Effect of puromycin and cycloheximide on negative-strand RNA synthesis within preinitiation RNA replication complexes. Preinitiation replication complexes were isolated from 50 μl of HeLa S10 translation-replication reactions containing T7-PV1(A)80 RNA (rather than virion RNA) and 2 mM guanidine HCl after incubation at 30°C for 5 h (method 6 in Materials and Methods). The preinitiation complexes were resuspended in 50-μl reaction mixtures containing HeLa S10 extract, translation initiation factors, 50 μCi of [α-32P]CTP, and 5 μM CTPfinal. The individual reactions contained 2 mM guanidine (lane 1), no additional drugs (lane 2), 200 μg of cycloheximide per ml (lane 3), or 100 μg of puromycin per ml (lane 4). The reactions were incubated at 30°C for 60 min (A) or for 90 min (B), and the labeled RNA was phenol extracted and ethanol precipitated. The labeled RNA products were fractionated by CH3HgOH-agarose gel electrophoresis. The dried gels were subjected to autoradiography for visualization of the labeled RNAs. The amount of labeled negative-strand RNA was quantitated by using a Molecular Dynamics PhosphorImager.
DISCUSSION
A dilemma common to all positive-strand RNA viruses is the potential for collisions between translating ribosomes and the viral polymerase during negative-strand synthesis (Fig. 1B). This is a dilemma specifically associated with positive-strand RNA viruses since the infecting genomic RNA must be translated to synthesize the viral replication proteins before RNA synthesis can initiate. At some point, the input viral RNA must switch from serving as a mRNA for translation and serve as the template for negative-strand synthesis. It would be advantageous for these viruses to prevent ribosome-polymerase collisions to avoid the dilemma of the continued presence of translating ribosomes inhibiting polymerase elongation during negative-strand synthesis. This dilemma may be especially important for poliovirus since many of the viral replication proteins appear to be predominantly cis-active in virus-infected cells (14, 24, 30).
In this study, we provide evidence that the presence of translating ribosomes on poliovirus RNA templates in preinitiation RNA replication complexes inhibits viral RNA replication by inhibiting negative-strand synthesis. Puromycin, which dissociates translating ribosomes from mRNA, increased viral RNA replication in preinitiation replication complexes. In contrast, “freezing” ribosomes on viral mRNA with cycloheximide, anisomycin, diphtheria toxin, or ricin A chain inhibited poliovirus RNA replication. Two mechanisms may mediate the inhibition of negative-strand RNA synthesis by translating ribosomes. One possibility is that negative-strand synthesis does not initiate in preinitiation replication complexes until the ribosomes have been cleared from the RNA. This mechanism infers that the replication machinery could sense the presence of ribosomes on the viral mRNA. A second possibility is that the polymerase does initiate synthesis on viral mRNAs containing translating ribosomes but is not able to displace ribosomes during negative-strand elongation. In either case, the results rule out a model for switching from translation to replication that would be based on the ability of the elongating polymerase to displace translating ribosomes from the input RNA. The inability of the poliovirus polymerase to dislodge translating ribosomes is consistent with previous studies with purified Qβ replicase where it was shown that ribosomes bound to Qβ RNA were also able to block negative-strand synthesis (25).
Some evidence exists to suggest that ribosome-polymerase collisions may occur during poliovirus replication in infected cells. Charini et al. (12) isolated a pseudorevertant of a poliovirus mutant that appears to have transduced a 15-nucleotide sequence from 28S rRNA into the genome of poliovirus. These authors hypothesize that an exposed region of 28S rRNA was used as a template for nonhomologous recombination during negative-strand RNA synthesis and that the recombination occurred as the result of a ribosome-polymerase collision (12). In this case, the polymerase may have switched to a second positive-strand viral RNA template free of ribosomes after the ribosome-polymerase collision. This raises the possibility of a mechanistic role for ribosome-polymerase collisions in normal viral RNA recombination events (i.e., the inducement of template switching during negative-strand synthesis). It will be important to investigate this idea further now that it is possible to measure RNA recombination in vitro (17, 37).
Poliovirus must have evolved a mechanism to clear viral RNA of translating ribosomes to either reduce or avoid ribosome-polymerase collisions. As shown in this study, preinitiation replication complexes were capable of replicating viral RNA independent of puromycin treatment. RNA replication occurring in these reactions was due to the availability of viral RNA templates that were naturally cleared of ribosomes. In addition, we found that given enough time essentially all of the positive-strand RNA templates in preinitiation replication complexes were cleared of translating ribosomes and used for negative-strand synthesis. Of course, we cannot exclude the possibility that there are differences in the mechanisms used to clear translating ribosomes from poliovirus RNA in vitro and in vivo. Borman and coworkers (9) identified mutations within the poliovirus IRES that inhibited viral RNA replication independent of their effects on viral protein synthesis. One IRES mutation characterized by these investigators, a 46-nucleotide duplication within the IRES, dramatically diminished RNA replication without significantly diminishing translation initiation. This mutation may have prevented the normal shutdown of translation initiation such that the continued presence of ribosomes on the viral RNA blocked negative-strand synthesis. Therefore, it appears likely that a mechanism exists to inhibit translation initiation so that translating ribosomes can clear via normal elongation and termination at the end of the polyprotein coding sequence.
The mechanism used to regulate the switch from translation to replication may involve the direct binding of a viral protein to the IRES or to a cellular protein factor that is required for translation initiation. For example, bacteriophage Qβ utilizes the competitive binding of viral proteins to the viral RNA to regulate the switch from translation to RNA replication (1, 25). At present, only limited information is available regarding the mechanisms involved in this fundamental step of poliovirus replication. Intriguingly, Hoffman and Palmenberg characterized a revertant of a small plaque mutant of encephalomyocarditis virus (EMCV) in which an IRES mutation that diminished translation was compensated by a mutation in the leader protein (22). The EMCV leader protein is a zinc-binding protein with homology to bacteriophage T4 gp32 (13), which is a protein capable of repressing the translation of its cognate mRNA by binding to a 5′-terminal pseudoknot (34). Chen et al. (13) speculated that the EMCV leader protein may feedback inhibit the EMCV IRES to help regulate the switch from translation to RNA replication. Hoffman and Palmenberg concur, suggesting that the second site reversion mutation in leader protein may have reduced the normal efficiency of the shutoff of viral translation, compensating for the diminished translation caused by the primary IRES mutation (22). Poliovirus protein 2A is similar to the EMCV leader protein in that it also contains a zinc-binding domain that may mediate RNA binding (36, 43). A number of studies have implicated poliovirus protein 2A in RNA replication without yet determining its specific function (28). It is interesting to speculate that protein 2A may mediate the shutoff of host protein synthesis (41) and that it may also play a role in the subsequent inhibition of viral translation initiation via IRES binding upon the accumulation of larger amounts of 2APro than those required for the shutoff of host translation.
Alternatively, a viral protein may accumulate during translation and inhibit continued initiation of translation by binding to the viral RNA outside the core IRES. Infectious poliovirus chimeras have been constructed in which the poliovirus IRES has been replaced by either the EMCV IRES or the hepatitis C virus IRES (27, 39). By visual inspection, the different IRES elements appear to be significantly different in terms of their individual sequences and predicted structures. This suggests the possibility that translation initiation might be regulated by viral proteins binding to sequences in the poliovirus genome that are not part of the core IRES sequence. For example, poliovirus protein 3CD binds to the 5′-terminal cloverleaf structure in poliovirus RNA (1, 2). Even though the 5′ cloverleaf is not considered an integral part of the IRES, some evidence indicates that mutations in the 5′ cloverleaf can affect translation initiation (35). Therefore, it is possible that the formation of the 5′-terminal ribonucleoprotein complex that is required for replication may also be involved in the regulation of translation initiation. Gamarnik and Andino (19) recently presented evidence that 3CDPro is able to inhibit poliovirus RNA translation. They suggest that 3CDPro is involved in the feedback inhibition of poliovirus mRNA translation necessary for the transition from translation to RNA replication (19). Clearly, further experimentation is required to elucidate the regulatory pathway involved in the viral RNA switching from translation to replication. Such a pathway is likely to involve interactions between the viral genome, viral and cellular proteins required for translation and replication, and membrane surfaces that are used for the assembly of viral RNA replication complexes. The cell-free replication reactions described herein should facilitate these studies regarding the molecular basis of the switch from the translation to RNA replication for poliovirus and other positive-strand RNA viruses.
ACKNOWLEDGMENTS
We thank Brian O’Donnell for excellent technical assistance.
This work was supported by Public Health Service grants AI15539 and AI32123 from the National Institute of Allergy and Infectious Diseases. David J. Barton was supported by Public Health Service training grant AI07110 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Andino R, Rieckhof G E, Achacoso P L, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 3.Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968;32:359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- 4.Barton D J, Black E P, Flanegan J B. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton D J, Flanegan J B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton D J, Flanegan J B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol. 1997;71:8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton D J, Morasco B J, Flanegan J B. Assays for poliovirus polymerase, 3DPol, and authentic RNA replication in HeLa S10 extracts. Methods Enzymol. 1996;275:35–57. doi: 10.1016/s0076-6879(96)75005-x. [DOI] [PubMed] [Google Scholar]
- 8.Blobel G, Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci USA. 1971;68:390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borman A M, Deliat F G, Kean K M. Sequences within the poliovirus internal ribosome entry segment control viral RNA synthesis. EMBO J. 1994;13:3149–3157. doi: 10.1002/j.1460-2075.1994.tb06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caliguiri L A, Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969;166:885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- 11.Carey J, Cameron V, Krug M, de Haseth P L, Uhlenbeck O C. Failure of translational repression in the phage f2 op3 mutant is not due to an altered coat protein-RNA interaction. J Biol Chem. 1984;259:20–22. [PubMed] [Google Scholar]
- 12.Charini W A, Todd S, Gutman G A, Semler B L. Transduction of a human RNA sequence by poliovirus. J Virol. 1994;68:6547–6552. doi: 10.1128/jvi.68.10.6547-6552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Kong W, Roos R P. The leader peptide of Theiler’s murine encephalomyelitis virus is a zinc-binding protein. J Virol. 1995;69:8076–8078. doi: 10.1128/jvi.69.12.8076-8078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collis P S, O’Donnell B J, Barton D J, Rogers J A, Flanegan J B. Replication of poliovirus RNA and subgenomic RNA transcripts in transfected cells. J Virol. 1992;66:6480–6488. doi: 10.1128/jvi.66.11.6480-6488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darnell J E, Levintow L, Thoren M M, Hooper J L. The time course of synthesis of poliovirus RNA. Virology. 1961;13:271–279. doi: 10.1016/0042-6822(61)90145-3. [DOI] [PubMed] [Google Scholar]
- 16.Drazin R, Kandel J, Collier R J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971;246:1504–1510. [PubMed] [Google Scholar]
- 17.Duggal R, Cuconati A, Gromeier M, Wimmer E. Genetic recombination of poliovirus in a cell-free system. Proc Natl Acad Sci USA. 1997;94:13786–13791. doi: 10.1073/pnas.94.25.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enders J F, Weller T H, Robbins F C. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85–87. doi: 10.1126/science.109.2822.85. [DOI] [PubMed] [Google Scholar]
- 19.Gamarnik A V, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluck A, Endo Y, Wool I G. Ribosomal RNA identity elements for ricin A-chain recognition and catalysis: analysis with tetraloop mutants. J Mol Biol. 1992;226:411–424. doi: 10.1016/0022-2836(92)90956-k. [DOI] [PubMed] [Google Scholar]
- 21.Grollman A P. Inhibitors of protein synthesis. II. Mode of action of anisomycin. J Biol Chem. 1967;242:3226–3233. [PubMed] [Google Scholar]
- 22.Hoffman M A, Palmenberg A C. Revertant analysis of J-K mutations in the encephalomyocarditis virus internal ribosome entry site detects an altered leader protein. J Virol. 1996;70:6425–6430. doi: 10.1128/jvi.70.9.6425-6430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honjo T, Nishizuka Y, Hayaishi O, Kato J. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and the inhibition of protein synthesis. J Biol Chem. 1968;243:3553–3555. [PubMed] [Google Scholar]
- 24.Johnson K L, Sarnow P. Three poliovirus 2B mutants exhibit noncomplementable defects in viral RNA amplification and display dosage-dependent dominance over wild-type poliovirus. J Virol. 1991;65:4341–4349. doi: 10.1128/jvi.65.8.4341-4349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolakofsky D, Weissmann C. Possible mechanism for transition of viral RNA from polysome to replication complex. Nat New Biol. 1971;231:42–46. doi: 10.1038/newbio231042a0. [DOI] [PubMed] [Google Scholar]
- 26.Lehninger A L, Nelson D L, Cox M M. Principles of biochemistry. New York, N.Y: Worth Publishers; 1993. pp. 927–928. [Google Scholar]
- 27.Lu H, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molla A, Paul A V, Schmid M, Jang S K, Wimmer E. Studies on dicistronic polioviruses implicate viral proteinase 2A in RNA replication. Virology. 1993;196:739–747. doi: 10.1006/viro.1993.1531. [DOI] [PubMed] [Google Scholar]
- 29.Molla A, Paul A V, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 30.Novak J E, Kirkegaard K. Coupling between genome translation and replication in an RNA virus. Genes Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 31.Roizman B. Multiplication of viruses: an overview. In: Fields B N, editor. Virology. New York, N.Y: Raven Press; 1990. pp. 87–94. [Google Scholar]
- 32.Roumiantzeff M, Summers D F, Maizel J V. In vitro protein synthetic activity of membrane-bound poliovirus polyribosomes. Virology. 1971;44:249–258. doi: 10.1016/0042-6822(71)90257-1. [DOI] [PubMed] [Google Scholar]
- 33.Sarnow P. Role of 3′-end sequences in infectivity of poliovirus transcripts made in vitro. J Virol. 1989;63:467–470. doi: 10.1128/jvi.63.1.467-470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamoo Y, Webster K R, Williams K R, Konigsberg W H. A retrovirus-like zinc domain is essential for translational repression of bacteriophage T4 gene 32. J Biol Chem. 1991;266:7967–7970. [PubMed] [Google Scholar]
- 35.Simoes E A, Sarnow P. An RNA hairpin at the extreme 5′ end of the poliovirus RNA genome modulates viral translation in human cells. J Virol. 1991;65:913–921. doi: 10.1128/jvi.65.2.913-921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sommergruber W, Casari G, Fessl F, Seipelt J, Skern T. The 2A proteinase of human rhinovirus is a zinc containing enzyme. Virology. 1994;204:815–818. doi: 10.1006/viro.1994.1599. [DOI] [PubMed] [Google Scholar]
- 37.Tang R S, Barton D J, Flanegan J B, Kirkegaard K. Poliovirus RNA recombination in cell free extracts. RNA. 1997;3:624–633. [PMC free article] [PubMed] [Google Scholar]
- 38.Villa-Komaroff L, McDowell M, Baltimore D, Lodish H F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qβ RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- 39.Wimmer E, Hellen C U T, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 40.Windholz M, editor. The Merck index. 10th ed. Rahway, N.J: Merck and Co., Inc.; 1983. p. 1146. [Google Scholar]
- 41.Wyckoff E, Lloyd R, Ehrenfeld E. Relationship of eukaryotic initiation factor 3 to poliovirus-induced p220 cleavage activity. J Virol. 1992;66:2943–2951. doi: 10.1128/jvi.66.5.2943-2951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young D C, Tuschall D M, Flanegan J B. Poliovirus RNA-dependent RNA polymerase and host cell protein synthesize product RNA twice the size of poliovirion RNA in vitro. J Virol. 1985;54:256–264. doi: 10.1128/jvi.54.2.256-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S F, Lloyd R E. Characterization of the roles of conserved cysteine and histidine residues in poliovirus 2A protease. Virology. 1992;186:725–735. doi: 10.1016/0042-6822(92)90039-r. [DOI] [PubMed] [Google Scholar]