Abstract
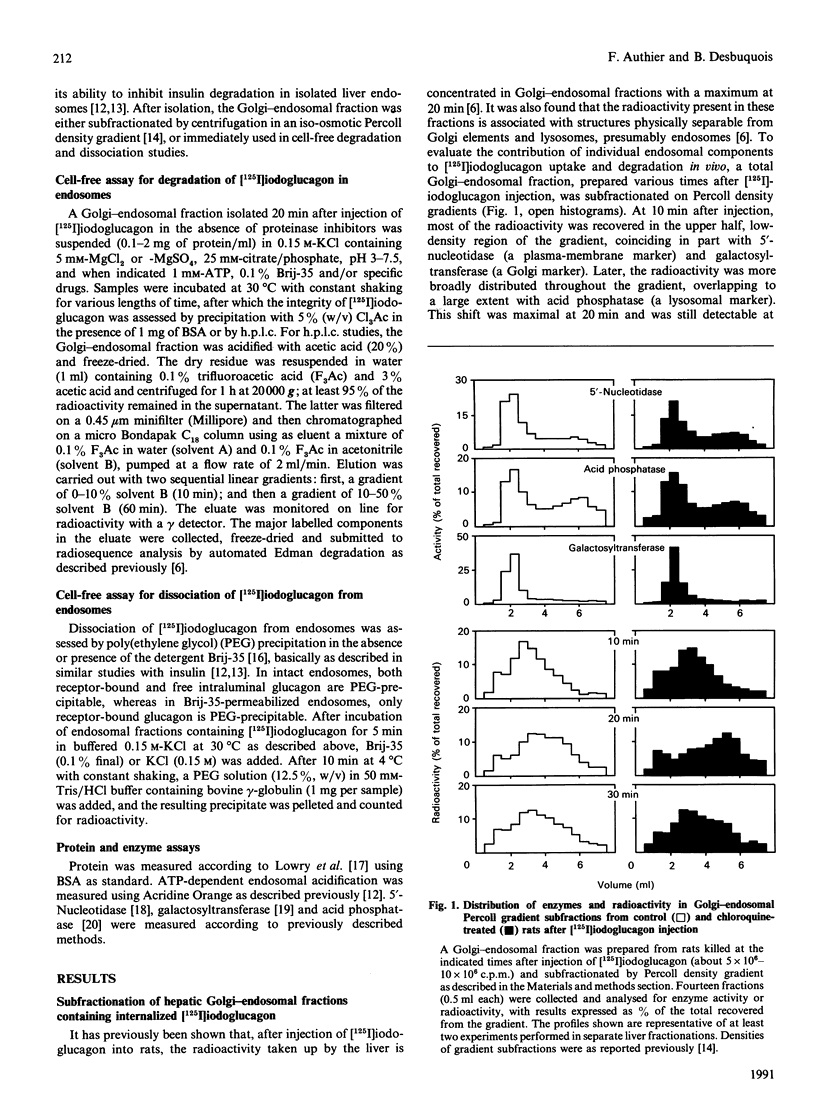
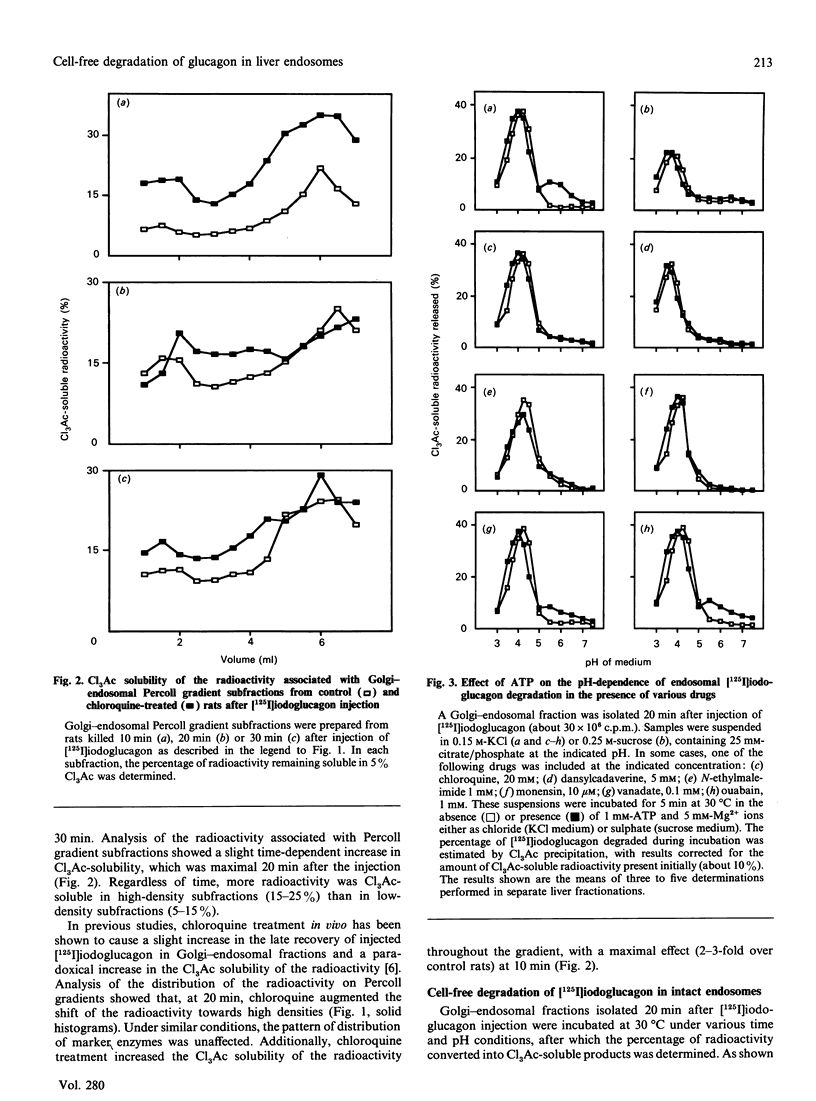
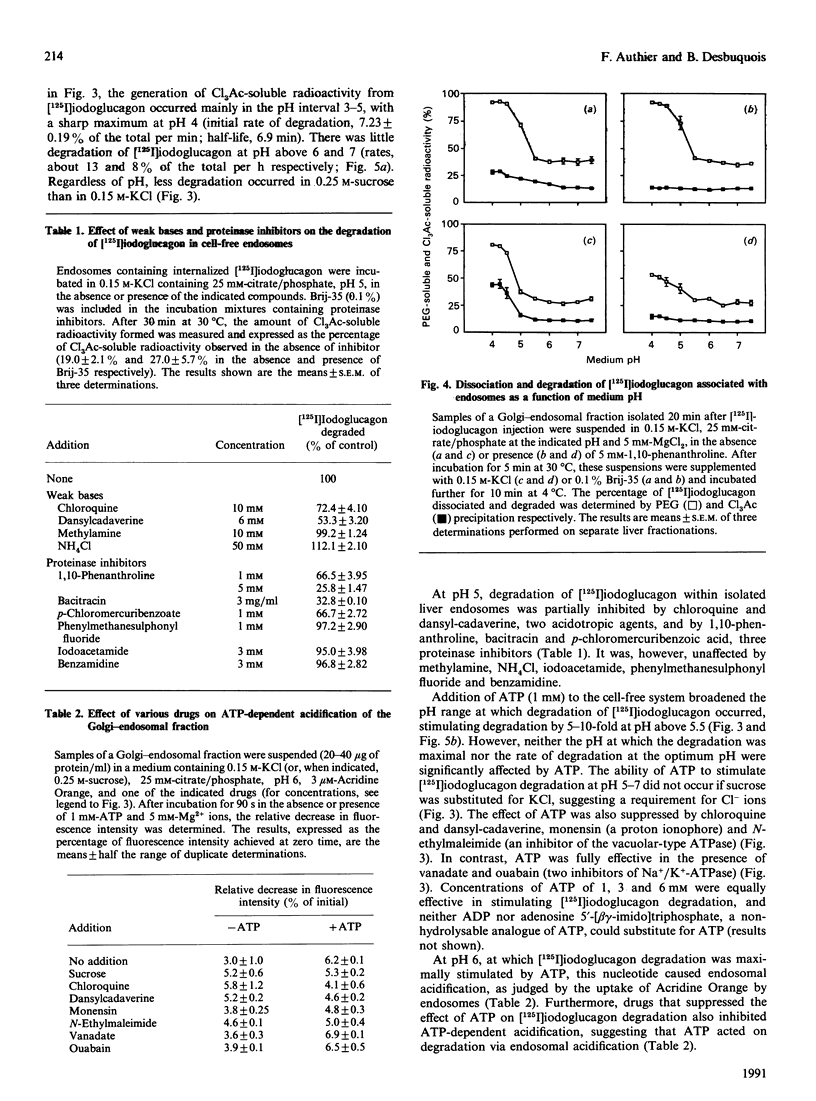
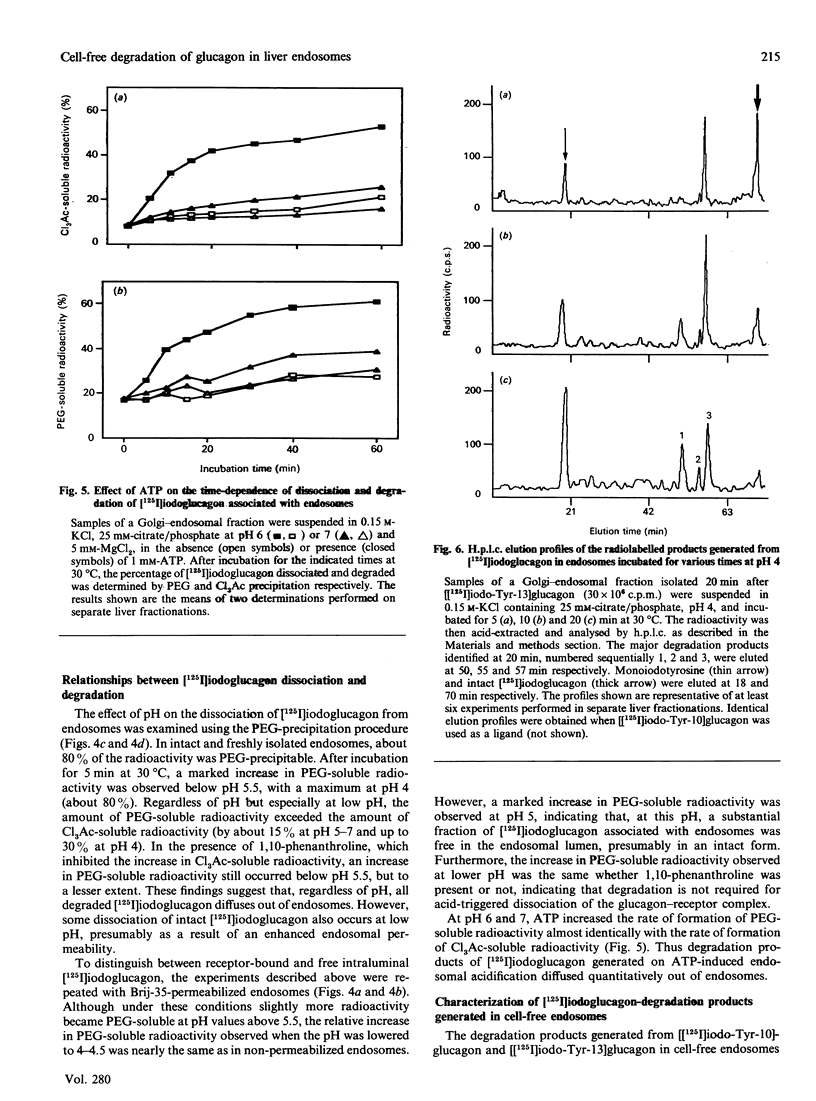
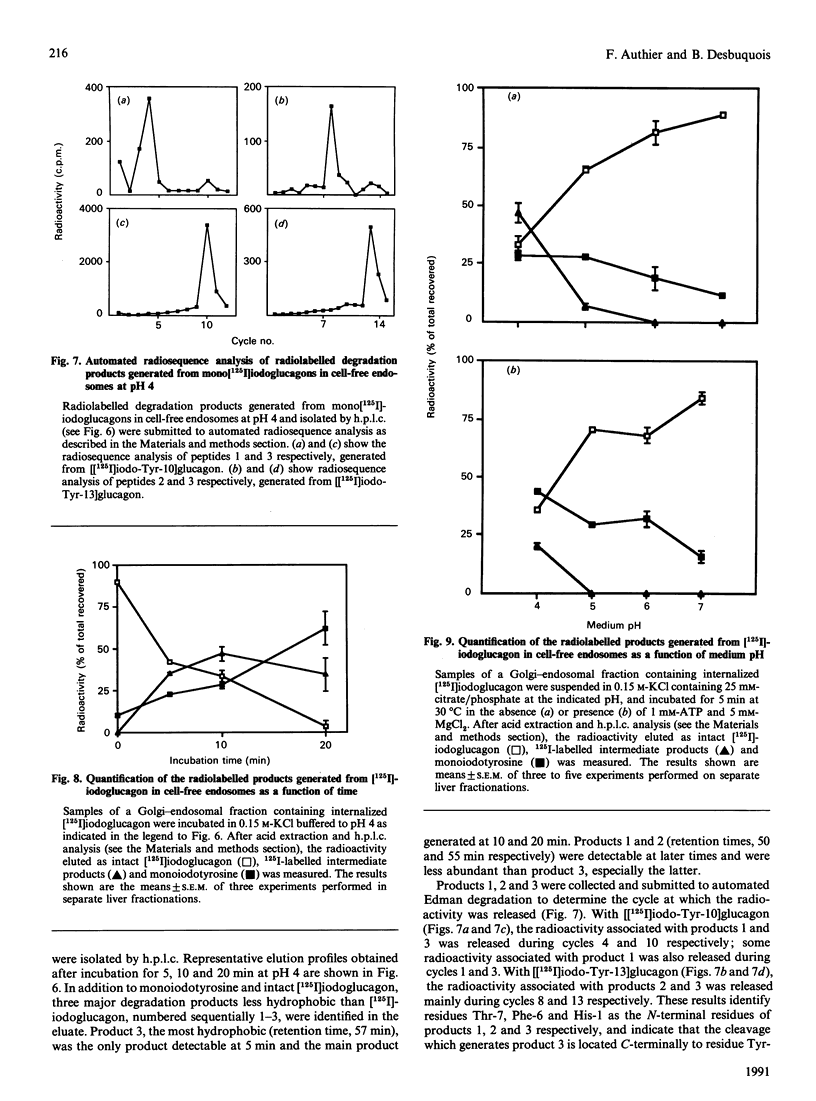
Endosomes have recently been identified as one major site of glucagon degradation in intact rat liver. In this study, a cell-free system has been used to assess the role of ATP-dependent acidification in endosomal glucagon degradation and identify the glucagon products generated. Percoll gradient fractionation of Golgi-endosomal fractions prepared 10-30 min after injection of [125I]iodoglucagon showed a time-dependent shift of the radioactivity towards high densities. Regardless of time, the radioactivity was less precipitable by trichloroacetic acid (Cl3Ac) at high densities than at low densities. Chloroquine treatment slightly increased the density shift of the radioactivity and decreased its Cl3Ac-precipitability throughout the gradient. Incubation of endosomal fractions containing [125I]iodoglucagon in 0.15 M-KCl at 30 degrees C resulted in a time- and pH-dependent generation of Cl3Ac-soluble radioactivity, with a maximum at pH 4 (t1/2, 7 min). At pH 5, 1,10-phenanthroline, bacitracin and p-chloromercuribenzoic acid partially inhibited [125I]iodoglucagon degradation. At pH 6-7, ATP stimulated [125I]iodoglucagon degradation by 5-10-fold and caused endosomal acidification as judged from Acridine Orange uptake. The effects of ATP were inhibited by chloroquine, monensin, N-ethylmaleimide and dansylcadaverine. Poly(ethylene glycol) (PEG) precipitation of the radioactivity associated with endosomes showed that lowering the pH below 5.5 caused dissociation of the glucagon-receptor complex, and that, regardless of incubation conditions, all degraded [125I]iodoglucagon diffused extraluminally. On h.p.l.c., at least three products less hydrophobic than [125I]iodoglucagon were identified in incubation mixtures along with monoiodotyrosine. Radiosequence analysis of the products revealed one major cleavage located C-terminally to Tyr-13 and two minor cleavages affecting Thr-5-Phe-6 and Phe-6-Thr-7 bonds. It is concluded that glucagon degradation in liver endosomes is functionally linked to ATP-dependent endosomal acidification and involves several cleavages in the glucagon sequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Authier F., Janicot M., Lederer F., Desbuquois B. Fate of injected glucagon taken up by rat liver in vivo. Degradation of internalized ligand in the endosomal compartment. Biochem J. 1990 Dec 15;272(3):703–712. doi: 10.1042/bj2720703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balage M., Grizard J., Grizard G. Binding and degradation of 125I-glucagon by highly purified rat liver plasma membranes. Biochim Biophys Acta. 1986 Oct 29;884(1):101–108. doi: 10.1016/0304-4165(86)90232-1. [DOI] [PubMed] [Google Scholar]
- Barazzone P., Gorden P., Carpentier J. L., Orci L., Freychet P., Canivet B. Binding, internalization, and lysosomal association of 125I-glucagon in isolated rat hepatocytes. A quantitative electron microscope autoradiographic study. J Clin Invest. 1980 Nov;66(5):1081–1093. doi: 10.1172/JCI109937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blache P., Kervran A., Dufour M., Martinez J., Le-Nguyen D., Lotersztajn S., Pavoine C., Pecker F., Bataille D. Glucagon-(19-29), a Ca2+ pump inhibitory peptide, is processed from glucagon in the rat liver plasma membrane by a thiol endopeptidase. J Biol Chem. 1990 Dec 15;265(35):21514–21519. [PubMed] [Google Scholar]
- Canivet B., Gorden P., Carpentier J. L., Orci L., Freychet P. Glucagon degradation in isolated rat hepatocytes: effect of ammonium chloride and chloroquine. Mol Cell Endocrinol. 1981 Sep;23(3):311–320. doi: 10.1016/0303-7207(81)90128-3. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Janicot M., Dupuis A. Degradation of insulin in isolated liver endosomes is functionally linked to ATP-dependent endosomal acidification. Eur J Biochem. 1990 Oct 24;193(2):501–512. doi: 10.1111/j.1432-1033.1990.tb19365.x. [DOI] [PubMed] [Google Scholar]
- Diment S., Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985 Dec 5;260(28):15311–15317. [PubMed] [Google Scholar]
- Doherty J. J., 2nd, Kay D. G., Lai W. H., Posner B. I., Bergeron J. J. Selective degradation of insulin within rat liver endosomes. J Cell Biol. 1990 Jan;110(1):35–42. doi: 10.1083/jcb.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- Hagopian W. A., Tager H. S. Hepatic glucagon metabolism. Correlation of hormone processing by isolated canine hepatocytes with glucagon metabolism in man and in the dog. J Clin Invest. 1987 Feb;79(2):409–417. doi: 10.1172/JCI112827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian W. A., Tager H. S. Receptor binding and cell-mediated metabolism of [125I]monoiodoglucagon by isolated canine hepatocytes. J Biol Chem. 1984 Jul 25;259(14):8986–8993. [PubMed] [Google Scholar]
- Janicot M., Desbuquois B. Fate of injected 125I-labeled cholera toxin taken up by rat liver in vivo. Generation of the active A1 peptide in the endosomal compartment. Eur J Biochem. 1987 Mar 2;163(2):433–442. doi: 10.1111/j.1432-1033.1987.tb10816.x. [DOI] [PubMed] [Google Scholar]
- Khan M. N., Posner B. I., Khan R. J., Bergeron J. J. Internalization of insulin into rat liver Golgi elements. Evidence for vesicle heterogeneity and the path of intracellular processing. J Biol Chem. 1982 May 25;257(10):5969–5976. [PubMed] [Google Scholar]
- Khan R. J., Khan M. N., Bergeron J. J., Posner B. I. Prolactin uptake into liver endocytic components. Reduced sensitivity to chloroquine. Biochim Biophys Acta. 1985 Jan 28;838(1):77–83. doi: 10.1016/0304-4165(85)90252-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pease R. J., Smith G. D., Peters T. J. Characterization of insulin degradation by rat-liver low-density vesicles. Eur J Biochem. 1987 Apr 1;164(1):251–257. doi: 10.1111/j.1432-1033.1987.tb11018.x. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Birnbaumer L., Rodbell M. Inactivation of glucagon by plasma membranes of rat liver. J Biol Chem. 1972 Apr 25;247(8):2295–2301. [PubMed] [Google Scholar]
- Rose K., Savoy L. A., Muir A. V., Davies J. G., Offord R. E., Turcatti G. Insulin proteinase liberates from glucagon a fragment known to have enhanced activity against Ca2+ + Mg2+-dependent ATPase. Biochem J. 1988 Dec 15;256(3):847–851. doi: 10.1042/bj2560847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouer E., Desbuquois B., Postel-Vinay M. C. Interactions of glucagon with isolated rat-liver cells. Fate and subcellular localization of cell-associated hormone. Mol Cell Endocrinol. 1980 Aug;19(2):143–164. doi: 10.1016/0303-7207(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. J., Tager H. S. Characterization of a glucagon receptor-linked protease from canine hepatic plasma membranes. Partial purification, kinetic analysis, and determination of sites for hormone processing. J Biol Chem. 1988 Dec 15;263(35):19210–19217. [PubMed] [Google Scholar]
- Sheetz M. J., Tager H. S. Receptor-linked proteolysis of membrane-bound glucagon yields a membrane-associated hormone fragment. J Biol Chem. 1988 Jun 15;263(17):8509–8514. [PubMed] [Google Scholar]
- Smith G. D., Christensen J. R., Rideout J. M., Peters T. J. Hepatic processing of insulin. Characterization of differential inhibition by weak bases. Eur J Biochem. 1989 May 1;181(2):287–294. doi: 10.1111/j.1432-1033.1989.tb14723.x. [DOI] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Wileman T., Boshans R., Stahl P. Uptake and transport of mannosylated ligands by alveolar macrophages. Studies on ATP-dependent receptor-ligand dissociation. J Biol Chem. 1985 Jun 25;260(12):7387–7393. [PubMed] [Google Scholar]


