Abstract
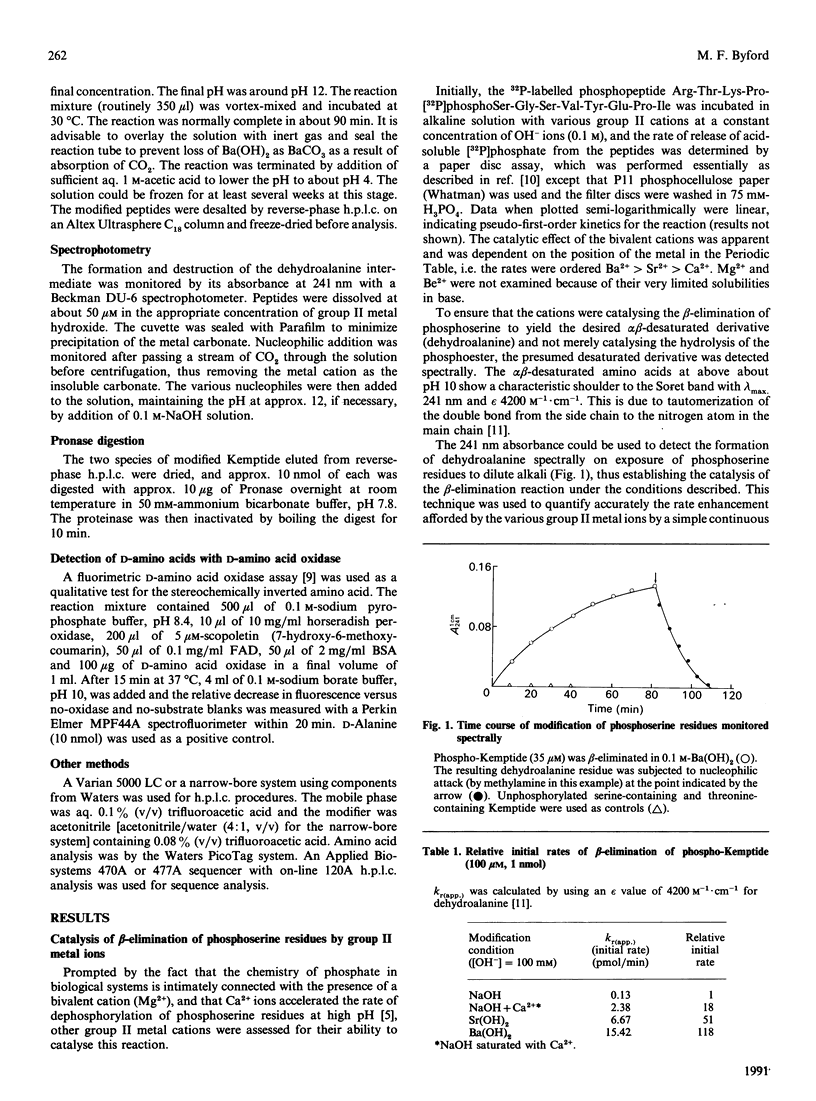
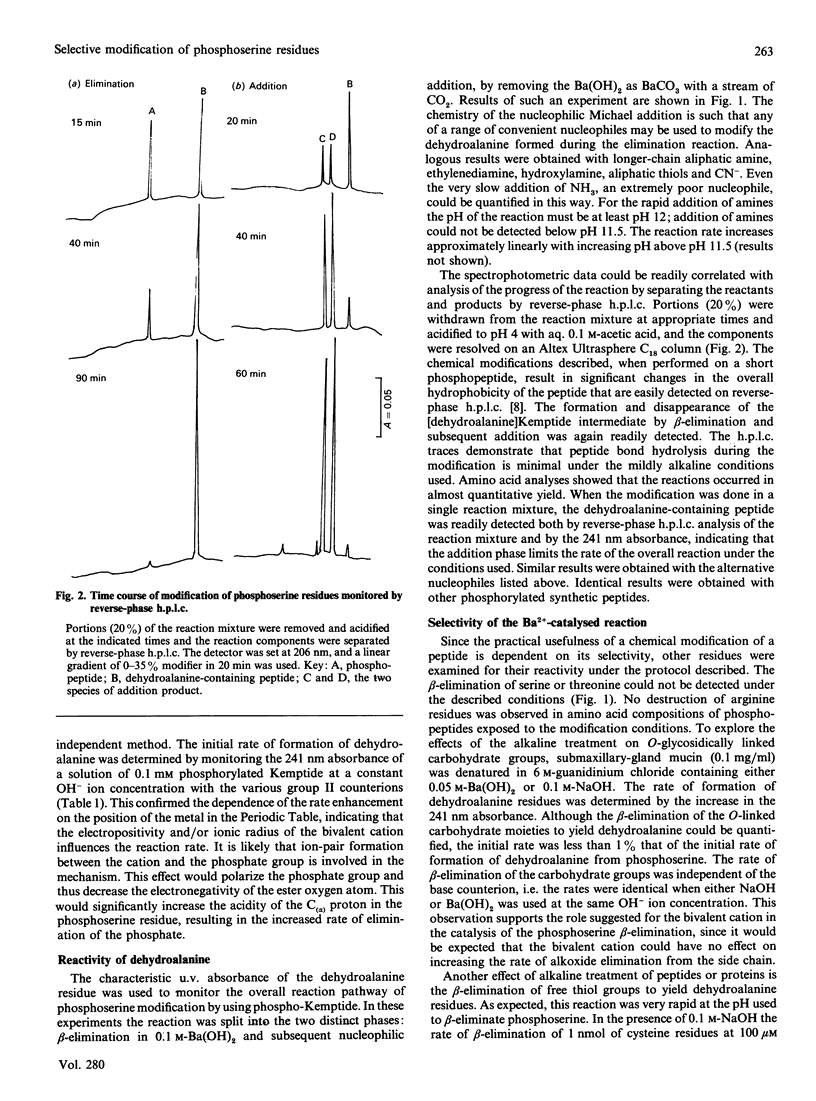
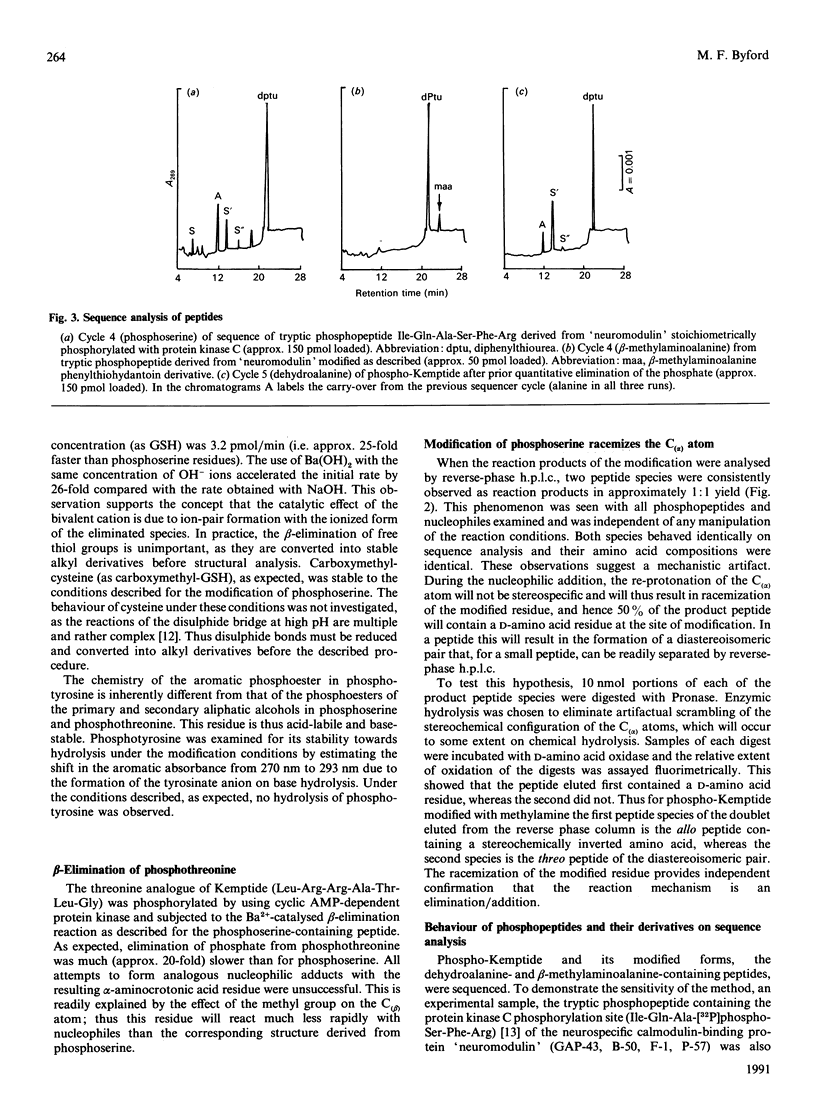
The beta-elimination of phosphoserine residues by dilute alkali is catalysed by the presence of group II metal ions. The use of 0.1 M-Ba (OH)2 catalysed the rate of beta-elimination of phosphoserine by more than two orders of magnitude compared with the use of NaOH at the same OH-ion concentration. Serine and threonine residues are unaffected by this treatment. Free thiol groups and disulphide bonds are labile to these conditions, but carboxymethylcysteine is stable. The rate of beta-elimination of O-glycosidically linked moieties is not catalysed under these conditions, and the rate of reaction is thus two orders of magnitude slower than for phosphoserine. This specific catalysis was readily exploited in the rapid and selective modification of phosphoserine residues under mildly alkaline conditions with the nucleophile methylamine via the alpha beta-desaturated dehydroalanine intermediate to yield the beta-methylaminoalanine residue. This modified residue could be easily detected on sequence analysis and in amino acid compositions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annan W. D., Manson W., Nimmo J. A. The identification of phosphoseryl residues during the determination amino acid sequence in phosphoproteins. Anal Biochem. 1982 Mar 15;121(1):62–68. doi: 10.1016/0003-2697(82)90557-7. [DOI] [PubMed] [Google Scholar]
- Apel E. D., Byford M. F., Au D., Walsh K. A., Storm D. R. Identification of the protein kinase C phosphorylation site in neuromodulin. Biochemistry. 1990 Mar 6;29(9):2330–2335. doi: 10.1021/bi00461a017. [DOI] [PubMed] [Google Scholar]
- Clark R. C., Dijkstra J. Chemical modification of phosvitin: preparation of dimethylaminovitin and methylmercaptovitin and their utility for elucidation of phosvitin primary structure. Int J Biochem. 1980;11(6):577–585. doi: 10.1016/0020-711x(80)90268-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985 Sep 16;151(3):439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The presence of dehydroalanine in the antibiotic nisin and its relationship to activity. J Am Chem Soc. 1967 May 24;89(11):2791–2792. doi: 10.1021/ja00987a084. [DOI] [PubMed] [Google Scholar]
- Holmes C. F. A new method for the selective isolation of phosphoserine-containing peptides. FEBS Lett. 1987 May 4;215(1):21–24. doi: 10.1016/0014-5793(87)80106-0. [DOI] [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Korte H., Heilmeyer L. M., Jr Sequence analysis of phosphoserine-containing peptides. Modification for picomolar sensitivity. FEBS Lett. 1986 Aug 11;204(1):61–66. doi: 10.1016/0014-5793(86)81388-6. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Cohen P. Amino acid sequence at the site on rabbit skeletal muscle glycogen synthase phosphorylated by the endogenous glycogen synthase kinase-2 activity. FEBS Lett. 1979 Feb 1;98(1):71–75. doi: 10.1016/0014-5793(79)80154-4. [DOI] [PubMed] [Google Scholar]


