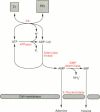
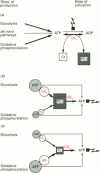
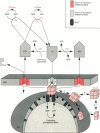
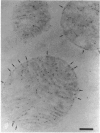
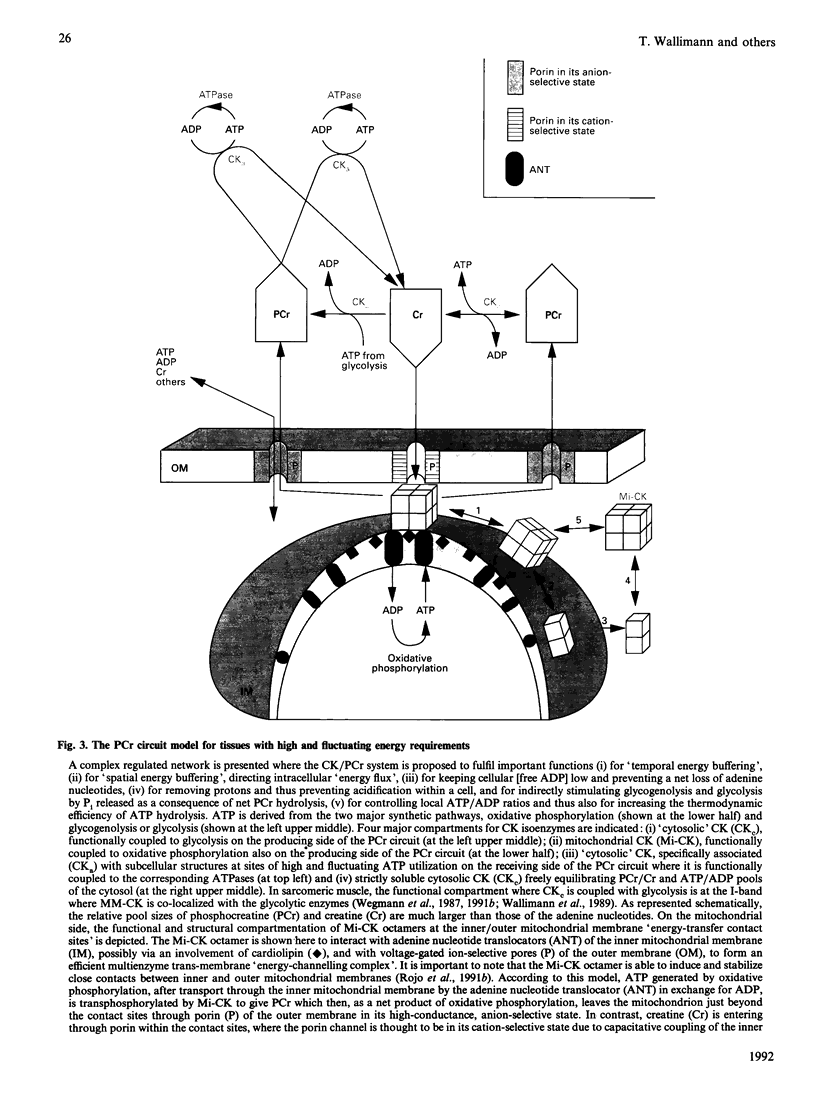
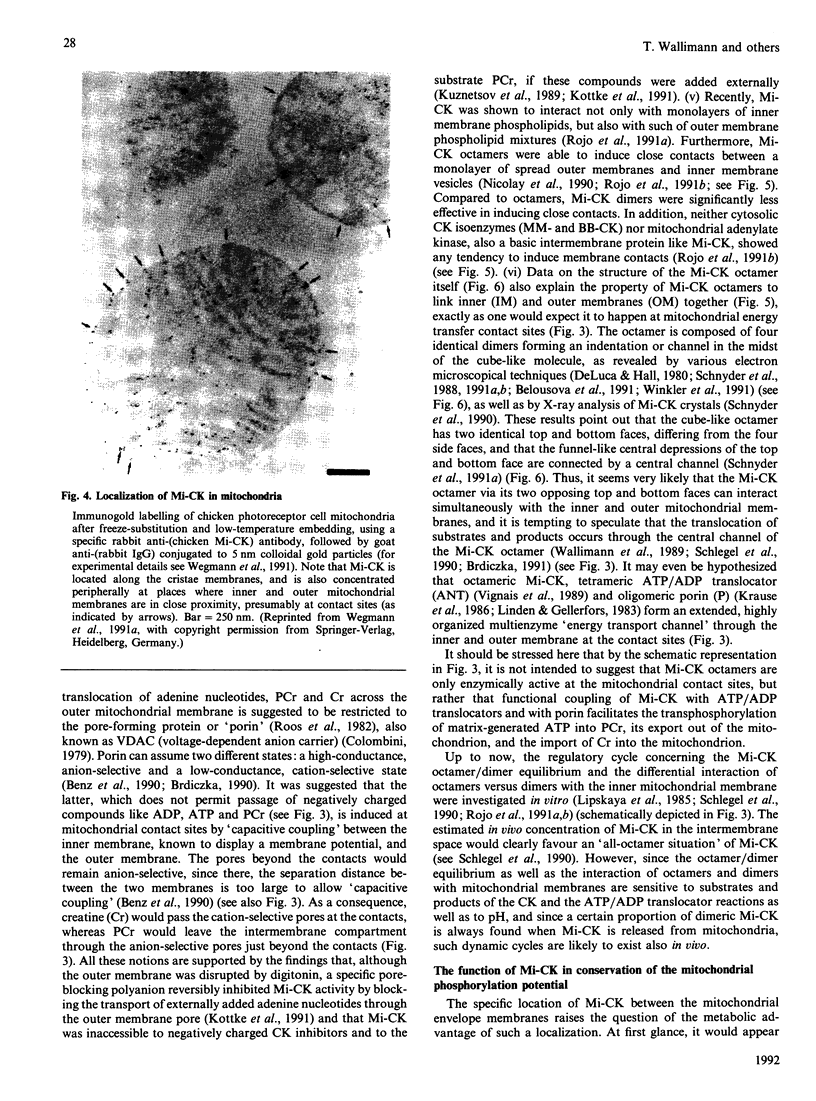
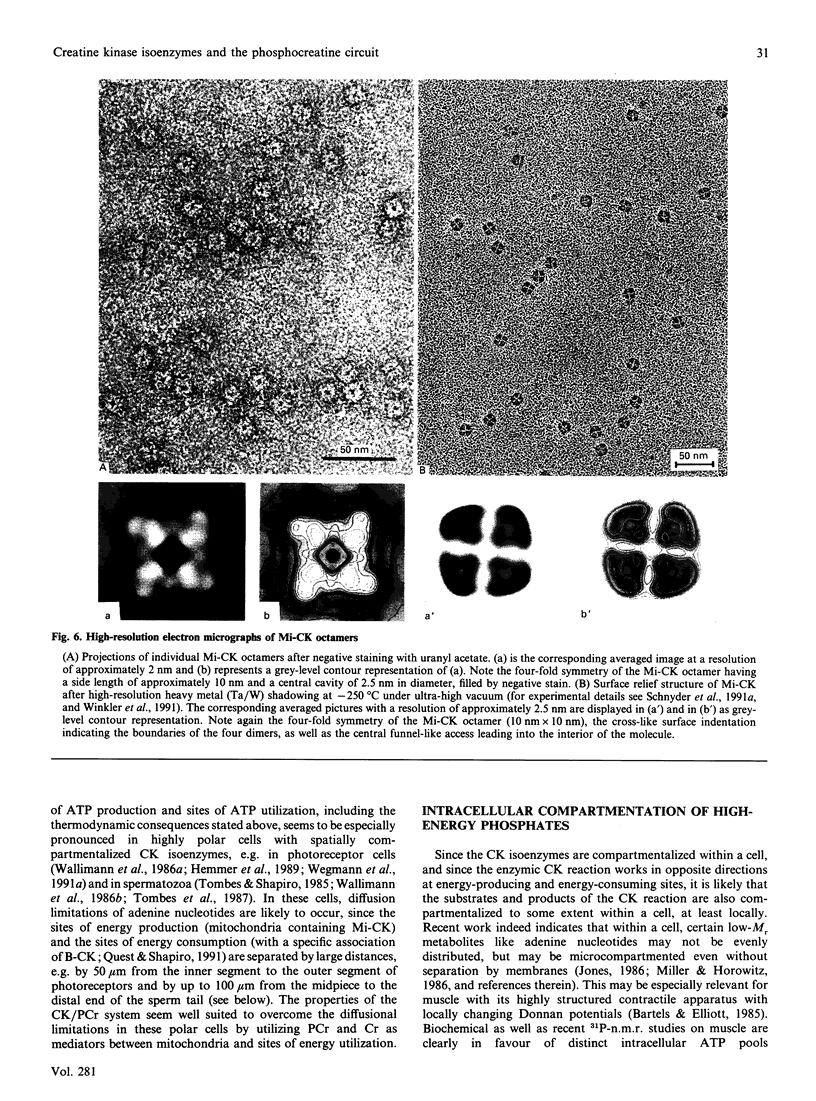
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Adams V., Bosch W., Schlegel J., Wallimann T., Brdiczka D. Further characterization of contact sites from mitochondria of different tissues: topology of peripheral kinases. Biochim Biophys Acta. 1989 Jun 6;981(2):213–225. doi: 10.1016/0005-2736(89)90031-x. [DOI] [PubMed] [Google Scholar]
- Altschuld R. A., Brierley G. P. Interaction between the creatine kinase of heart mitochondria and oxidative phosphorylation. J Mol Cell Cardiol. 1977 Nov;9(11):875–896. doi: 10.1016/s0022-2828(77)80009-6. [DOI] [PubMed] [Google Scholar]
- Apple F. S., Rogers M. A. Mitochondrial creatine kinase activity alterations in skeletal muscle during long-distance running. J Appl Physiol (1985) 1986 Aug;61(2):482–485. doi: 10.1152/jappl.1986.61.2.482. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of aldolase and triosephosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase. Eur J Biochem. 1970 Aug;15(2):360–366. doi: 10.1111/j.1432-1033.1970.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Arrio-Dupont M. An example of substrate channeling between co-immobilized enzymes. Coupled activity of myosin ATPase and creatine kinase bound to frog heart myofilaments. FEBS Lett. 1988 Nov 21;240(1-2):181–185. doi: 10.1016/0014-5793(88)80364-8. [DOI] [PubMed] [Google Scholar]
- Ashby B., Frieden C., Bischoff R. Immunofluorescent and histochemical localization of AMP deaminase in skeletal muscle. J Cell Biol. 1979 May;81(2):361–373. doi: 10.1083/jcb.81.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbitt P. C., West B. L., Buechter D. D., Kuntz I. D., Kenyon G. L. Removal of a proteolytic activity associated with aggregates formed from expression of creatine kinase in Escherichia coli leads to improved recovery of active enzyme. Biotechnology (N Y) 1990 Oct;8(10):945–949. doi: 10.1038/nbt1090-945. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Balaban R. S. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990 Mar;258(3 Pt 1):C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Barbour R. L., Ribaudo J., Chan S. H. Effect of creatine kinase activity on mitochondrial ADP/ATP transport. Evidence for a functional interaction. J Biol Chem. 1984 Jul 10;259(13):8246–8251. [PubMed] [Google Scholar]
- Barbour R. L., Sotak C. H., Levy G. C., Chan S. H. Use of gated perfusion to study early effects of anoxia on cardiac energy metabolism: a new 31P NMR method. Biochemistry. 1984 Dec 4;23(25):6053–6062. doi: 10.1021/bi00320a024. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J., Braceras A., Caldironi H. A., Mieskes G., Moser H., Toren E. C., Jr, Roque M. E., Wallimann T., Zechel A. Isolation and characterization of acetylcholine receptor membrane-associated (nonreceptor v2-protein) and soluble electrocyte creatine kinases. J Biol Chem. 1985 Mar 10;260(5):3024–3034. [PubMed] [Google Scholar]
- Barrantes F. J., Mieskes G., Wallimann T. A membrane-associated creatine kinase (EC 2.7.3.2) identified as an acidic species of the non-receptor, peripheral nu-proteins in Torpedo acetylcholine receptor membranes. FEBS Lett. 1983 Feb 21;152(2):270–276. doi: 10.1016/0014-5793(83)80394-9. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J., Mieskes G., Wallimann T. Creatine kinase activity in the Torpedo electrocyte and in the nonreceptor, peripheral v proteins from acetylcholine receptor-rich membranes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5440–5444. doi: 10.1073/pnas.80.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E. M., Elliott G. F. Donnan potentials from the A- and I-bands of glycerinated and chemically skinned muscles, relaxed and in rigor. Biophys J. 1985 Jul;48(1):61–76. doi: 10.1016/S0006-3495(85)83760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin R. J., Deamer D. W. A membrane-bound creatine phosphokinase in fragmented sarcoplasmic reticulum. J Biol Chem. 1970 Mar 25;245(6):1345–1347. [PubMed] [Google Scholar]
- Belousova L. V., Fedosov S. N., Orlova E. V., Stel'mashchuk VYa The structural features of beef heart mitochondrial creatine kinase. Biochem Int. 1991 May;24(1):51–58. [PubMed] [Google Scholar]
- Benz R., Kottke M., Brdiczka D. The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim Biophys Acta. 1990 Mar;1022(3):311–318. doi: 10.1016/0005-2736(90)90279-w. [DOI] [PubMed] [Google Scholar]
- Berger S. J., DeVries G. W., Carter J. G., Schulz D. W., Passonneau P. N., Lowry O. H., Ferrendelli J. A. The distribution of the components of the cyclic GMP cycle in retina. J Biol Chem. 1980 Apr 10;255(7):3128–3133. [PubMed] [Google Scholar]
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Fonyo A. The possible role of the mitochondrial bound creatine kinase in regulation of mitochondrial respiration. Biochem Biophys Res Commun. 1966 Mar 8;22(5):597–602. doi: 10.1016/0006-291x(66)90317-2. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Biermans W., Bakker A., Jacob W. Contact site between inner and outer mitochondrial membrane: a dynamic microcompartment for creatine kinase activity. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):225–228. doi: 10.1016/0005-2728(90)90254-2. [DOI] [PubMed] [Google Scholar]
- Biermans W., Bernaert I., De Bie M., Nijs B., Jacob W. Ultrastructural localisation of creatine kinase activity in the contact sites between inner and outer mitochondrial membranes of rat myocardium. Biochim Biophys Acta. 1989 Apr 17;974(1):74–80. doi: 10.1016/s0005-2728(89)80167-7. [DOI] [PubMed] [Google Scholar]
- Binderman I., Harel S., Earon Y., Tomer A., Weisman Y., Kaye A. M., Sömjen D. Acute stimulation of creatine kinase activity by vitamin D metabolites in the developing cerebellum. Biochim Biophys Acta. 1988 Oct 28;972(1):9–16. doi: 10.1016/0167-4889(88)90096-1. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Blum H., Balschi J. A., Johnson R. G., Jr Coupled in vivo activity of creatine phosphokinase and the membrane-bound (Na+,K+)-ATPase in the resting and stimulated electric organ of the electric fish Narcine brasiliensis. J Biol Chem. 1991 Jun 5;266(16):10254–10259. [PubMed] [Google Scholar]
- Blum H., Nioka S., Johnson R. G., Jr Activation of the Na+, K(+)-ATPase in Narcine brasiliensis. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1247–1251. doi: 10.1073/pnas.87.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. Studies on the mitochondrially bound form of rat brain creatine kinase. Biochem J. 1978 Jan 15;170(1):145–151. doi: 10.1042/bj1700145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni E. Role of creatine phosphate in the discharge of the electric organ of Torpedo marmorata. J Neurochem. 1984 Sep;43(3):795–798. doi: 10.1111/j.1471-4159.1984.tb12801.x. [DOI] [PubMed] [Google Scholar]
- Bowditch J., Nigdikar S., Brown A. K., Dow J. W. 5'-Nucleotidase activity of isolated mature rat cardiac myocytes. Biochim Biophys Acta. 1985 Apr 22;845(1):21–26. doi: 10.1016/0167-4889(85)90049-7. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J. Nerve-specific enolase and creatine phosphokinase in axonal transport: soluble proteins and the axoplasmic matrix. Cell. 1981 Feb;23(2):515–523. doi: 10.1016/0092-8674(81)90147-1. [DOI] [PubMed] [Google Scholar]
- Brdiczka D. Contact sites between mitochondrial envelope membranes. Structure and function in energy- and protein-transfer. Biochim Biophys Acta. 1991 Nov 13;1071(3):291–312. doi: 10.1016/0304-4157(91)90018-r. [DOI] [PubMed] [Google Scholar]
- Brdiczka D. Interaction of mitochondrial porin with cytosolic proteins. Experientia. 1990 Feb 15;46(2):161–167. doi: 10.1007/BF02027312. [DOI] [PubMed] [Google Scholar]
- Brindle K., Braddock P., Fulton S. 31P NMR measurements of the ADP concentration in yeast cells genetically modified to express creatine kinase. Biochemistry. 1990 Apr 3;29(13):3295–3302. doi: 10.1021/bi00465a021. [DOI] [PubMed] [Google Scholar]
- Bronstein W. W., Knull H. R. Interaction of muscle glycolytic enzymes with thin filament proteins. Can J Biochem. 1981 Jul;59(7):494–499. doi: 10.1139/o81-069. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Storey K. B. Reevaluation of the "glycolytic complex" in muscle: a multitechnique approach using trout white muscle. Arch Biochem Biophys. 1988 Nov 15;267(1):13–22. doi: 10.1016/0003-9861(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Suelter C. H. Association of chicken mitochondrial creatine kinase with the inner mitochondrial membrane. Arch Biochem Biophys. 1987 Feb 15;253(1):122–132. doi: 10.1016/0003-9861(87)90644-8. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Suelter C. H. Compartmented coupling of chicken heart mitochondrial creatine kinase to the nucleotide translocase requires the outer mitochondrial membrane. Arch Biochem Biophys. 1987 Aug 15;257(1):144–153. doi: 10.1016/0003-9861(87)90553-4. [DOI] [PubMed] [Google Scholar]
- Brosnan M. J., Chen L. H., Wheeler C. E., Van Dyke T. A., Koretsky A. P. Phosphocreatine protects ATP from a fructose load in transgenic mouse liver expressing creatine kinase. Am J Physiol. 1991 Jun;260(6 Pt 1):C1191–C1200. doi: 10.1152/ajpcell.1991.260.6.C1191. [DOI] [PubMed] [Google Scholar]
- Brumback R. A., Gerst J. W., Knull H. R. High energy phosphate depletion in a model of defective muscle glycolysis. Muscle Nerve. 1983 Jan;6(1):52–55. doi: 10.1002/mus.880060109. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Bücheler K., Adams V., Brdiczka D. Localization of the ATP/ADP translocator in the inner membrane and regulation of contact sites between mitochondrial envelope membranes by ADP. A study on freeze-fractured isolated liver mitochondria. Biochim Biophys Acta. 1991 Feb 8;1056(3):233–242. doi: 10.1016/s0005-2728(05)80054-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Cadoux-Hudson T. A., Blackledge M. J., Radda G. K. Imaging of human brain creatine kinase activity in vivo. FASEB J. 1989 Dec;3(14):2660–2666. doi: 10.1096/fasebj.3.14.2629743. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Chabre M., Deterre P. Molecular mechanism of visual transduction. Eur J Biochem. 1989 Feb 1;179(2):255–266. doi: 10.1111/j.1432-1033.1989.tb14549.x. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Clark B. J., Maris J., Kent J., Nioka S., Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Kent J., McCully K., Nioka S., Clark B. J., Maris J. M., Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. L., Fine J. S., Emery M., Weaver D., Reichenbach D., Clayson K. J. Regional creatine kinase, adenylate kinase, and lactate dehydrogenase in normal canine brain. Stroke. 1988 Feb;19(2):251–255. doi: 10.1161/01.str.19.2.251. [DOI] [PubMed] [Google Scholar]
- Chen L. H., Babbitt P. C., Vásquez J. R., West B. L., Kenyon G. L. Cloning and expression of functional rabbit muscle creatine kinase in Escherichia coli. Addressing the problem of microheterogeneity. J Biol Chem. 1991 Jun 25;266(18):12053–12057. [PubMed] [Google Scholar]
- Cheneval D., Carafoli E. Identification and primary structure of the cardiolipin-binding domain of mitochondrial creatine kinase. Eur J Biochem. 1988 Jan 15;171(1-2):1–9. doi: 10.1111/j.1432-1033.1988.tb13750.x. [DOI] [PubMed] [Google Scholar]
- Chevli R., Fitch C. D. beta-Guanidinopropionate and phosphorylated beta-guanidinopropionate as substrates for creatine kinase. Biochem Med. 1979 Apr;21(2):162–167. doi: 10.1016/0006-2944(79)90068-1. [DOI] [PubMed] [Google Scholar]
- Chida K., Kasahara K., Tsunenaga M., Kohno Y., Yamada S., Ohmi S., Kuroki T. Purification and identification of creatine phosphokinase B as a substrate of protein kinase C in mouse skin in vivo. Biochem Biophys Res Commun. 1990 Nov 30;173(1):351–357. doi: 10.1016/s0006-291x(05)81064-2. [DOI] [PubMed] [Google Scholar]
- Chida K., Tsunenaga M., Kasahara K., Kohno Y., Kuroki T. Regulation of creatine phosphokinase B activity by protein kinase C. Biochem Biophys Res Commun. 1990 Nov 30;173(1):346–350. doi: 10.1016/s0006-291x(05)81063-0. [DOI] [PubMed] [Google Scholar]
- Christen R., Schackmann R. W., Dahlquist F. W., Shapiro B. M. 31P-NMR analysis of sea urchin sperm activation. Reversible formation of high energy phosphate compounds by changes in intracellular pH. Exp Cell Res. 1983 Nov;149(1):289–294. doi: 10.1016/0014-4827(83)90400-7. [DOI] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Connett R. J. Analysis of metabolic control: new insights using scaled creatine kinase model. Am J Physiol. 1988 Jun;254(6 Pt 2):R949–R959. doi: 10.1152/ajpregu.1988.254.6.R949. [DOI] [PubMed] [Google Scholar]
- Cooper J., Trinick J. Binding and location of AMP deaminase in rabbit psoas muscle myofibrils. J Mol Biol. 1984 Jul 25;177(1):137–152. doi: 10.1016/0022-2836(84)90061-5. [DOI] [PubMed] [Google Scholar]
- Dillon P. F., Clark J. F. The theory of diazymes and functional coupling of pyruvate kinase and creatine kinase. J Theor Biol. 1990 Mar 22;143(2):275–284. doi: 10.1016/s0022-5193(05)80272-3. [DOI] [PubMed] [Google Scholar]
- Doorey A. J., Barry W. H. The effects of inhibition of oxidative phosphorylation and glycolysis on contractility and high-energy phosphate content in cultured chick heart cells. Circ Res. 1983 Aug;53(2):192–201. doi: 10.1161/01.res.53.2.192. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Loctin F., Marsal J., Muller D., Parducz A., Rabasseda X. Energy metabolism and quantal acetylcholine release: effects of botulinum toxin, 1-fluoro-2,4-dinitrobenzene, and diamide in the Torpedo electric organ. J Neurochem. 1988 Feb;50(2):431–439. doi: 10.1111/j.1471-4159.1988.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Eggleton G. P. Further observations on phosphagen. J Physiol. 1928 Mar 30;65(1):15–24. doi: 10.1113/jphysiol.1928.sp002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington W. R. Phosphocreatine represents a thermodynamic and functional improvement over other muscle phosphagens. J Exp Biol. 1989 May;143:177–194. doi: 10.1242/jeb.143.1.177. [DOI] [PubMed] [Google Scholar]
- Eppenberger-Eberhardt M., Riesinger I., Messerli M., Schwarb P., Müller M., Eppenberger H. M., Wallimann T. Adult rat cardiomyocytes cultured in creatine-deficient medium display large mitochondria with paracrystalline inclusions, enriched for creatine kinase. J Cell Biol. 1991 Apr;113(2):289–302. doi: 10.1083/jcb.113.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Eppenberger H. M., Perriard J. C., Wallimann T. Analysis of creatine kinase isozymes during muscle differentiation. Isozymes Curr Top Biol Med Res. 1983;7:19–38. [PubMed] [Google Scholar]
- Erickson-Viitanen S., Geiger P. J., Viitanen P., Bessman S. P. Compartmentation of mitochondrial creatine phosphokinase. II. The importance of the outer mitochondrial membrane for mitochondrial compartmentation. J Biol Chem. 1982 Dec 10;257(23):14405–14411. [PubMed] [Google Scholar]
- Erickson-Viitanen S., Viitanen P., Geiger P. J., Yang W. C., Bessman S. P. Compartmentation of mitochondrial creatine phosphokinase. I. Direct demonstration of compartmentation with the use of labeled precursors. J Biol Chem. 1982 Dec 10;257(23):14395–14404. [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Pfitzer G. Rapid myosin phosphorylation transients in phasic contractions in chicken gizzard smooth muscle. FEBS Lett. 1989 Nov 20;258(1):59–62. doi: 10.1016/0014-5793(89)81615-1. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Jellinek M., Mueller E. J. Experimental depletion of creatine and phosphocreatine from skeletal muscle. J Biol Chem. 1974 Feb 25;249(4):1060–1063. [PubMed] [Google Scholar]
- Fitch C. D., Shields R. P., Payne W. F., Dacus J. M. Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J Biol Chem. 1968 Apr 25;243(8):2024–2027. [PubMed] [Google Scholar]
- Font B., Eichenberger D., Goldschmidt D., Vial C. Interaction of creatine kinase and hexokinase with the mitochondrial membranes, and self-association of creatine kinase: crosslinking studies. Mol Cell Biochem. 1987 Dec;78(2):131–140. doi: 10.1007/BF00229687. [DOI] [PubMed] [Google Scholar]
- Font B., Vial C., Goldschmidt D., Eichenberger D., Gautheron D. C. Heart mitochondrial creatine kinase solubilization. Effect of mitochondrial swelling and SH group reagents. Arch Biochem Biophys. 1981 Nov;212(1):195–203. doi: 10.1016/0003-9861(81)90359-3. [DOI] [PubMed] [Google Scholar]
- Friedhoff A. J., Lerner M. H. Creatine kinase isoenzyme associated with synaptosomal membrane and synaptic vesicles. Life Sci. 1977 Mar 1;20(5):867–873. doi: 10.1016/0024-3205(77)90039-x. [DOI] [PubMed] [Google Scholar]
- From A. H., Zimmer S. D., Michurski S. P., Mohanakrishnan P., Ulstad V. K., Thoma W. J., Uğurbil K. Regulation of the oxidative phosphorylation rate in the intact cell. Biochemistry. 1990 Apr 17;29(15):3731–3743. doi: 10.1021/bi00467a020. [DOI] [PubMed] [Google Scholar]
- Gellerich F. N., Schlame M., Bohnensack R., Kunz W. Dynamic compartmentation of adenine nucleotides in the mitochondrial intermembrane space of rat-heart mitochondria. Biochim Biophys Acta. 1987 Feb 11;890(2):117–126. doi: 10.1016/0005-2728(87)90012-0. [DOI] [PubMed] [Google Scholar]
- Gellerich F., Saks V. A. Control of heart mitochondrial oxygen consumption by creatine kinase: the importance of enzyme localization. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1473–1481. doi: 10.1016/0006-291x(82)90954-8. [DOI] [PubMed] [Google Scholar]
- Gerbitz K. D., Deufel T., Summer J., Thallemer J., Wieland O. H. Brain specific proteins: creatine kinase BB isoenzyme is cochromatographed during preparation of neuron-specific enolase from human brain. Clin Chim Acta. 1983 Sep 30;133(2):233–239. doi: 10.1016/0009-8981(83)90410-2. [DOI] [PubMed] [Google Scholar]
- Gercken G., Schlette U. Metabolite status of the heart in acute insufficiency due to 1-fluoro-2,4-dinitrobenzene. Experientia. 1968 Jan 15;24(1):17–19. doi: 10.1007/BF02136764. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Perriard J. C., Changeux J. P. Complete nucleotide sequence of Torpedo marmorata mRNA coding for the 43,000-dalton nu 2 protein: muscle-specific creatine kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7313–7317. doi: 10.1073/pnas.81.23.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R., Spitzer E., Kupriyanov V. V., Saks V. A., Repke K. R. Coordinate interplay between (Na+ + K+)-ATPase and creatine phosphokinase optimizes (Na+/K+)-antiport across the membrane of vesicles formed from the plasma membrane of cardiac muscle cell. Biochim Biophys Acta. 1980 Dec 2;603(1):142–156. doi: 10.1016/0005-2736(80)90397-1. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S., Mathes P., Ravens K. G. Functional compartmentation of ATP and creatine phosphate in heart muscle. J Mol Cell Cardiol. 1970 Sep;1(3):325–339. doi: 10.1016/0022-2828(70)90009-x. [DOI] [PubMed] [Google Scholar]
- Gysin R., Yost B., Flanagan S. D. Creatine kinase isoenzymes in Torpedo californica: absence of the major brain isoenzyme from nicotinic acetylcholine receptor membranes. Biochemistry. 1986 Mar 25;25(6):1271–1278. doi: 10.1021/bi00354a012. [DOI] [PubMed] [Google Scholar]
- Gyulai L., Roth Z., Leigh J. S., Jr, Chance B. Bioenergetic studies of mitochondrial oxidative phosphorylation using 31phosphorus NMR. J Biol Chem. 1985 Apr 10;260(7):3947–3954. [PubMed] [Google Scholar]
- Haas R. C., Korenfeld C., Zhang Z. F., Perryman B., Roman D., Strauss A. W. Isolation and characterization of the gene and cDNA encoding human mitochondrial creatine kinase. J Biol Chem. 1989 Feb 15;264(5):2890–2897. [PubMed] [Google Scholar]
- Haas R. C., Strauss A. W. Separate nuclear genes encode sarcomere-specific and ubiquitous human mitochondrial creatine kinase isoenzymes. J Biol Chem. 1990 Apr 25;265(12):6921–6927. [PubMed] [Google Scholar]
- Hall N., DeLuca M. Developmental changes in creatine phosphokinase isoenzymes in neonatal mouse hearts. Biochem Biophys Res Commun. 1975 Oct 6;66(3):988–994. doi: 10.1016/0006-291x(75)90737-8. [DOI] [PubMed] [Google Scholar]
- Hall N., DeLuca M. Electrophoretic separation and quantitation of creatine kinase isozymes. Anal Biochem. 1976 Dec;76(2):561–567. doi: 10.1016/0003-2697(76)90350-x. [DOI] [PubMed] [Google Scholar]
- Hall N., Deluca M. Binding of creatine kinase to heart and liver mitochondria in vitro. Arch Biochem Biophys. 1980 May;201(2):674–677. doi: 10.1016/0003-9861(80)90558-5. [DOI] [PubMed] [Google Scholar]
- Hamada M., Kuby S. A. Studies on adenosine triphosphate transphosphorylases. XIII. Kinetic properties of the crystalline rabbit muscle ATP-AMP transphorphorylase (adenylate kinase) and a comparison with the crystalline calf muscle and liver adenylate kinases. Arch Biochem Biophys. 1978 Oct;190(2):772–779. doi: 10.1016/0003-9861(78)90338-7. [DOI] [PubMed] [Google Scholar]
- Hamburg R. J., Friedman D. L., Olson E. N., Ma T. S., Cortez M. D., Goodman C., Puleo P. R., Perryman M. B. Muscle creatine kinase isoenzyme expression in adult human brain. J Biol Chem. 1990 Apr 15;265(11):6403–6409. [PubMed] [Google Scholar]
- Hebisch S., Sies H., Soboll S. Function dependent changes in the subcellular distribution of high energy phosphates in fast and slow rat skeletal muscles. Pflugers Arch. 1986 Jan;406(1):20–24. doi: 10.1007/BF00582947. [DOI] [PubMed] [Google Scholar]
- Hellstrand P., Vogel H. J. Phosphagens and intracellular pH in intact rabbit smooth muscle studied by 31P-NMR. Am J Physiol. 1985 Mar;248(3 Pt 1):C320–C329. doi: 10.1152/ajpcell.1985.248.3.C320. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Hoerter J. A., Kuznetsov A., Ventura-Clapier R. Functional development of the creatine kinase system in perinatal rabbit heart. Circ Res. 1991 Sep;69(3):665–676. doi: 10.1161/01.res.69.3.665. [DOI] [PubMed] [Google Scholar]
- Hoerter J. A., Lauer C., Vassort G., Guéron M. Sustained function of normoxic hearts depleted in ATP and phosphocreatine: a 31P-NMR study. Am J Physiol. 1988 Aug;255(2 Pt 1):C192–C201. doi: 10.1152/ajpcell.1988.255.2.C192. [DOI] [PubMed] [Google Scholar]
- Holtzman D., Herman M. M., Desautel M., Lewiston N. Effects of altered osmolality on respiration and morphology of mitochondria from the developing brain. J Neurochem. 1979 Aug;33(2):453–460. doi: 10.1111/j.1471-4159.1979.tb05175.x. [DOI] [PubMed] [Google Scholar]
- Holtzman D., McFarland E. W., Jacobs D., Offutt M. C., Neuringer L. J. Maturational increase in mouse brain creatine kinase reaction rates shown by phosphorus magnetic resonance. Brain Res Dev Brain Res. 1991 Feb 22;58(2):181–188. doi: 10.1016/0165-3806(91)90004-3. [DOI] [PubMed] [Google Scholar]
- Holtzman D., McFarland E., Moerland T., Koutcher J., Kushmerick M. J., Neuringer L. J. Brain creatine phosphate and creatine kinase in mice fed an analogue of creatine. Brain Res. 1989 Mar 27;483(1):68–77. doi: 10.1016/0006-8993(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Hossle J. P., Schlegel J., Wegmann G., Wyss M., Böhlen P., Eppenberger H. M., Wallimann T., Perriard J. C. Distinct tissue specific mitochondrial creatine kinases from chicken brain and striated muscle with a conserved CK framework. Biochem Biophys Res Commun. 1988 Feb 29;151(1):408–416. doi: 10.1016/0006-291x(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Hovius R., Lambrechts H., Nicolay K., de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta. 1990 Jan 29;1021(2):217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Berkich D., Williams G. D., LaNoue K. F., Briggs R. W. 31P NMR visibility and characterization of rat liver mitochondrial matrix adenine nucleotides. Biochemistry. 1989 May 16;28(10):4325–4332. doi: 10.1021/bi00436a030. [DOI] [PubMed] [Google Scholar]
- Ikeda K. Localization of brain type creatine kinase in kidney epithelial cell subpopulations in rat. Experientia. 1988 Sep 15;44(9):734–735. doi: 10.1007/BF01959143. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Tomonaga M. Creatine kinase immunoreactivity: localization in nerve terminals in the hypothalamic area and superior colliculus of the mouse brain. Neurosci Lett. 1988 Feb 15;85(1):51–55. doi: 10.1016/0304-3940(88)90427-2. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Tomonaga M. The presence of creatine kinase (CK)-immunoreactive neurons in the zona incerta and lateral hypothalamic area of the mouse brain. Brain Res. 1987 Dec 1;435(1-2):348–350. doi: 10.1016/0006-8993(87)91622-2. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- Ingwall J. S., Atkinson D. E., Clarke K., Fetters J. K. Energetic correlates of cardiac failure: changes in the creatine kinase system in the failing myocardium. Eur Heart J. 1990 Apr;11 (Suppl B):108–115. doi: 10.1093/eurheartj/11.suppl_b.108. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Evidence for compartmentation of high energy phosphagens in smooth muscle. Prog Clin Biol Res. 1989;315:417–428. [PubMed] [Google Scholar]
- Ishida Y., Wyss M., Hemmer W., Wallimann T. Identification of creatine kinase isoenzymes in the guinea-pig. Presence of mitochondrial creatine kinase in smooth muscle. FEBS Lett. 1991 May 20;283(1):37–43. doi: 10.1016/0014-5793(91)80548-h. [DOI] [PubMed] [Google Scholar]
- Iyengar M. R. Creatine kinase as an intracellular regulator. J Muscle Res Cell Motil. 1984 Oct;5(5):527–534. doi: 10.1007/BF00713259. [DOI] [PubMed] [Google Scholar]
- Iyengar M. R., Fluellen C. E., Iyengar C. Creatine kinase from the bovine myometrium: purification and characterization. J Muscle Res Cell Motil. 1982 Jun;3(2):231–246. doi: 10.1007/BF00711944. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Heldt H. W., Klingenberg M. High activity of creatine kinase in mitochondria from muscle and brain and evidence for a separate mitochondrial isoenzyme of creatine kinase. Biochem Biophys Res Commun. 1964 Aug 11;16(6):516–521. doi: 10.1016/0006-291x(64)90185-8. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Diffley D. M. Creatine kinase of heart mitochondria. Control of oxidative phosphorylation by the extramitochondrial concentrations of creatine and phosphocreatine. J Biol Chem. 1986 Dec 15;261(35):16579–16583. [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973 Jul 10;248(13):4803–4810. [PubMed] [Google Scholar]
- Jacobus W. E., Moreadith R. W., Vandegaer K. M. Control of heart oxidative phosphorylation by creatine kinase in mitochondrial membranes. Ann N Y Acad Sci. 1983;414:73–89. doi: 10.1111/j.1749-6632.1983.tb31676.x. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E. Respiratory control and the integration of heart high-energy phosphate metabolism by mitochondrial creatine kinase. Annu Rev Physiol. 1985;47:707–725. doi: 10.1146/annurev.ph.47.030185.003423. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E. Theoretical support for the heart phosphocreatine energy transport shuttle based on the intracellular diffusion limited mobility of ADP. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1035–1041. doi: 10.1016/0006-291x(85)91240-9. [DOI] [PubMed] [Google Scholar]
- James P., Wyss M., Lutsenko S., Wallimann T., Carafoli E. ATP binding site of mitochondrial creatine kinase. Affinity labelling of Asp-335 with C1RATP. FEBS Lett. 1990 Oct 29;273(1-2):139–143. doi: 10.1016/0014-5793(90)81069-z. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Hill M. L., Mayer S. E. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res. 1981 Oct;49(4):892–900. doi: 10.1161/01.res.49.4.892. [DOI] [PubMed] [Google Scholar]
- Jockers-Wretou E., Giebel W., Pfleiderer G. Immunohistochemische Lokalisierung der Isoenzyme der Creatinkinase im menschlichen Gewebe. Histochemistry. 1977 Oct 3;54(1):83–95. doi: 10.1007/BF00493332. [DOI] [PubMed] [Google Scholar]
- Jones D. P. Intracellular diffusion gradients of O2 and ATP. Am J Physiol. 1986 May;250(5 Pt 1):C663–C675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- Kammermeier H. Why do cells need phosphocreatine and a phosphocreatine shuttle. J Mol Cell Cardiol. 1987 Jan;19(1):115–118. doi: 10.1016/s0022-2828(87)80550-3. [DOI] [PubMed] [Google Scholar]
- Kartner N., Ling V. Multidrug resistance in cancer. Sci Am. 1989 Mar;260(3):44–51. doi: 10.1038/scientificamerican0389-44. [DOI] [PubMed] [Google Scholar]
- Kato K., Suzuki F., Shimizu A., Shinohara H., Semba R. Highly sensitive immunoassay for rat brain-type creatine kinase: determination in isolated Purkinje cells. J Neurochem. 1986 Jun;46(6):1783–1788. doi: 10.1111/j.1471-4159.1986.tb08496.x. [DOI] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Gordon P. V. Discrete subcellular localization of a cytoplasmic and a mitochondrial isozyme of creatine kinase in intestinal epithelial cells. Cell Motil Cytoskeleton. 1991;19(3):169–179. doi: 10.1002/cm.970190305. [DOI] [PubMed] [Google Scholar]
- Kenyon G. L., Reed G. H. Creatine kinase: structure-activity relationships. Adv Enzymol Relat Areas Mol Biol. 1983;54:367–426. doi: 10.1002/9780470122990.ch6. [DOI] [PubMed] [Google Scholar]
- Khan M. A. Effect of calcium on creatine kinase activity of cerebellum. Histochemistry. 1976 Jul 30;48(1):29–32. doi: 10.1007/BF00489713. [DOI] [PubMed] [Google Scholar]
- Khan M. A., Holt P. G., Papadimitriou J. M., Knight J. O., Kakulas B. A. Creatine kinase, a histochemical study by the gelatin film-lead precipitation technique. Histochemie. 1972;32(1):49–58. doi: 10.1007/BF00277470. [DOI] [PubMed] [Google Scholar]
- Klein S. C., Haas R. C., Perryman M. B., Billadello J. J., Strauss A. W. Regulatory element analysis and structural characterization of the human sarcomeric mitochondrial creatine kinase gene. J Biol Chem. 1991 Sep 25;266(27):18058–18065. [PubMed] [Google Scholar]
- Klingenberg M. Muskelmitochondrien. Ergeb Physiol. 1964;55:131–189. [PubMed] [Google Scholar]
- Klingenberg M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. J Membr Biol. 1980 Sep 30;56(2):97–105. doi: 10.1007/BF01875961. [DOI] [PubMed] [Google Scholar]
- Knoll G., Brdiczka D. Changes in freeze-fractured mitochondrial membranes correlated to their energetic state. Dynamic interactions of the boundary membranes. Biochim Biophys Acta. 1983 Aug 24;733(1):102–110. doi: 10.1016/0005-2736(83)90095-0. [DOI] [PubMed] [Google Scholar]
- Koretsky A. P., Basus V. J., James T. L., Klein M. P., Weiner M. W. Detection of exchange reactions involving small metabolite pools using NMR magnetization transfer techniques: relevance to subcellular compartmentation of creatine kinase. Magn Reson Med. 1985 Dec;2(6):586–594. doi: 10.1002/mrm.1910020610. [DOI] [PubMed] [Google Scholar]
- Koretsky A. P., Brosnan M. J., Chen L. H., Chen J. D., Van Dyke T. NMR detection of creatine kinase expressed in liver of transgenic mice: determination of free ADP levels. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3112–3116. doi: 10.1073/pnas.87.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretsky A. P., Traxler B. A. The B isozyme of creatine kinase is active as a fusion protein in Escherichia coli: in vivo detection by 31P NMR. FEBS Lett. 1989 Jan 16;243(1):8–12. doi: 10.1016/0014-5793(89)81206-2. [DOI] [PubMed] [Google Scholar]
- Koretsky A. P., Wang S., Klein M. P., James T. L., Weiner M. W. 31P NMR saturation transfer measurements of phosphorus exchange reactions in rat heart and kidney in situ. Biochemistry. 1986 Jan 14;25(1):77–84. doi: 10.1021/bi00349a012. [DOI] [PubMed] [Google Scholar]
- Kottke M., Adam V., Riesinger I., Bremm G., Bosch W., Brdiczka D., Sandri G., Panfili E. Mitochondrial boundary membrane contact sites in brain: points of hexokinase and creatine kinase location, and control of Ca2+ transport. Biochim Biophys Acta. 1988 Aug 17;935(1):87–102. doi: 10.1016/0005-2728(88)90111-9. [DOI] [PubMed] [Google Scholar]
- Kottke M., Adams V., Wallimann T., Nalam V. K., Brdiczka D. Location and regulation of octameric mitochondrial creatine kinase in the contact sites. Biochim Biophys Acta. 1991 Jan 30;1061(2):215–225. doi: 10.1016/0005-2736(91)90287-i. [DOI] [PubMed] [Google Scholar]
- Krause J., Hay R., Kowollik C., Brdiczka D. Cross-linking analysis of yeast mitochondrial outer membrane. Biochim Biophys Acta. 1986 Sep 11;860(3):690–698. doi: 10.1016/0005-2736(86)90568-7. [DOI] [PubMed] [Google Scholar]
- Kupriyanov V. V., Seppet E. K., Emelin I. V., Saks V. A. Phosphocretine production coupled to the glycolytic reactions in the cytosol of cardiac cells. Biochim Biophys Acta. 1980 Sep 5;592(2):197–210. doi: 10.1016/0005-2728(80)90181-4. [DOI] [PubMed] [Google Scholar]
- Kupriyanov V. V., Ya Steinschneider A., Ruuge E. K., Kapel'ko V. I., Yu Zueva M., Lakomkin V. L., Smirnov V. N., Saks V. A. Regulation of energy flux through the creatine kinase reaction in vitro and in perfused rat heart. 31P-NMR studies. Biochim Biophys Acta. 1984 Dec 11;805(4):319–331. doi: 10.1016/0167-4889(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. V., Khuchua Z. A., Vassil'eva E. V., Medved'eva N. V., Saks V. A. Heart mitochondrial creatine kinase revisited: the outer mitochondrial membrane is not important for coupling of phosphocreatine production to oxidative phosphorylation. Arch Biochem Biophys. 1989 Jan;268(1):176–190. doi: 10.1016/0003-9861(89)90578-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., SCHULZ D. W., CLOW J. E., CLARK J. R. Quantitative histochemistry of retina. II. Enzymes of glucose metabolism. J Biol Chem. 1961 Oct;236:2813–2820. [PubMed] [Google Scholar]
- Levin R. M., Longhurst P. A., Levin S. S., Haugaard N., Wein A. J. Creatine kinase activity of urinary bladder and skeletal muscle from control and streptozotocin-diabetic rats. Mol Cell Biochem. 1990 Sep 21;97(2):153–159. doi: 10.1007/BF00221057. [DOI] [PubMed] [Google Scholar]
- Levitskii D. O., Levchenko T. S., Saks V. A., Sharov V. G., Smirnov V. N. Funktsional'noe sopriazhenie mezhdu Ca2+-ATPazoi i kreatinfosfokinazoi v sarkoplazmaticheskom retikulume serdechnoi myshtsy. Biokhimiia. 1977 Oct;42(10):1766–1773. [PubMed] [Google Scholar]
- Levitsky D. O., Levchenko T. S., Saks V. A., Sharov V. G., Smirnov V. N. The role of creatine phosphokinase in supplying energy for the calcium pump system of heart sarcoplasmic reticulum. Membr Biochem. 1978;2(1):81–96. doi: 10.3109/09687687809063859. [DOI] [PubMed] [Google Scholar]
- Lim L., Hall C., Leung T., Mahadevan L., Whatley S. Neurone-specific enolase and creatine phosphokinase are protein components of rat brain synaptic plasma membranes. J Neurochem. 1983 Oct;41(4):1177–1182. doi: 10.1111/j.1471-4159.1983.tb09069.x. [DOI] [PubMed] [Google Scholar]
- Lindsey G. G., Diamond E. M. Evidence for significant quantities of creatine kinase MM isoenzyme in human brain. Biochim Biophys Acta. 1978 May 11;524(1):78–84. doi: 10.1016/0005-2744(78)90105-5. [DOI] [PubMed] [Google Scholar]
- Lindén M., Gellerfors P. Hydrodynamic properties of porin isolated from outer membranes of rat liver mitochondria. Biochim Biophys Acta. 1983 Dec 7;736(1):125–129. doi: 10.1016/0005-2736(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Lipskaya TYu, Trofimova M. E. Study on heart mitochondrial creatine kinase using a cross-linking bifunctional reagent. I. The binding involves the octameric form of the enzyme. Biochem Int. 1989 May;18(5):1029–1039. [PubMed] [Google Scholar]
- Lipskaya TYu, Trofimova M. E. Study on heart mitochondrial creatine kinase using a cross-linking bifunctional reagent. II. The binding sites for the creatine kinase octamer on mitochondrial membranes have different properties. Biochem Int. 1989 Jun;18(6):1149–1159. [PubMed] [Google Scholar]
- Luther P., Squire J. Three-dimensional structure of the vertebrate muscle M-region. J Mol Biol. 1978 Nov 5;125(3):313–324. doi: 10.1016/0022-2836(78)90405-9. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Whatley S. A., Leung T. K., Lim L. The brain isoform of a key ATP-regulating enzyme, creatine kinase, is a phosphoprotein. Biochem J. 1984 Aug 15;222(1):139–144. doi: 10.1042/bj2220139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. J Gen Physiol. 1985 Jul;86(1):135–165. doi: 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwood G. W., Rakusan K. A model for intracellular energy transport. Can J Physiol Pharmacol. 1982 Jan;60(1):98–102. doi: 10.1139/y82-016. [DOI] [PubMed] [Google Scholar]
- Maker H. S., Lehrer G. M., Silides D. J., Weiss C. Regional changes in cerebellar creatine phosphate metabolism during late maturation. Exp Neurol. 1973 Feb;38(2):295–300. doi: 10.1016/0014-4886(73)90153-2. [DOI] [PubMed] [Google Scholar]
- Manos P., Bryan G. K., Edmond J. Creatine kinase activity in postnatal rat brain development and in cultured neurons, astrocytes, and oligodendrocytes. J Neurochem. 1991 Jun;56(6):2101–2107. doi: 10.1111/j.1471-4159.1991.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Marcillat O., Goldschmidt D., Eichenberger D., Vial C. Only one of the two interconvertible forms of mitochondrial creatine kinase binds to heart mitoplasts. Biochim Biophys Acta. 1987 Feb 11;890(2):233–241. doi: 10.1016/0005-2728(87)90024-7. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Bland J. L., Gadian D. G., Radda G. K. A 31P-NMR saturation transfer study of the regulation of creatine kinase in the rat heart. Biochim Biophys Acta. 1982 Nov 17;721(3):312–320. doi: 10.1016/0167-4889(82)90084-2. [DOI] [PubMed] [Google Scholar]
- Maughan D., Wegner E. On the organization and diffusion of glycolytic enzymes in skeletal muscle. Prog Clin Biol Res. 1989;315:137–147. [PubMed] [Google Scholar]
- McAuliffe J. J., Perry S. B., Brooks E. E., Ingwall J. S. Kinetics of the creatine kinase reaction in neonatal rabbit heart: an empirical analysis of the rate equation. Biochemistry. 1991 Mar 12;30(10):2585–2593. doi: 10.1021/bi00224a004. [DOI] [PubMed] [Google Scholar]
- McAuliffe J. J., Perry S. B., Brooks E. E., Ingwall J. S. The kinetics of the creatine kinase reaction in neonatal rabbit heart: does the rate equation accurately describe the kinetics observed in the isolated perfused heart? Prog Clin Biol Res. 1989;315:581–592. [PubMed] [Google Scholar]
- McClellan G., Weisberg A., Winegrad S. Energy transport from mitochondria to myofibril by a creatine phosphate shuttle in cardiac cells. Am J Physiol. 1983 Nov;245(5 Pt 1):C423–C427. doi: 10.1152/ajpcell.1983.245.5.C423. [DOI] [PubMed] [Google Scholar]
- McGilvery R. W., Murray T. W. Calculated equilibria of phosphocreatine and adenosine phosphates during utilization of high energy phosphate by muscle. J Biol Chem. 1974 Sep 25;249(18):5845–5850. [PubMed] [Google Scholar]
- Meyer R. A. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988 Apr;254(4 Pt 1):C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Krilowicz B. L., Kushmerick M. J. Phosphagen and intracellular pH changes during contraction of creatine-depleted rat muscle. Am J Physiol. 1986 Feb;250(2 Pt 1):C264–C274. doi: 10.1152/ajpcell.1986.250.2.C264. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Sweeney H. L., Kushmerick M. J. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984 May;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Miller D. S., Horowitz S. B. Intracellular compartmentalization of adenosine triphosphate. J Biol Chem. 1986 Oct 25;261(30):13911–13915. [PubMed] [Google Scholar]
- Milner-White E. J., Watts D. C. Inhibition of adenosine 5'-triphosphate-creatine phosphotransferase by substrate-anion complexes. Evidence for the transition-state organization of the catalytic site. Biochem J. 1971 May;122(5):727–740. doi: 10.1042/bj1220727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommaerts W. F. Energetics of muscular contraction. Physiol Rev. 1969 Jul;49(3):427–508. doi: 10.1152/physrev.1969.49.3.427. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F., Wallner A. The break-down of adenosine triphosphate in the contraction cycle of the frog sartorius muscle. J Physiol. 1967 Nov;193(2):343–357. doi: 10.1113/jphysiol.1967.sp008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen C. T., van Zijl P. C., Frank J. A., Le Bihan D., Becker E. D. Functional magnetic resonance imaging in medicine and physiology. Science. 1990 Oct 5;250(4977):53–61. doi: 10.1126/science.2218514. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Jacobus W. E. Creatine kinase of heart mitochondria. Functional coupling of ADP transfer to the adenine nucleotide translocase. J Biol Chem. 1982 Jan 25;257(2):899–905. [PubMed] [Google Scholar]
- Murphy E., Gabel S. A., Funk A., London R. E. NMR observability of ATP: preferential depletion of cytosolic ATP during ischemia in perfused rat liver. Biochemistry. 1988 Jan 26;27(2):526–528. doi: 10.1021/bi00402a003. [DOI] [PubMed] [Google Scholar]
- Möller A., Hamprecht B. Creatine transport in cultured cells of rat and mouse brain. J Neurochem. 1989 Feb;52(2):544–550. doi: 10.1111/j.1471-4159.1989.tb09154.x. [DOI] [PubMed] [Google Scholar]
- Müller M., Moser R., Cheneval D., Carafoli E. Cardiolipin is the membrane receptor for mitochondrial creatine phosphokinase. J Biol Chem. 1985 Mar 25;260(6):3839–3843. [PubMed] [Google Scholar]
- Newsholme E. A., Beis I., Leech A. R., Zammit V. A. The role of creatine kinase and arginine kinase in muscle. Biochem J. 1978 Jun 15;172(3):533–537. doi: 10.1042/bj1720533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay K., Rojo M., Wallimann T., Demel R., Hovius R. The role of contact sites between inner and outer mitochondrial membrane in energy transfer. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):229–233. doi: 10.1016/0005-2728(90)90255-3. [DOI] [PubMed] [Google Scholar]
- Norwood W. I., Ingwall J. S., Norwood C. R., Fossel E. T. Developmental changes of creatine kinase metabolism in rat brain. Am J Physiol. 1983 Mar;244(3):C205–C210. doi: 10.1152/ajpcell.1983.244.3.C205. [DOI] [PubMed] [Google Scholar]
- Nunnally R. L., Hollis D. P. Adenosine triphosphate compartmentation in living hearts: a phosphorus nuclear magnetic resonance saturation transfer study. Biochemistry. 1979 Aug 7;18(16):3642–3646. doi: 10.1021/bi00583a032. [DOI] [PubMed] [Google Scholar]
- Nägle S. Die Bedeutung von Kreatinphosphat und Adenosintriphosphat im Hinblick auf Energiebereitstellung, -transport und -verwertung im normalen und insuffizienten Herzmuskel. Klin Wochenschr. 1970 Mar 15;48(6):332–341. doi: 10.1007/BF01484859. [DOI] [PubMed] [Google Scholar]
- Oblinger M. M., Brady S. T., McQuarrie I. G., Lasek R. J. Cytotypic differences in the protein composition of the axonally transported cytoskeleton in mammalian neurons. J Neurosci. 1987 Feb;7(2):453–462. doi: 10.1523/JNEUROSCI.07-02-00453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M. Proton stoichiometry of adenosine 5'-triphosphate synthesis in rat liver mitochondria studied by phosphorus-31 nuclear magnetic resonance. Biochemistry. 1982 Aug 31;21(18):4467–4473. doi: 10.1021/bi00261a042. [DOI] [PubMed] [Google Scholar]
- Otsu N., Hirata M., Tuboi S., Miyazawa K. Immunocytochemical localization of creatine kinase M in canine myocardial cells: most creatine kinase M is distributed in the A-band. J Histochem Cytochem. 1989 Oct;37(10):1465–1470. doi: 10.1177/37.10.2778305. [DOI] [PubMed] [Google Scholar]
- Payne R. M., Haas R. C., Strauss A. W. Structural characterization and tissue-specific expression of the mRNAs encoding isoenzymes from two rat mitochondrial creatine kinase genes. Biochim Biophys Acta. 1991 Jul 23;1089(3):352–361. doi: 10.1016/0167-4781(91)90176-m. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perry S. B., McAuliffe J., Balschi J. A., Hickey P. R., Ingwall J. S. Velocity of the creatine kinase reaction in the neonatal rabbit heart: role of mitochondrial creatine kinase. Biochemistry. 1988 Mar 22;27(6):2165–2172. doi: 10.1021/bi00406a052. [DOI] [PubMed] [Google Scholar]
- Perryman M. B., Knell J. D., Ifegwu J., Roberts R. Identification of a 43-kDa polypeptide associated with acetylcholine receptor-enriched membranes as MM creatine kinase. J Biol Chem. 1985 Aug 5;260(16):9399–9404. [PubMed] [Google Scholar]
- Perryman M. B., Strauss A. W., Olson J., Roberts R. In vitro translation of canine mitochondrial creatine kinase messenger RNA. Biochem Biophys Res Commun. 1983 Feb 10;110(3):967–972. doi: 10.1016/0006-291x(83)91057-4. [DOI] [PubMed] [Google Scholar]
- Quemeneur E., Eichenberger D., Goldschmidt D., Vial C., Beauregard G., Potier M. The radiation inactivation method provides evidence that membrane-bound mitochondrial creatine kinase is an oligomer. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1310–1314. doi: 10.1016/s0006-291x(88)81371-8. [DOI] [PubMed] [Google Scholar]
- Quest A. F., Eppenberger H. M., Wallimann T. Purification of brain-type creatine kinase (B-CK) from several tissues of the chicken: B-CK subspecies. Enzyme. 1989;41(1):33–42. doi: 10.1159/000469048. [DOI] [PubMed] [Google Scholar]
- Quest A. F., Eppenberger H. M., Wallimann T. Two different B-type creatine kinase subunits dimerize in a tissue-specific manner. FEBS Lett. 1990 Mar 26;262(2):299–304. doi: 10.1016/0014-5793(90)80214-4. [DOI] [PubMed] [Google Scholar]
- Quest A. F., Shapiro B. M. Membrane association of flagellar creatine kinase in the sperm phosphocreatine shuttle. J Biol Chem. 1991 Oct 15;266(29):19803–19811. [PubMed] [Google Scholar]
- Quest A. F., Soldati T., Hemmer W., Perriard J. C., Eppenberger H. M., Wallimann T. Phosphorylation of chicken brain-type creatine kinase affects a physiologically important kinetic parameter and gives rise to protein microheterogeneity in vivo. FEBS Lett. 1990 Sep 3;269(2):457–464. doi: 10.1016/0014-5793(90)81215-a. [DOI] [PubMed] [Google Scholar]
- Reiss N. A., Kaye A. M. Identification of the major component of the estrogen-induced protein of rat uterus as the BB isozyme of creatine kinase. J Biol Chem. 1981 Jun 10;256(11):5741–5749. [PubMed] [Google Scholar]
- Rojo M., Hovius R., Demel R. A., Nicolay K., Wallimann T. Mitochondrial creatine kinase mediates contact formation between mitochondrial membranes. J Biol Chem. 1991 Oct 25;266(30):20290–20295. [PubMed] [Google Scholar]
- Rojo M., Hovius R., Demel R., Wallimann T., Eppenberger H. M., Nicolay K. Interaction of mitochondrial creatine kinase with model membranes. A monolayer study. FEBS Lett. 1991 Apr 9;281(1-2):123–129. doi: 10.1016/0014-5793(91)80374-c. [DOI] [PubMed] [Google Scholar]
- Roos N., Benz R., Brdiczka D. Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. Biochim Biophys Acta. 1982 Apr 7;686(2):204–214. doi: 10.1016/0005-2736(82)90114-6. [DOI] [PubMed] [Google Scholar]
- Rossi A. M., Eppenberger H. M., Volpe P., Cotrufo R., Wallimann T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem. 1990 Mar 25;265(9):5258–5266. [PubMed] [Google Scholar]
- Saks V. A., Chernousova G. B., Vetter R., Smirnov V. N., Chazov E. I. Kinetic properties and the functional role of particulate MM-isoenzyme of creatine phosphokinase bound to heart muscle myofibrils. FEBS Lett. 1976 Mar 1;62(3):293–296. doi: 10.1016/0014-5793(76)80078-6. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Khuchua Z. A., Kuznetsov A. V., Veksler V. I., Sharov V. G. Heart mitochondria in physiological salt solution: not ionic strength but salt composition is important for association of creatine kinase with the inner membrane surface. Biochem Biophys Res Commun. 1986 Sep 30;139(3):1262–1271. doi: 10.1016/s0006-291x(86)80314-x. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Kupriyanov V. V., Elizarova G. V., Jacobus W. E. Studies of energy transport in heart cells. The importance of creatine kinase localization for the coupling of mitochondrial phosphorylcreatine production to oxidative phosphorylation. J Biol Chem. 1980 Jan 25;255(2):755–763. [PubMed] [Google Scholar]
- Saks V. A., Kuznetsov A. V., Kupriyanov V. V., Miceli M. V., Jacobus W. E. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J Biol Chem. 1985 Jun 25;260(12):7757–7764. [PubMed] [Google Scholar]
- Saks V. A., Lipina N. V., Sharov V. G., Smirnov V. N., Chazov E., Grosse R. The localization of the MM isozyme of creatine phosphokinase on the surface membrane of myocardial cells and its functional coupling to ouabain-inhibited (Na+, K+)-ATPase. Biochim Biophys Acta. 1977 Mar 17;465(3):550–558. doi: 10.1016/0005-2736(77)90272-3. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Lipina N. V., Smirnov V. N., Chazov E. I. Studies of energy transport in heart cells. The functional coupling between mitochondrial creatine phosphokinase and ATP ADP translocase: kinetic evidence. Arch Biochem Biophys. 1976 Mar;173(1):34–41. doi: 10.1016/0003-9861(76)90231-9. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Rosenshtraukh L. V., Smirnov V. N., Chazov E. I. Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharmacol. 1978 Oct;56(5):691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Ventura-Clapier R., Huchua Z. A., Preobrazhensky A. N., Emelin I. V. Creatine kinase in regulation of heart function and metabolism. I. Further evidence for compartmentation of adenine nucleotides in cardiac myofibrillar and sarcolemmal coupled ATPase-creatine kinase systems. Biochim Biophys Acta. 1984 Apr 16;803(4):254–264. doi: 10.1016/0167-4889(84)90115-0. [DOI] [PubMed] [Google Scholar]
- Savabi F. Free creatine available to the creatine phosphate energy shuttle in isolated rat atria. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7476–7480. doi: 10.1073/pnas.85.20.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabi F., Geiger P. J., Bessman S. P. Myofibrillar end of the creatine phosphate energy shuttle. Am J Physiol. 1984 Nov;247(5 Pt 1):C424–C432. doi: 10.1152/ajpcell.1984.247.5.C424. [DOI] [PubMed] [Google Scholar]
- Schlame M., Augustin W. Association of creatine kinase with rat heart mitochondria: high and low affinity binding sites and the involvement of phospholipids. Biomed Biochim Acta. 1985;44(7-8):1083–1088. [PubMed] [Google Scholar]
- Schlegel J., Wyss M., Eppenberger H. M., Wallimann T. Functional studies with the octameric and dimeric form of mitochondrial creatine kinase. Differential pH-dependent association of the two oligomeric forms with the inner mitochondrial membrane. J Biol Chem. 1990 Jun 5;265(16):9221–9227. [PubMed] [Google Scholar]
- Schlegel J., Wyss M., Schürch U., Schnyder T., Quest A., Wegmann G., Eppenberger H. M., Wallimann T. Mitochondrial creatine kinase from cardiac muscle and brain are two distinct isoenzymes but both form octameric molecules. J Biol Chem. 1988 Nov 15;263(32):16963–16969. [PubMed] [Google Scholar]
- Schlegel J., Zurbriggen B., Wegmann G., Wyss M., Eppenberger H. M., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. I. Isolation of two interconvertible mitochondrial creatine kinase forms, dimeric and octameric mitochondrial creatine kinase: characterization, localization, and structure-function relationships. J Biol Chem. 1988 Nov 15;263(32):16942–16953. [PubMed] [Google Scholar]
- Schmitt T., Pette D. Increased mitochondrial creatine kinase in chronically stimulated fast-twitch rabbit muscle. FEBS Lett. 1985 Sep 2;188(2):341–344. doi: 10.1016/0014-5793(85)80399-9. [DOI] [PubMed] [Google Scholar]
- Schnyder T., Engel A., Lustig A., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. II. Characterization of dimers and octamers by ultracentrifugation, direct mass measurements by scanning transmission electron microscopy, and image analysis of single mitochondrial creatine kinase octamers. J Biol Chem. 1988 Nov 15;263(32):16954–16962. [PubMed] [Google Scholar]
- Schnyder T., Gross H., Winkler H., Eppenberger H. M., Wallimann T. Structure of the mitochondrial creatine kinase octamer: high-resolution shadowing and image averaging of single molecules and formation of linear filaments under specific staining conditions. J Cell Biol. 1991 Jan;112(1):95–101. doi: 10.1083/jcb.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder T., Sargent D. F., Richmond T. J., Eppenberger H. M., Wallimann T. Crystallization and preliminary X-ray analysis of two different forms of mitochondrial creatine kinase from chicken cardiac muscle. J Mol Biol. 1990 Dec 20;216(4):809–812. doi: 10.1016/S0022-2836(99)80002-3. [DOI] [PubMed] [Google Scholar]
- Schnyder T., Winkler H., Gross H., Eppenberger H. M., Wallimann T. Crystallization of mitochondrial creatine kinase. Growing of large protein crystals and electron microscopic investigation of microcrystals consisting of octamers. J Biol Chem. 1991 Mar 15;266(8):5318–5322. [PubMed] [Google Scholar]
- Scholte H. R. On the triple localization of creatine kinase in heart and skeletal muscle cells of the rat: evidence for the existence of myofibrillar and mitochondrial isoenzymes. Biochim Biophys Acta. 1973 May 30;305(2):413–427. doi: 10.1016/0005-2728(73)90187-4. [DOI] [PubMed] [Google Scholar]
- Scholte H. R., Weijers P. J., Wit-Peeters E. M. The localization of mitochondrial creatine kinase, and its use for the determination of the sidedness of submitochondrial particles. Biochim Biophys Acta. 1973 Feb 16;291(3):764–773. doi: 10.1016/0005-2736(73)90479-3. [DOI] [PubMed] [Google Scholar]
- Schäfer B. W., Perriard J. C. Intracellular targeting of isoproteins in muscle cytoarchitecture. J Cell Biol. 1988 Apr;106(4):1161–1170. doi: 10.1083/jcb.106.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Studies with a reconstituted muscle glycolytic system. The rate and extent of creatine phosphorylation by anaerobic glycolysis. Biochem J. 1973 May;134(1):197–208. doi: 10.1042/bj1340197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraydarian M. W., Abbott B. C. The role of the creatine--phosphorylcreatine system in muscle. J Mol Cell Cardiol. 1976 Oct;8(10):741–746. doi: 10.1016/0022-2828(76)90081-x. [DOI] [PubMed] [Google Scholar]
- Sharov V. G., Saks V. A., Smirnov V. N., Chazov E. I. An electron microscopic histochemical investigation of the localization of creatine phosphokinase in heart cells. Biochim Biophys Acta. 1977 Aug 1;468(3):495–501. doi: 10.1016/0005-2736(77)90299-1. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Challiss R. A., Hayes D. J., Radda G. K. Biochemical adaptation in the skeletal muscle of rats depleted of creatine with the substrate analogue beta-guanidinopropionic acid. Biochem J. 1985 Nov 15;232(1):125–131. doi: 10.1042/bj2320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge E. A., Jeffry F. M., Keogh J. M., Radda G. K., Seymour A. M. Creatine kinase kinetics, ATP turnover, and cardiac performance in hearts depleted of creatine with the substrate analogue beta-guanidinopropionic acid. Biochim Biophys Acta. 1985 Oct 30;847(1):25–32. doi: 10.1016/0167-4889(85)90148-x. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Radda G. K. A 31P-nuclear magnetic resonance study of skeletal muscle metabolism in rats depleted of creatine with the analogue beta-guanidinopropionic acid. Biochim Biophys Acta. 1984 Sep 14;805(1):79–88. doi: 10.1016/0167-4889(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Soboll S., Scholz R., Heldt H. W. Subcellular metabolite concentrations. Dependence of mitochondrial and cytosolic ATP systems on the metabolic state of perfused rat liver. Eur J Biochem. 1978 Jun 15;87(2):377–390. doi: 10.1111/j.1432-1033.1978.tb12387.x. [DOI] [PubMed] [Google Scholar]
- Soldati T., Schäfer B. W., Perriard J. C. Alternative ribosomal initiation gives rise to chicken brain-type creatine kinase isoproteins with heterogeneous amino termini. J Biol Chem. 1990 Mar 15;265(8):4498–4506. [PubMed] [Google Scholar]
- Spande J. I., Schottelius B. A. Chemical basis of fatigue in isolated mouse soleus muscle. Am J Physiol. 1970 Nov;219(5):1490–1495. doi: 10.1152/ajplegacy.1970.219.5.1490. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Biophysical chemistry of metabolic reaction sequences in concentrated enzyme solution and in the cell. Annu Rev Biophys Biophys Chem. 1987;16:175–204. doi: 10.1146/annurev.bb.16.060187.001135. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Metabolite transfer via enzyme-enzyme complexes. Science. 1986 Nov 28;234(4780):1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- Strehler E. E., Carlsson E., Eppenberger H. M., Thornell L. E. Ultrastructural localization of M-band proteins in chicken breast muscle as revealed by combined immunocytochemistry and ultramicrotomy. J Mol Biol. 1983 May 15;166(2):141–158. doi: 10.1016/s0022-2836(83)80003-5. [DOI] [PubMed] [Google Scholar]
- Swanson P. D. The particulate adenosine triphosphate-creatine phosphotransferase from brain: its distribution in subcellular fractions and its properties. J Neurochem. 1967 Mar;14(3):343–356. doi: 10.1111/j.1471-4159.1967.tb09531.x. [DOI] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Thompson R. J., Kynoch P. A., Sarjant J. Immunohistochemical localization of creatine kinase-BB isoenzyme to astrocytes in human brain. Brain Res. 1980 Nov 17;201(2):423–426. doi: 10.1016/0006-8993(80)91046-x. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., du Boulay G. H., Duchen L. W., Radda G. A 31P nuclear magnetic resonance in vivo study of cerebral ischaemia in the gerbil. J Cereb Blood Flow Metab. 1982 Sep;2(3):299–306. doi: 10.1038/jcbfm.1982.31. [DOI] [PubMed] [Google Scholar]
- Tofts P., Wray S. Changes in brain phosphorus metabolites during the post-natal development of the rat. J Physiol. 1985 Feb;359:417–429. doi: 10.1113/jphysiol.1985.sp015593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes R. M., Brokaw C. J., Shapiro B. M. Creatine kinase-dependent energy transport in sea urchin spermatozoa. Flagellar wave attenuation and theoretical analysis of high energy phosphate diffusion. Biophys J. 1987 Jul;52(1):75–86. doi: 10.1016/S0006-3495(87)83190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes R. M., Farr A., Shapiro B. M. Sea urchin sperm creatine kinase: the flagellar isozyme is a microtubule-associated protein. Exp Cell Res. 1988 Oct;178(2):307–317. doi: 10.1016/0014-4827(88)90401-6. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Enzyme termini of a phosphocreatine shuttle. Purification and characterization of two creatine kinase isozymes from sea urchin sperm. J Biol Chem. 1987 Nov 25;262(33):16011–16019. [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Metabolite channeling: a phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985 May;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Toyo-oka T., Nagayama K., Umeda M., Eguchi K., Hosoda S. Rhythmic change of myocardial phosphate metabolite content in cardiac cycle observed by depth-selected and EKG-gated in vivo 31P-NMR spectroscopy in a whole animal. Biochem Biophys Res Commun. 1986 Mar 28;135(3):808–815. doi: 10.1016/0006-291x(86)91000-4. [DOI] [PubMed] [Google Scholar]
- Trask R. V., Strauss A. W., Billadello J. J. Developmental regulation and tissue-specific expression of the human muscle creatine kinase gene. J Biol Chem. 1988 Nov 15;263(32):17142–17149. [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Holmsen H., Shulman R. G. Adenine nucleotide storage and secretion in platelets as studied by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1979 May;76(5):2227–2231. doi: 10.1073/pnas.76.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Racker E. Regulatory mechanisms in carbohydrate metabolism. VII. Hexokinase and phosphofructokinase. J Biol Chem. 1965 Dec;240(12):4682–4688. [PubMed] [Google Scholar]
- Uğurbil K., Petein M., Maidan R., Michurski S., From A. H. Measurement of an individual rate constant in the presence of multiple exchanges: application to myocardial creatine kinase reaction. Biochemistry. 1986 Jan 14;25(1):100–107. doi: 10.1021/bi00349a015. [DOI] [PubMed] [Google Scholar]
- Van Waarde A., Van den Thillart G., Erkelens C., Addink A., Lugtenburg J. Functional coupling of glycolysis and phosphocreatine utilization in anoxic fish muscle. An in vivo 31P NMR study. J Biol Chem. 1990 Jan 15;265(2):914–923. [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Ventura-Clapier R., Mekhfi H., Vassort G. Role of creatine kinase in force development in chemically skinned rat cardiac muscle. J Gen Physiol. 1987 May;89(5):815–837. doi: 10.1085/jgp.89.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R., Saks V. A., Vassort G., Lauer C., Elizarova G. V. Reversible MM-creatine kinase binding to cardiac myofibrils. Am J Physiol. 1987 Sep;253(3 Pt 1):C444–C455. doi: 10.1152/ajpcell.1987.253.3.C444. [DOI] [PubMed] [Google Scholar]
- Vial C., Font B., Goldschmidt D., Gautheron D. C. Dissociation and reassociation of creatine kinase with heart mitochondria; pH and phosphate dependence. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1352–1359. doi: 10.1016/0006-291x(79)91129-x. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Doetschman T. C., Eppenberger H. M. Novel staining pattern of skeletal muscle M-lines upon incubation with antibodies against MM-creatine kinase. J Cell Biol. 1983 Jun;96(6):1772–1779. doi: 10.1083/jcb.96.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. The subcellular compartmentation of creatine kinase isozymes as a precondition for a proposed phosphoryl-creatine circuit. Prog Clin Biol Res. 1990;344:877–889. [PubMed] [Google Scholar]
- Wallimann T., Moser H., Eppenberger H. M. Isoenzyme-specific localization of M-line bound creatine kinase in myogenic cells. J Muscle Res Cell Motil. 1983 Aug;4(4):429–441. doi: 10.1007/BF00711948. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Moser H., Zurbriggen B., Wegmann G., Eppenberger H. M. Creatine kinase isoenzymes in spermatozoa. J Muscle Res Cell Motil. 1986 Feb;7(1):25–34. doi: 10.1007/BF01756199. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Pelloni G., Turner D. C., Eppenberger H. M. Monovalent antibodies against MM-creatine kinase remove the M line from myofibrils. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4296–4300. doi: 10.1073/pnas.75.9.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Schlösser T., Eppenberger H. M. Function of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscle. J Biol Chem. 1984 Apr 25;259(8):5238–5246. [PubMed] [Google Scholar]
- Wallimann T., Schnyder T., Schlegel J., Wyss M., Wegmann G., Rossi A. M., Hemmer W., Eppenberger H. M., Quest A. F. Subcellular compartmentation of creatine kinase isoenzymes, regulation of CK and octameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res. 1989;315:159–176. [PubMed] [Google Scholar]
- Wallimann T., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Walzthöny D., Wegmann G., Moser H., Eppenberger H. M., Barrantes F. J. Subcellular localization of creatine kinase in Torpedo electrocytes: association with acetylcholine receptor-rich membranes. J Cell Biol. 1985 Apr;100(4):1063–1072. doi: 10.1083/jcb.100.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Wegmann G., Moser H., Huber R., Eppenberger H. M. High content of creatine kinase in chicken retina: compartmentalized localization of creatine kinase isoenzymes in photoreceptor cells. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3816–3819. doi: 10.1073/pnas.83.11.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann G., Huber R., Zanolla E., Eppenberger H. M., Wallimann T. Differential expression and localization of brain-type and mitochondrial creatine kinase isoenzymes during development of the chicken retina: Mi-CK as a marker for differentiation of photoreceptor cells. Differentiation. 1991 Mar;46(2):77–87. doi: 10.1111/j.1432-0436.1991.tb00868.x. [DOI] [PubMed] [Google Scholar]
- West B. L., Babbitt P. C., Mendez B., Baxter J. D. Creatine kinase protein sequence encoded by a cDNA made from Torpedo californica electric organ mRNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7007–7011. doi: 10.1073/pnas.81.22.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. J., Nagy B., Gergely J. Free adenosine diphosphate as an intermediary in the phosphorylation by creatine phosphate of adenosine diphosphate bound to actin. J Biol Chem. 1967 Mar 25;242(6):1140–1145. [PubMed] [Google Scholar]
- Wevers R. A., Reutelingsperger C. P., Dam B., Soons J. B. Mitochondrial creatine kinase (EC 2.7.3.2) in the brain. Clin Chim Acta. 1982 Mar 12;119(3):209–223. doi: 10.1016/0009-8981(82)90333-3. [DOI] [PubMed] [Google Scholar]
- Winegrad S., Weisberg A., Lin L. E., McClellan G. A calcium independent on-off switch for cardiac force generators. Prog Clin Biol Res. 1989;315:473–479. [PubMed] [Google Scholar]
- Winkler H., Gross H., Schnyder T., Kunath W. Circular harmonic averaging of rotary-shadowed and negatively stained creatine kinase macromolecules. J Electron Microsc Tech. 1991 Jun;18(2):135–141. doi: 10.1002/jemt.1060180207. [DOI] [PubMed] [Google Scholar]
- Wirz T., Brändle U., Soldati T., Hossle J. P., Perriard J. C. A unique chicken B-creatine kinase gene gives rise to two B-creatine kinase isoproteins with distinct N termini by alternative splicing. J Biol Chem. 1990 Jul 15;265(20):11656–11666. [PubMed] [Google Scholar]
- Witzemann V. Creatine phosphokinase: isoenzymes in Torpedo marmorata. Eur J Biochem. 1985 Jul 1;150(1):201–210. doi: 10.1111/j.1432-1033.1985.tb09008.x. [DOI] [PubMed] [Google Scholar]
- Wothe D. D., Charbonneau H., Shapiro B. M. The phosphocreatine shuttle of sea urchin sperm: flagellar creatine kinase resulted from a gene triplication. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5203–5207. doi: 10.1073/pnas.87.13.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M., Schlegel J., James P., Eppenberger H. M., Wallimann T. Mitochondrial creatine kinase from chicken brain. Purification, biophysical characterization, and generation of heterodimeric and heterooctameric molecules with subunits of other creatine kinase isoenzymes. J Biol Chem. 1990 Sep 15;265(26):15900–15908. [PubMed] [Google Scholar]
- Yamada T., Sugi H. Regulation of glycogenolysis in contracting frog skeletal muscle studied by 31P nuclear magnetic resonance. Prog Clin Biol Res. 1989;315:149–157. [PubMed] [Google Scholar]
- Yamashita K., Yoshioka T. Profiles of creatine kinase isoenzyme compositions in single muscle fibres of different types. J Muscle Res Cell Motil. 1991 Feb;12(1):37–44. doi: 10.1007/BF01781172. [DOI] [PubMed] [Google Scholar]
- Yang W. C., Geiger P. J., Besman S. P. Formation of creatine phosphate from creatine and 32P-labelled ATP by isolated rabbit heart mitochondria. Biochem Biophys Res Commun. 1977 Jun 6;76(3):882–887. doi: 10.1016/0006-291x(77)91583-2. [DOI] [PubMed] [Google Scholar]
- Yoshimine T., Morimoto K., Homburger H. A., Yanagihara T. Immunohistochemical localization of creatine kinase BB-isoenzyme in human brain: comparison with tubulin and astroprotein. Brain Res. 1983 Apr 11;265(1):101–108. doi: 10.1016/0006-8993(83)91338-0. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Watari H., Radda G. K. Role of phosphocreatine in energy transport in skeletal muscle of bullfrog studied by 31P-NMR. Biochim Biophys Acta. 1990 Feb 19;1051(2):144–150. doi: 10.1016/0167-4889(90)90186-h. [DOI] [PubMed] [Google Scholar]
- Zahler R., Bittl J. A., Ingwall J. S. Analysis of compartmentation of ATP in skeletal and cardiac muscle using 31P nuclear magnetic resonance saturation transfer. Biophys J. 1987 Jun;51(6):883–893. doi: 10.1016/S0006-3495(87)83416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Jacobus W. E., Korecky B., Brandejs-Barry Y. Bioenergetic consequences of cardiac phosphocreatine depletion induced by creatine analogue feeding. J Biol Chem. 1991 Oct 25;266(30):20296–20304. [PubMed] [Google Scholar]