Abstract
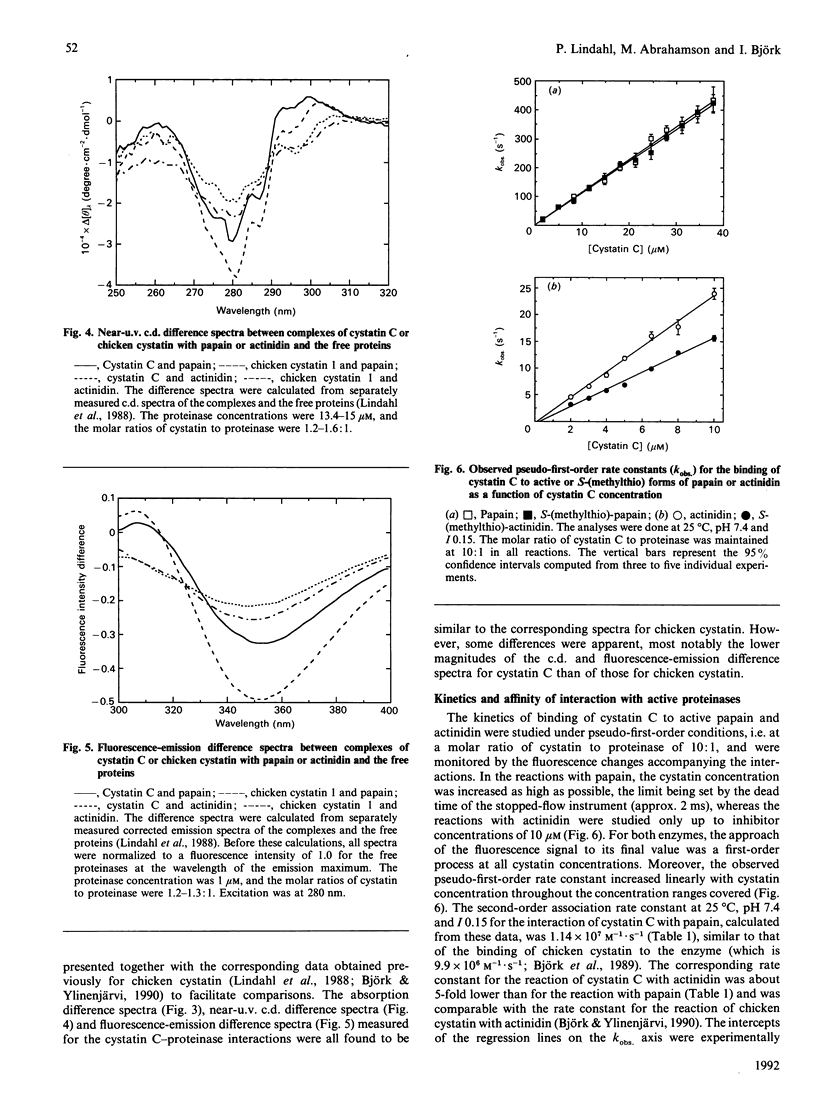
The interaction between recombinant human cystatin C and the cysteine proteinases papain and actinidin was studied by spectroscopic, kinetic and equilibrium methods. The absorption, near-u.v.c.d. and fluorescence-emission difference spectra for the cystatin C-proteinase interactions were all found to be similar to the corresponding spectra for chicken cystatin. The kinetics of binding of cystatin C to the two enzymes were best described by a simple reversible one-step bimolecular mechanism, like the kinetics of the reaction of chicken cystatin with several cysteine proteinases. Moreover, the second-order association rate constants at 25 degrees C, pH 7.4 and I0.15, of 1.1 x 10(7) and 2.4 x 10(6) M-1.s-1 for the reactions of cystatin C with papain and actinidin respectively, were similar to the corresponding rate constants for the chicken inhibitor and close to the value expected for a diffusion-controlled rate. The dissociation equilibrium constants, approx. 11 fM and approx. 19 nM for the binding of cystatin C to papain and actinidin respectively, were also comparable with the dissociation constants for chicken cystatin. The affinity between cystatin C and several inactivated papains or actinidins decreased with increasing size of the inactivating group in a manner similar to that in earlier studies with the chicken inhibitor. Together, these results strongly indicate that the mechanisms of the reactions of cystatin C and chicken cystatin with cysteine proteinases are identical or highly similar, but differ from that of reactions between serine-proteinase inhibitors and their target enzymes. The model for the proteinase-inhibitor interaction, based on the X-ray structure of chicken cystatin, therefore should be largely applicable also to human cystatin C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Barrett A. J., Salvesen G., Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986 Aug 25;261(24):11282–11289. [PubMed] [Google Scholar]
- Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988 Aug 15;236(1):14–18. doi: 10.1016/0014-5793(88)80276-x. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Grubb A., Olafsson I., Lundwall A. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human cysteine proteinase inhibitor cystatin C. FEBS Lett. 1987 Jun 1;216(2):229–233. doi: 10.1016/0014-5793(87)80695-6. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Alriksson E., Ylinenjärvi K. Kinetics of binding of chicken cystatin to papain. Biochemistry. 1989 Feb 21;28(4):1568–1573. doi: 10.1021/bi00430a022. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction between chicken cystatin and the cysteine proteinases actinidin, chymopapain A, and ficin. Biochemistry. 1990 Feb 20;29(7):1770–1776. doi: 10.1021/bi00459a016. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction of chicken cystatin with inactivated papains. Biochem J. 1989 May 15;260(1):61–68. doi: 10.1042/bj2600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. E., Lewis S. D., Shafer J. A. A two-step procedure for purification of papain from extract of papaya latex. Arch Biochem Biophys. 1974 Sep;164(1):30–36. doi: 10.1016/0003-9861(74)90004-6. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Ritonja A., Dando P. M., Abrahamson M., Shaw E. N., Wikstrom P., Turk V., Barrett A. J. Interactions of papaya proteinase IV with inhibitors. FEBS Lett. 1990 Mar 12;262(1):58–60. doi: 10.1016/0014-5793(90)80153-a. [DOI] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbøge H., Jensen E. B., Tøttrup H., Grubb A., Abrahamson M., Olafsson I., Carlsen S. High-level expression of active human cystatin C in Escherichia coli. Gene. 1989 Jul 15;79(2):325–332. doi: 10.1016/0378-1119(89)90214-x. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Jensson O., Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc Natl Acad Sci U S A. 1986 May;83(9):2974–2978. doi: 10.1073/pnas.83.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb A., Jensson O., Gudmundsson G., Arnason A., Löfberg H., Malm J. Abnormal metabolism of gamma-trace alkaline microprotein. The basic defect in hereditary cerebral hemorrhage with amyloidosis. N Engl J Med. 1984 Dec 13;311(24):1547–1549. doi: 10.1056/NEJM198412133112406. [DOI] [PubMed] [Google Scholar]
- Grubb A., Löfberg H. Human gamma-trace, a basic microprotein: amino acid sequence and presence in the adenohypophysis. Proc Natl Acad Sci U S A. 1982 May;79(9):3024–3027. doi: 10.1073/pnas.79.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Completion of the amino acid sequence of papain. Biochem J. 1969 Sep;114(2):279–288. doi: 10.1042/bj1140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Alriksson E., Jörnvall H., Björk I. Interaction of the cysteine proteinase inhibitor chicken cystatin with papain. Biochemistry. 1988 Jul 12;27(14):5074–5082. doi: 10.1021/bi00414a019. [DOI] [PubMed] [Google Scholar]
- Luthy J. A., Praissman M., Finkenstadt W. R., Laskowski M., Jr Detailed mechanism of interaction of bovine -trypsin with soybean trypsin inhibitor (Kunitz). I. Stopped flow measurements. J Biol Chem. 1973 Mar 10;248(5):1760–1771. [PubMed] [Google Scholar]
- Löfberg H., Grubb A. O., Nilsson E. K., Jensson O., Gudmundsson G., Blöndal H., Arnason A., Thorsteinsson L. Immunohistochemical characterization of the amyloid deposits and quantitation of pertinent cerebrospinal fluid proteins in hereditary cerebral hemorrhage with amyloidosis. Stroke. 1987 Mar-Apr;18(2):431–440. doi: 10.1161/01.str.18.2.431. [DOI] [PubMed] [Google Scholar]
- McDowall M. A. Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme. Eur J Biochem. 1970 Jun;14(2):214–221. doi: 10.1111/j.1432-1033.1970.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Moreau T., Hoebeke J., Lalamanach G., Hattab M., Gauthier F. Simulation of the inhibitory cystatin surface by a synthetic peptide. Biochem Biophys Res Commun. 1990 Feb 28;167(1):117–122. doi: 10.1016/0006-291x(90)91738-e. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Barrett A. J. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984 Oct 1;223(1):245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Binding of low-affinity and high-affinity heparin to antithrombin. Ultraviolet difference spectroscopy and circular dichroism studies. Biochemistry. 1978 Aug 8;17(16):3339–3344. doi: 10.1021/bi00609a026. [DOI] [PubMed] [Google Scholar]
- Nycander M., Björk I. Evidence by chemical modification that tryptophan-104 of the cysteine-proteinase inhibitor chicken cystatin is located in or near the proteinase-binding site. Biochem J. 1990 Oct 1;271(1):281–284. doi: 10.1042/bj2710281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo I., Kurachi K., Takasawa T., Shiokawa H., Sasaki M. Isolation of a human cDNA for alpha 2-thiol proteinase inhibitor and its identity with low molecular weight kininogen. Biochemistry. 1984 Nov 20;23(24):5691–5697. doi: 10.1021/bi00319a005. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Shore J. D. Demonstration of a two-step reaction mechanism for inhibition of alpha-thrombin by antithrombin III and identification of the step affected by heparin. J Biol Chem. 1982 Dec 25;257(24):14891–14895. [PubMed] [Google Scholar]
- Quast U., Engel J., Heumann H., Krause G., Steffen E. Kinetics of the interaction of bovine pancreatic trypsin inhibitor (Kunitz) with alpha-chymotrypsin. Biochemistry. 1974 Jun 4;13(12):2512–2520. doi: 10.1021/bi00709a600. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Lewis S. D., Ballou D. P., Olson S. T., Shafer J. A. Reactivity of small thiolate anions and cysteine-25 in papain toward methyl methanethiosulfonate. Biochemistry. 1986 Sep 23;25(19):5595–5601. doi: 10.1021/bi00367a038. [DOI] [PubMed] [Google Scholar]
- Schwabe C., Anastasi A., Crow H., McDonald J. K., Barrett A. J. Cystatin. Amino acid sequence and possible secondary structure. Biochem J. 1984 Feb 1;217(3):813–817. doi: 10.1042/bj2170813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M. Trypsin-pancreatic trypsin inhibitor association. Dynamics of the interaction and role of disulfide bridges. Biochemistry. 1972 Aug 1;11(16):2967–2977. doi: 10.1021/bi00766a007. [DOI] [PubMed] [Google Scholar]


