Abstract
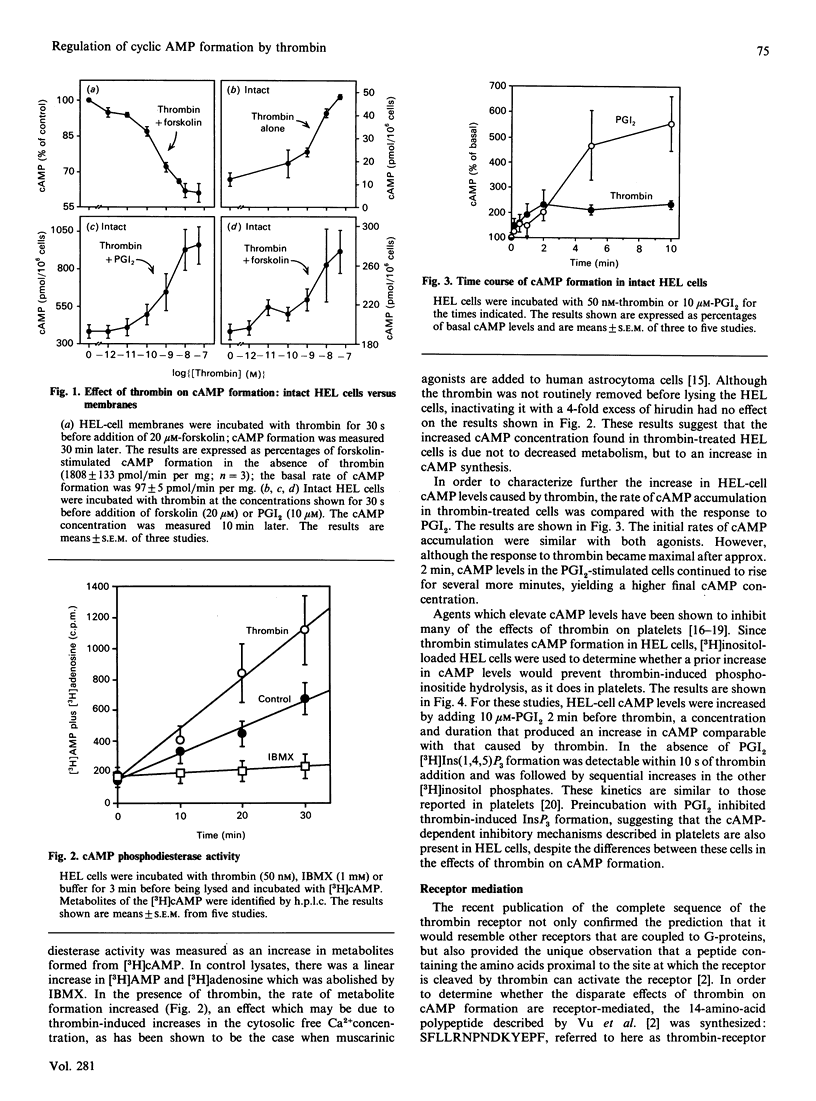
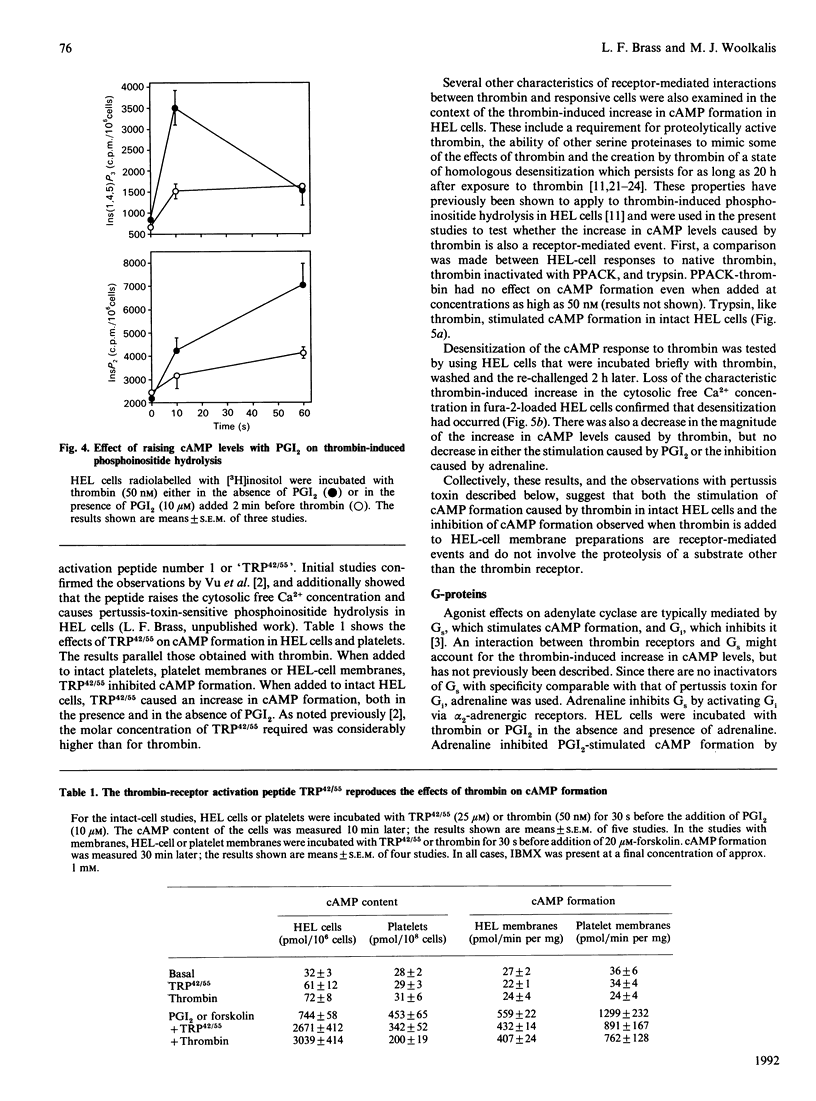
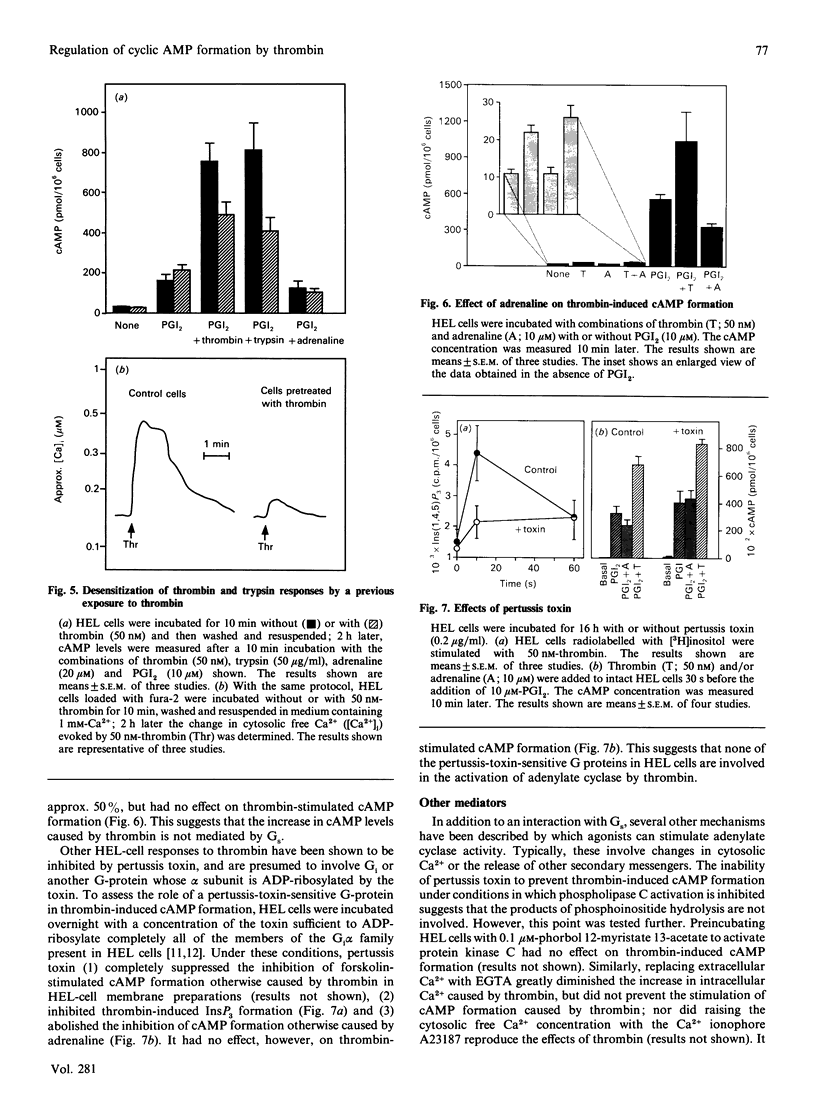
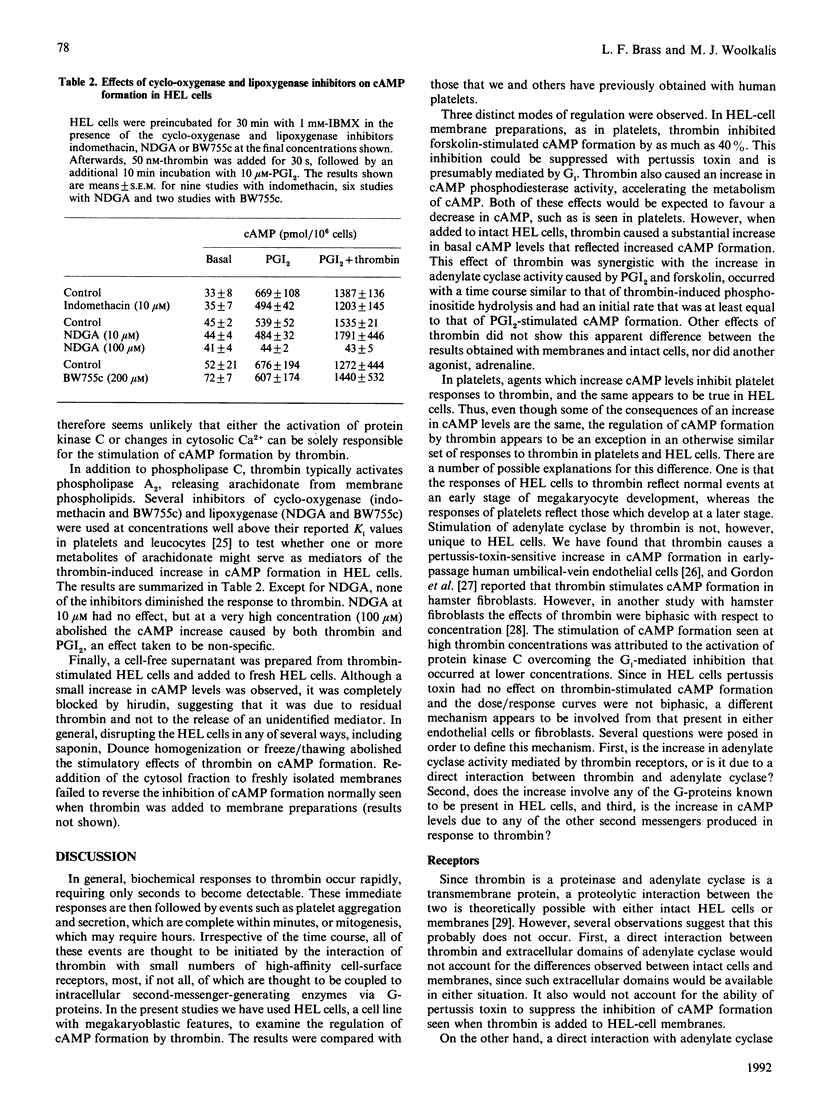
Thrombin is thought to stimulate responsive cells by cleaving cell-surface receptors coupled to intracellular second-messenger-generating enzymes via G-proteins. In order to understand this process better, we have examined the regulation of adenylate cyclase by thrombin in the megakaryoblastic HEL cell line and compared it with platelets. A notable difference was found. In HEL-cell membrane preparations, thrombin inhibited cyclic AMP (cAMP) formation by a pertussis-toxin-sensitive mechanism comparable with that observed in platelets. In contrast, when added to intact HEL cells, thrombin activated adenylate cyclase and caused an increase in cAMP formation synergistic with that produced by forskolin and prostaglandin I2. This increase, which was not seen with platelets, was accompanied by an increase in cAMP metabolism by phosphodiesterase. Like other responses to thrombin, the increase in cAMP formation required proteolytically active thrombin and was subject to homologous desensitization. An equivalent response could be evoked by the addition of a polypeptide, derived from the N-terminus of the thrombin receptor, that has been shown to activate the receptor. The effects of thrombin could not, however, be reproduced by the addition of phorbol ester and the Ca2+ ionophore, A23187, nor be prevented with inhibitors of arachidonate metabolism. Preincubation of the cells with adrenaline, which inhibited Gs-mediated activation of adenylate cyclase, or pertussis toxin, which inhibited phospholipase C activation, had no effect on thrombin-induced cAMP formation. These results suggest that thrombin can regulate cAMP formation by two different mechanisms. First, thrombin can inhibit adenylate cyclase in a Gi-dependent manner. This effect predominates in HEL-cell membrane preparations, as it does in platelets, but is not detectable when thrombin is added to intact HEL cells. Instead, in intact HEL cells thrombin activates adenylate cyclase. Although clearly receptor-mediated, this response does not appear to involve Gi, Gs, protein kinase C, eicosanoid formation or changes in the cytosolic Ca2+ concentration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Jaworski C. J., Vlahakis G. Proteolytic activation of adenylate cyclase from cultured fibroblasts. J Biol Chem. 1978 May 10;253(9):2921–2926. [PubMed] [Google Scholar]
- Brass L. F., Manning D. R., Shattil S. J. GTP-binding proteins and platelet activation. Prog Hemost Thromb. 1991;10:127–174. [PubMed] [Google Scholar]
- Brass L. F., Manning D. R., Williams A. G., Woolkalis M. J., Poncz M. Receptor and G protein-mediated responses to thrombin in HEL cells. J Biol Chem. 1991 Jan 15;266(2):958–965. [PubMed] [Google Scholar]
- Brock T. A., Capasso E. L. GTP gamma S increases thrombin-mediated inositol trisphosphate accumulation in permeabilized human endothelial cells. Am Rev Respir Dis. 1989 Oct;140(4):1121–1125. doi: 10.1164/ajrccm/140.4.1121. [DOI] [PubMed] [Google Scholar]
- Cronin M. J., Summers S. T., Sortino M. A., Hewlett E. L. Protein kinase C enhances growth hormone releasing factor (1-40)-stimulated cyclic AMP levels in anterior pituitary. Actions of somatostatin and pertussis toxin. J Biol Chem. 1986 Oct 25;261(30):13932–13935. [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J Biol Chem. 1989 Dec 5;264(34):20356–20362. [PubMed] [Google Scholar]
- Gagnon A. W., Manning D. R., Catani L., Gewirtz A., Poncz M., Brass L. F. Identification of Gz alpha as a pertussis toxin-insensitive G protein in human platelets and megakaryocytes. Blood. 1991 Sep 1;78(5):1247–1253. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gordon E. A., Fenton J. W., 2nd, Carney D. H. Thrombin-receptor occupancy initiates a transient increase in cAMP levels in mitogenically responsive hamster (NIL) fibroblasts. Ann N Y Acad Sci. 1986;485:249–263. doi: 10.1111/j.1749-6632.1986.tb34587.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harden T. K., Evans T., Hepler J. R., Hughes A. R., Martin M. W., Meeker R. B., Smith M. M., Tanner L. I. Regulation of cyclic AMP metabolism by muscarinic cholinergic receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:207–220. [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Jones L. G., McDonough P. M., Brown J. H. Thrombin and trypsin act at the same site to stimulate phosphoinositide hydrolysis and calcium mobilization. Mol Pharmacol. 1989 Jul;36(1):142–149. [PubMed] [Google Scholar]
- Kieffer N., Debili N., Wicki A., Titeux M., Henri A., Mishal Z., Breton-Gorius J., Vainchenker W., Clemetson K. J. Expression of platelet glycoprotein Ib alpha in HEL cells. J Biol Chem. 1986 Dec 5;261(34):15854–15862. [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Lacombe M. L., Stengel D., Hanoune J. Proteolytic activation of adenylate cyclase from rat-liver plasma membranes. FEBS Lett. 1977 May 15;77(2):159–163. doi: 10.1016/0014-5793(77)80225-1. [DOI] [PubMed] [Google Scholar]
- Lerea K. M., Glomset J. A., Krebs E. G. Agents that elevate cAMP levels in platelets decrease thrombin binding. J Biol Chem. 1987 Jan 5;262(1):282–288. [PubMed] [Google Scholar]
- Magnaldo I., Pouysségur J., Paris S. Thrombin exerts a dual effect on stimulated adenylate cyclase in hamster fibroblasts, an inhibition via a GTP-binding protein and a potentiation via activation of protein kinase C. Biochem J. 1988 Aug 1;253(3):711–719. doi: 10.1042/bj2530711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak D. R., Neer E. J. The site of alpha-chymotryptic activation of pigeon erythrocyte adenylate cyclase. J Biol Chem. 1980 May 25;255(10):4781–4785. [PubMed] [Google Scholar]
- Martin B. M., Feinman R. D., Detwiler T. C. Platelet stimulation by thrombin and other proteases. Biochemistry. 1975 Mar 25;14(6):1308–1314. doi: 10.1021/bi00677a032. [DOI] [PubMed] [Google Scholar]
- Michel M. C., Brass L. F., Williams A., Bokoch G. M., LaMorte V. J., Motulsky H. J. Alpha 2-adrenergic receptor stimulation mobilizes intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1989 Mar 25;264(9):4986–4991. [PubMed] [Google Scholar]
- Newton D. L., Klee W. A. Guanine nucleotide dependent and independent reconstitution of G-proteins with adenylate cyclase: stimulation or attenuation of the enzyme by Gi alpha subunits. FEBS Lett. 1990 Oct 1;271(1-2):207–210. doi: 10.1016/0014-5793(90)80407-a. [DOI] [PubMed] [Google Scholar]
- Paris S., Magnaldo I., Pouysségur J. Homologous desensitization of thrombin-induced phosphoinositide breakdown in hamster lung fibroblasts. J Biol Chem. 1988 Aug 15;263(23):11250–11256. [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Poncz M., Surrey S., LaRocco P., Weiss M. J., Rappaport E. F., Conway T. M., Schwartz E. Cloning and characterization of platelet factor 4 cDNA derived from a human erythroleukemic cell line. Blood. 1987 Jan;69(1):219–223. [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. Inhibition by forskolin of cytosolic calcium rise, shape change and aggregation in quin2-loaded human platelets. FEBS Lett. 1985 Aug 19;188(1):135–140. doi: 10.1016/0014-5793(85)80890-5. [DOI] [PubMed] [Google Scholar]
- Salari H., Braquet P., Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984 Jan;13(1):53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Stengel D., Lad P. M., Nielsen T. B., Rodbell M., Hanoune J. Proteolysis activates adenylate cyclase in rat liver and AC-lymphoma cell independently of the guanine nucleotide regulatory site. FEBS Lett. 1980 Jun 30;115(2):260–264. doi: 10.1016/0014-5793(80)81182-3. [DOI] [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Wiener E., Scarpa A. Activation of protein kinase C modulates the adenylate cyclase effector system of B-lymphocytes. J Biol Chem. 1989 Mar 15;264(8):4324–4328. [PubMed] [Google Scholar]
- Williams A. G., Woolkalis M. J., Poncz M., Manning D. R., Gewirtz A. M., Brass L. F. Identification of the pertussis toxin-sensitive G proteins in platelets, megakaryocytes, and human erythroleukemia cells. Blood. 1990 Aug 15;76(4):721–730. [PubMed] [Google Scholar]
- Yeo E., Furie B. C., Furie B. PADGEM protein in human erythroleukemia cells. Blood. 1989 Feb 15;73(3):722–728. [PubMed] [Google Scholar]
- Zimrin A. B., Eisman R., Vilaire G., Schwartz E., Bennett J. S., Poncz M. Structure of platelet glycoprotein IIIa. A common subunit for two different membrane receptors. J Clin Invest. 1988 May;81(5):1470–1475. doi: 10.1172/JCI113478. [DOI] [PMC free article] [PubMed] [Google Scholar]