Abstract
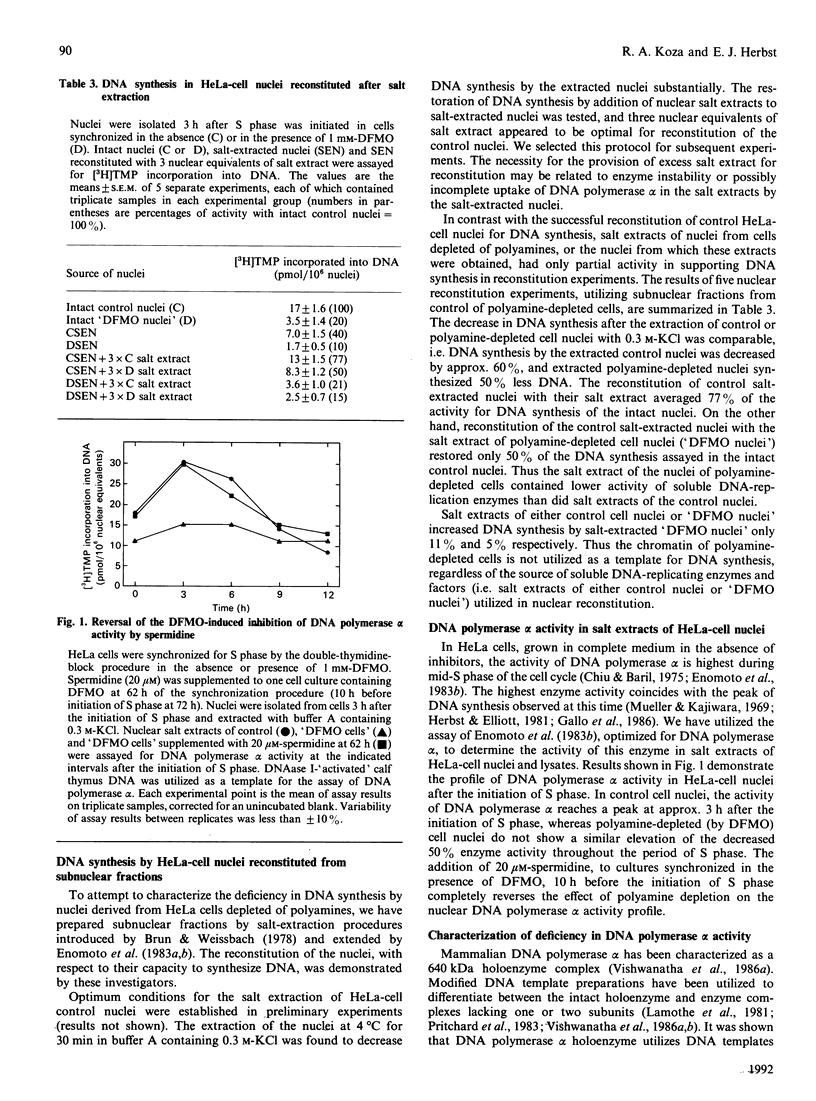
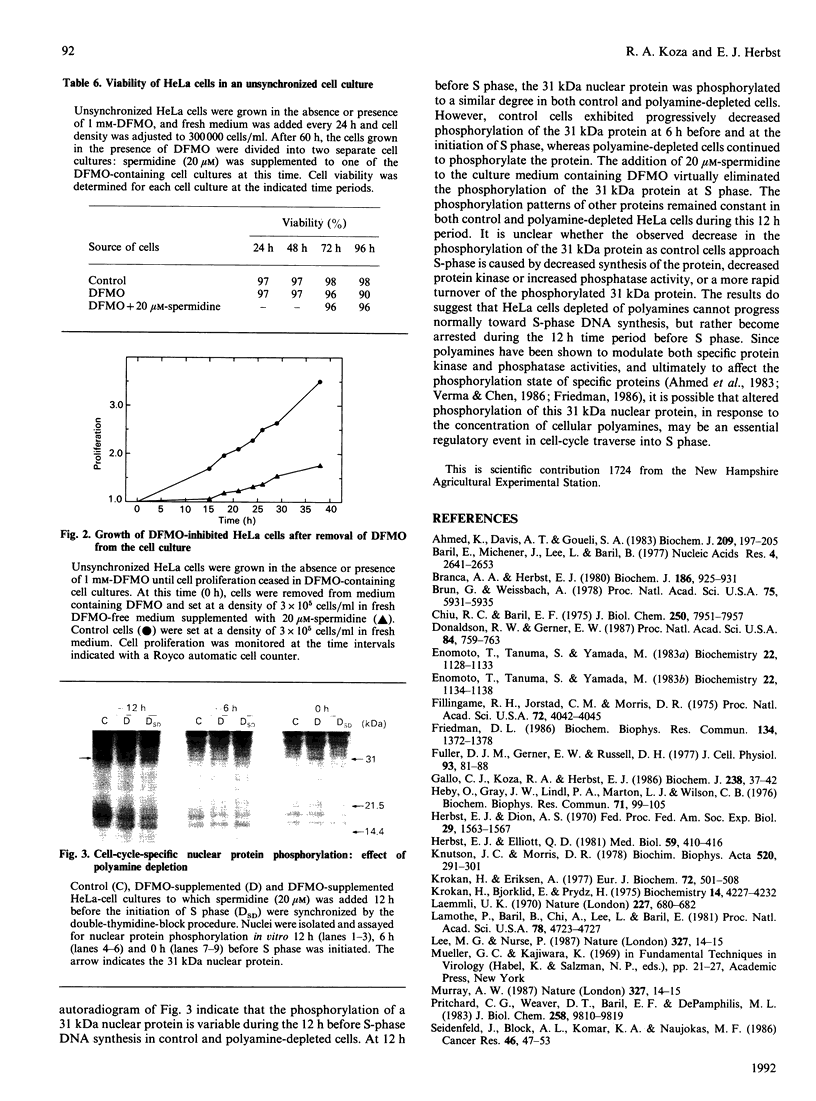
Synchronized HeLa cells depleted of polyamines by alpha-difluoromethylornithine exhibited substantially decreased DNA synthesis, and proliferation ceased after the release of the cells into S phase. Nuclei from these cells synthesized 70-80% less DNA than did nuclei from control cells. Extraction of isolated nuclei with 0.3 M-KCl decreased DNA synthesis by about 60%, which was recovered almost completely in control cell nuclei by reconstitution with the salt extracts of these nuclei. On the other hand, salt extracts of polyamine-depleted nuclei restored only 50% of DNA synthesis in extracted control nuclei. Salt extracts of control cell nuclei contained twice the DNA polymerase alpha activity of polyamine-depleted nuclear extracts. Extracts of cell lysates of both control and polyamine-depleted HeLa cells exhibited similar DNA polymerase alpha activity, suggesting that uptake of the enzyme or its retention by the nuclei of polyamine-depleted cells was decreased. Polyamine-depleted nuclei also showed altered phosphorylation of a 31 kDa protein as compared with control nuclei. Almost normal DNA synthesis, cell proliferation, DNA polymerase alpha activity and nuclear protein phosphorylation were restored in polyamine-depleted cells grown in medium supplemented with 20 microM-spermidine at least 10-12 h before S phase. Cultures in which proliferation was blocked by alpha-difluoromethylornithine did not exhibit synchronous growth after the block was removed. Thus it may be concluded that HeLa cells depleted of polyamines are not inhibited at a single control point in the cell cycle, but are arrested at diverse sites throughout G1 phase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Davis A. T., Goueli S. A. Differential effects of polyamines on the phosphorylation of chromatin-associated proteins. Biochem J. 1983 Jan 1;209(1):197–205. doi: 10.1042/bj2090197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril E., Mitchener J., Lee L., Baril B. Action of pancreatic DNase: requirements for activation of DNA as a template-primer for DNA polymerase. Nucleic Acids Res. 1977 Aug;4(8):2641–2653. doi: 10.1093/nar/4.8.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca A. A., Herbst E. J. Inhibition of ornithine decarboxylase of HeLa cells by diamines and polyamines. Effect on cell proliferation. Biochem J. 1980 Mar 15;186(3):925–931. doi: 10.1042/bj1860925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun G., Weissbach A. Initiation of HeLa cell DNA synthesis in a subnuclear system. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5931–5935. doi: 10.1073/pnas.75.12.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R. W., Baril E. F. Nuclear DNA polymerases and the HeLa cell cycle. J Biol Chem. 1975 Oct 10;250(19):7951–7957. [PubMed] [Google Scholar]
- Donaldson R. W., Gerner E. W. Phosphorylation of a high molecular weight DNA polymerase alpha. Proc Natl Acad Sci U S A. 1987 Feb;84(3):759–763. doi: 10.1073/pnas.84.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T., Tanuma S., Yamada M. Characterization of deoxyribonucleic acid synthesis in reconstituted nuclear systems. Biochemistry. 1983 Mar 1;22(5):1128–1133. doi: 10.1021/bi00274a021. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Tanuma S., Yamada M. Purification and characterization of two forms of DNA polymerase alpha from HeLa cell nuclei. Biochemistry. 1983 Mar 1;22(5):1134–1138. doi: 10.1021/bi00274a022. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. L. Polyamine-activated protein phosphatase activity in HeLa cell nuclei. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1372–1378. doi: 10.1016/0006-291x(86)90401-8. [DOI] [PubMed] [Google Scholar]
- Fuller D. J., Gerner E. W., Russell D. H. Polyamine biosynthesis and accumulation during the G1 to S phase transition. J Cell Physiol. 1977 Oct;93(1):81–88. doi: 10.1002/jcp.1040930111. [DOI] [PubMed] [Google Scholar]
- Gallo C. J., Koza R. A., Herbst E. J. Polyamines and HeLa-cell DNA replication. Biochem J. 1986 Aug 15;238(1):37–42. doi: 10.1042/bj2380037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O., Gray J. W., Lindl P. A., Marton L. J., Wilson C. B. Changes in L-ornithine decarboxylase activity during the cell cycle. Biochem Biophys Res Commun. 1976 Jul 12;71(1):99–105. doi: 10.1016/0006-291x(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Herbst E. J., Dion A. S. Polyamine changes during development of Drosophila melanogaster. Fed Proc. 1970 Jul-Aug;29(4):1563–1567. [PubMed] [Google Scholar]
- Herbst E. J., Elliot Q. D. Role of polyamines in HeLa cell proliferation. Med Biol. 1981 Dec;59(5-6):410–416. [PubMed] [Google Scholar]
- Knutson J. C., Morris D. R. Cellular polyamine depletion reduces DNA synthesis in isolated lymphocyte nuclei. Biochim Biophys Acta. 1978 Sep 27;520(2):291–301. doi: 10.1016/0005-2787(78)90228-9. [DOI] [PubMed] [Google Scholar]
- Krokan H., Bjorklid E., Prydz H. DNA synthesis in isolated HeLa cell nuclei. Optimalization of the system and characterization of the product. Biochemistry. 1975 Sep 23;14(19):4227–4232. doi: 10.1021/bi00690a012. [DOI] [PubMed] [Google Scholar]
- Krokan H., Eriksen A. DNA synthesis in HeLa cells and isolated nuclei after treatment with an inhibitor of spermidine synthesis, methyl glyoxal bis(guanylhydrazone). Eur J Biochem. 1977 Feb;72(3):501–508. doi: 10.1111/j.1432-1033.1977.tb11273.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamothe P., Baril B., Chi A., Lee L., Baril E. Accessory proteins for DNA polymerase alpha activity with single-strand DNA templates. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4723–4727. doi: 10.1073/pnas.78.8.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle control. A cycle is a cycle is a cycle. Nature. 1987 May 7;327(6117):14–15. doi: 10.1038/327014a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle control. A cycle is a cycle is a cycle. Nature. 1987 May 7;327(6117):14–15. doi: 10.1038/327014a0. [DOI] [PubMed] [Google Scholar]
- Pritchard C. G., Weaver D. T., Baril E. F., DePamphilis M. L. DNA polymerase alpha cofactors C1C2 function as primer recognition proteins. J Biol Chem. 1983 Aug 25;258(16):9810–9819. [PubMed] [Google Scholar]
- Seidenfeld J., Block A. L., Komar K. A., Naujokas M. F. Altered cell cycle phase distributions in cultured human carcinoma cells partially depleted of polyamines by treatment with difluoromethylornithine. Cancer Res. 1986 Jan;46(1):47–53. [PubMed] [Google Scholar]
- Seyfried C. E., Morris D. R. Relationship between inhibition of polyamine biosynthesis and DNA replication in activated lymphocytes. Cancer Res. 1979 Dec;39(12):4861–4867. [PubMed] [Google Scholar]
- Song M. K., Adolph K. W. Phosphorylation of nonhistone proteins during the HeLa cell cycle. Relationship to DNA synthesis and mitotic chromosome condensation. J Biol Chem. 1983 Mar 10;258(5):3309–3318. [PubMed] [Google Scholar]
- Stimac E., Morris D. R. Messenger RNAs coding for enzymes of polyamine biosynthesis are induced during the G0-G1 transition but not during traverse of the normal G1 phase. J Cell Physiol. 1987 Dec;133(3):590–594. doi: 10.1002/jcp.1041330323. [DOI] [PubMed] [Google Scholar]
- Verma R., Chen K. Y. Spermine inhibits the phosphorylation of the 11,000- and 10,000-dalton nuclear proteins catalyzed by nuclear protein kinase NI in NB-15 mouse neuroblastoma cells. J Biol Chem. 1986 Feb 25;261(6):2890–2896. [PubMed] [Google Scholar]
- Vishwanatha J. K., Coughlin S. A., Wesolowski-Owen M., Baril E. F. A multiprotein form of DNA polymerase alpha from HeLa cells. Resolution of its associated catalytic activities. J Biol Chem. 1986 May 15;261(14):6619–6628. [PubMed] [Google Scholar]
- Vishwanatha J. K., Yamaguchi M., DePamphilis M. L., Baril E. F. Selection of template initiation sites and the lengths of RNA primers synthesized by DNA primase are strongly affected by its organization in a multiprotein DNA polymerase alpha complex. Nucleic Acids Res. 1986 Sep 25;14(18):7305–7323. doi: 10.1093/nar/14.18.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]



