Abstract
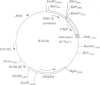
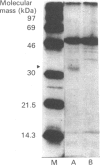
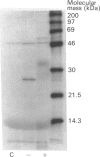
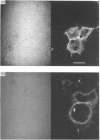
A naturally secreted protein, tissue inhibitor of metalloproteinases (TIMP), has been transiently expressed on the surface of transfected COS cells and stably on transfected murine BW 5147 thymoma cells, by linkage of the entire coding sequence of the cDNA to the last exon of Thy-1. Thy-1 is a glycophospholipid-linked protein. In COS cells the chimaeric protein can be labelled by [3H]ethanolamine, which is a component of glycophospholipid anchors. Ltk- cells cannot anchor proteins by glycan phosphatidylinositol linkage and were found to be unable to express the engineered protein extracellularly on their plasma membranes. Phosphatidylinositol-specific phospholipase C treatment released 90% of the protein from all BW 5147 cells, but very little from the COS-1 cells. It is concluded that the last exon of Thy-1 has conferred the property of glycophospholipid anchorage on the normally secreted protein TIMP.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J., Howard A. D., Brink L., Gerber L., Hauber J., Cullen B. R., Udenfriend S. COOH-terminal requirements for the correct processing of a phosphatidylinositol-glycan anchored membrane protein. J Biol Chem. 1988 Jul 15;263(20):10016–10021. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brown D. A., Crise B., Rose J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989 Sep 29;245(4925):1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Williams S. R. Analysis of the signal for attachment of a glycophospholipid membrane anchor. J Cell Biol. 1989 Apr;108(4):1387–1396. doi: 10.1083/jcb.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockett M. I., Bebbington C. R., Yarranton G. T. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology (N Y) 1990 Jul;8(7):662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Selvaraj P., Mattaliano R. J., Springer T. A. Anchoring mechanisms for LFA-3 cell adhesion glycoprotein at membrane surface. 1987 Oct 29-Nov 4Nature. 329(6142):846–848. doi: 10.1038/329846a0. [DOI] [PubMed] [Google Scholar]
- Edidin M., Stroynowski I. Differences between the lateral organization of conventional and inositol phospholipid-anchored membrane proteins. A further definition of micrometer scale membrane domains. J Cell Biol. 1991 Mar;112(6):1143–1150. doi: 10.1083/jcb.112.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Gibney G., Taylor P. Biosynthesis of Torpedo acetylcholinesterase in mammalian cells. Functional expression and mutagenesis of the glycophospholipid-anchored form. J Biol Chem. 1990 Jul 25;265(21):12576–12583. [PubMed] [Google Scholar]
- Giguére V., Isobe K., Grosveld F. Structure of the murine Thy-1 gene. EMBO J. 1985 Aug;4(8):2017–2024. doi: 10.1002/j.1460-2075.1985.tb03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. L., Selvaraj P., Carpén O., Springer T. A., Kuster H., Jouvin M. H., Kinet J. P. Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16). Science. 1989 Dec 22;246(4937):1608–1611. doi: 10.1126/science.2531918. [DOI] [PubMed] [Google Scholar]
- Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W., Anand R., Williams A. F. Complete structure of the glycosyl phosphatidylinositol membrane anchor of rat brain Thy-1 glycoprotein. Nature. 1988 May 19;333(6170):269–272. doi: 10.1038/333269a0. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1988 Mar 15;250(3):865–869. doi: 10.1042/bj2500865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. Somatic genetic analysis of the expression of cell surface molecules. Trends Genet. 1988 Jan;4(1):5–8. doi: 10.1016/0168-9525(88)90120-5. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Ravetch J. V. A single amino acid in the glycosyl phosphatidylinositol attachment domain determines the membrane topology of Fc gamma RIII. Nature. 1989 Dec 14;342(6251):805–807. doi: 10.1038/342805a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Cwirla S., Yu G., Testi R., Phillips J. H. Membrane anchoring of a human IgG Fc receptor (CD16) determined by a single amino acid. Science. 1989 Dec 22;246(4937):1611–1613. doi: 10.1126/science.2531919. [DOI] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Raab H., Kohr W. J., Caras I. W. Glycophospholipid membrane anchor attachment. Molecular analysis of the cleavage/attachment site. J Biol Chem. 1991 Jan 15;266(2):1250–1257. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont S., Regnier-Vigouroux A., Naquet P., Blanc D., Pierres A., Marchetto S., Pierres M. Analysis of the Thy-1 pathway of T cell hybridoma activation using 17 rat monoclonal antibodies reactive with distinct Thy-1 epitopes. Eur J Immunol. 1985 Dec;15(12):1222–1228. doi: 10.1002/eji.1830151215. [DOI] [PubMed] [Google Scholar]
- Post T. W., Arce M. A., Liszewski M. K., Thompson E. S., Atkinson J. P., Lublin D. M. Structure of the gene for human complement protein decay accelerating factor. J Immunol. 1990 Jan 15;144(2):740–744. [PubMed] [Google Scholar]
- Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988 Nov 1;174(2):361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Krall J. A., Dever T. E., Haas R., Louvard D., Merrick W. C. Biosynthetic incorporation of [3H]ethanolamine into protein synthesis elongation factor 1 alpha reveals a new post-translational protein modification. J Biol Chem. 1989 May 5;264(13):7096–7099. [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Moriuchi T., Chang H. C., Denome R., Silver J. Structural organization of the rat thy-1 gene. Nature. 1985 Feb 7;313(6002):485–487. doi: 10.1038/313485a0. [DOI] [PubMed] [Google Scholar]
- Seki T., Spurr N., Obata F., Goyert S., Goodfellow P., Silver J. The human Thy-1 gene: structure and chromosomal location. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6657–6661. doi: 10.1073/pnas.82.19.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Rosse W. F., Silber R., Springer T. A. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988 Jun 9;333(6173):565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- Singh N., Singleton D., Tartakoff A. M. Anchoring and degradation of glycolipid-anchored membrane proteins by L929 versus by LM-TK- mouse fibroblasts: implications for anchor biosynthesis. Mol Cell Biol. 1991 May;11(5):2362–2374. doi: 10.1128/mcb.11.5.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Southern P. J. Analysis of gene expression using simian virus 40 vectors. Anal Biochem. 1983 Nov;135(1):1–15. doi: 10.1016/0003-2697(83)90723-6. [DOI] [PubMed] [Google Scholar]
- Toutant J. P., Richards M. K., Krall J. A., Rosenberry T. L. Molecular forms of acetylcholinesterase in two sublines of human erythroleukemia K562 cells. Sensitivity or resistance to phosphatidylinositol-specific phospholipase C and biosynthesis. Eur J Biochem. 1990 Jan 12;187(1):31–38. doi: 10.1111/j.1432-1033.1990.tb15274.x. [DOI] [PubMed] [Google Scholar]
- Toutant J. P., Roberts W. L., Murray N. R., Rosenberry T. L. Conversion of human erythrocyte acetylcholinesterase from an amphiphilic to a hydrophilic form by phosphatidylinositol-specific phospholipase C and serum phospholipase D. Eur J Biochem. 1989 Apr 1;180(3):503–508. doi: 10.1111/j.1432-1033.1989.tb14674.x. [DOI] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Ulker N., Lewis K. D., Hood L. E., Stroynowski I. Activated T cells transcribe an alternatively spliced mRNA encoding a soluble form of Qa-2 antigen. EMBO J. 1990 Dec;9(12):3839–3847. doi: 10.1002/j.1460-2075.1990.tb07602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P., Khokha R., Denhardt D. T. Modulation of translation by the 5' leader sequence of the mRNA encoding murine tissue inhibitor of metalloproteinases. J Biol Chem. 1990 Apr 5;265(10):5585–5589. [PubMed] [Google Scholar]