Abstract
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is a demyelinating disorder, distinct from multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). MOGAD most frequently presents with optic neuritis (MOG-ON), often with characteristic clinical and radiological features. Bilateral involvement, disc swelling clinically and radiologically, and longitudinally extensive optic nerve hyperintensity with associated optic perineuritis on MRI are key characteristics that can help distinguish MOG-ON from optic neuritis due to other aetiologies. The detection of serum MOG immunoglobulin G utilising a live cell-based assay in a patient with a compatible clinical phenotype is highly specific for the diagnosis of MOGAD. This review will highlight the key clinical and radiological features which expedite diagnosis, as well as ancillary investigations such as visual fields, visual evoked potentials and cerebrospinal fluid analysis, which may be less discriminatory. Optical coherence tomography can identify optic nerve swelling acutely, and atrophy chronically, and may transpire to have utility as a diagnostic and prognostic biomarker. MOG-ON appears to be largely responsive to corticosteroids, which are often the mainstay of acute management. However, relapses are common in patients in whom follow-up is prolonged, often in the context of early or rapid corticosteroid tapering. Establishing optimal acute therapy, the role of maintenance steroid-sparing immunotherapy for long-term relapse prevention, and identifying predictors of relapsing disease remain key research priorities in MOG-ON.
Subject terms: Optic nerve diseases, Eye manifestations
Abstract
髓鞘少突胶质细胞糖蛋白 (MOG)抗体相关疾病(MOGAD)是一种脱髓鞘疾病, 有别于多发性硬化症 (MS) 和视神经脊髓炎视神经疾病 (NMOSD) 。MOGAD最常表现为视神经炎 (MOG-ON), 通常具有特征性的临床和影像学特征。MOGAD双侧受累、临床和影像学表现为视盘肿胀以及纵向MRI上广泛视神经高信号伴相关视神经束膜炎是有助于区分MOG-ON与其他病因所致视神经炎的关键特征。在具有相同临床表型的患者中, 利用基于活细胞的测定法检测血清MOG免疫球蛋白G对于MOGAD的诊断具有高度特异性。本综述将重点介绍加快诊断的关键临床和影像学特征及辅助检查, 如视野, 视觉诱发电位和脑脊液分析。这些辅助检查的诊断价值相对较弱, OCT可以识别急性视神经肿胀和慢性视神经萎缩, 并可能作为诊断和预后的生物标志物。MOG-ON似乎对皮质类固醇较敏感, 皮质类固醇通常是急性期治疗的一线药物。然而, 在随访时间延长的患者中很容易复发, 通常是在早期或快速减少皮质类固醇的情况下。最佳策略的的急性期治疗, 维持类固醇保留免疫疗法在长期预防复发中的作用, 以及确定疾病复发的预测因子仍然是MOG-ON的关键研究重点。
Introduction
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a demyelinating disorder of the central nervous system (CNS) distinct from multiple sclerosis (MS) and aquaporin 4 (AQP4) immunoglobulin G (IgG) antibody-associated neuromyelitis optica spectrum disorder (NMOSD). MOGAD refers to a demyelinating syndrome in association with IgG autoantibodies targeting myelin oligodendrocyte glycoprotein (MOG), a minor transmembrane surface protein found on the outermost lamellae of CNS myelin and oligodendrocytes [1, 2]. The annual incidence of MOGAD worldwide is approximately 1.6–4.8 per million people, with a prevalence estimated at 1.3–2.5 per 100,000 people [3–5]. There is a biphasic distribution of the age of onset, peaking in children aged 5–10 years as well as in adults aged 20–45 years. The median age of onset overall is 20–30 years [6, 7]. MOGAD accounts for nearly 50% of acute demyelinating syndromes in children under 11 years of age [8]. Optic neuritis (ON) is the most common initial manifestation of MOGAD in adults (~30–60%), followed by transverse myelitis (~10–25%), and is the second most common manifestation in children, after acute disseminated encephalomyelitis (ADEM; ~45% in children <11 years of age vs. <5% in adults) [1, 9]. This review aims to summarise current knowledge on MOG antibody-associated optic neuritis (MOG-ON), from presentation to diagnosis and treatment, in order to assist clinicians in managing this increasingly recognised condition.
Historical evolution and pathophysiology of MOGAD in human demyelination
Early studies of MOG IgG were hampered by the use of Western blots and enzyme linked immunosorbent assays (ELISAs), which denature and linearise the MOG peptide respectively, while it was identified that only MOG IgG that binds to MOG in its native conformational state is potentially pathogenic [10–16]. O’Connor et al., using a MOG tetramer radioimmunoassay, first identified the presence of MOG-IgG in children presenting with ADEM [17]. Since then, it has been the advent of live cell-based assays, which has enabled native human MOG to be transduced or transfected into a mammalian cell line for surface expression, incubated with sera and a secondary anti-human IgG antibody, and analysed qualitatively (with microscopy) or quantitatively (with flow cytometry) for MOG-specific antibody binding [18–22]. This methodology has enabled the detection of clinically relevant MOG-IgG and accurate identification of children and adults with a demyelinating syndrome distinct from MS and NMOSD.
Histopathological data on MOGAD in humans is limited. MOGAD is an oligodendrogliopathy rather than an astrocytopathy like NMOSD, where the antigen is AQP4 expressed on astrocytic end feet. The immune response in MOGAD-related inflammatory plaques appears to be dominated by CD4-positive T cells with lesser infiltration of CD8-positive T cells and B cells [23]. Other histopathological features include variable infiltration of granulocytes, MOG-laden macrophages, some complement and Ig deposition in active white matter lesions, variable oligodendrocyte and axonal destruction, astrogliosis, and some overlapping features with MS and ADEM [23, 24]. There are divergent findings regarding whether or not there is preferential loss of MOG [23, 24]. Moreover, pre-myelinating oligodendrocytes are sometimes visible without signs of active remyelination [23].
The CSF profile of MOGAD patients can display elevated levels of both B cell and T cell-related pro-inflammatory cytokines and chemokines, supporting complex innate and adaptive immune activation [25]. Some of these cytokines, such as IL-6, have potential as therapeutic targets. Indeed, the off-label use of tocilizumab (an IL-6 receptor antibody) in MOGAD has been shown to prevent relapses over a duration of up to 29 months [26].
One study observed specific B-cells in the periphery of MOGAD patients, though a correlation with MOG-IgG titres was absent [27]. More detailed analyses revealed increased memory B-cells and T-follicular helper cells, as well as decreased regulatory B-cells, in MOGAD [28]. The search for MOG specific T cells in MOGAD has been challenging, and stimulation of peripheral blood mononuclear cells with different MOG peptides has not been shown to lead to antigen specific responses thus far [29].
Antibodies taken from the sera of patients with MOG-ON, which are primarily of the IgG1 isotype, induce demyelination in animal models with involvement of the complement pathway if they are cross-reactive with rodent MOG in combination with myelin basic protein-specific T cells; together with MOG-specific T cells, they evoke enhanced T cell infiltration [30]. Studies examining the potential for MOG-IgG to activate the complement system in humans made divergent observations and different complement-independent mechanisms have also been demonstrated [31, 32].
The trigger for antibody production in MOGAD is unknown. As with many other cell surface antibody-associated CNS disorders, it is believed that a break in immune tolerance may be triggered in the peripheral circulatory compartment, with subsequent passage of immune cells into the intrathecal compartment and the CNS, and ongoing trafficking of immune cells between the brain parenchyma, CSF and circulation [33]. Cases of vaccination and infection preceding MOGAD are documented [34–36]. These may trigger the autoimmune cascade by mechanisms such as bystander activation, whereby chemokines and cytokines released in the immune response to a foreign pathogen activate autoreactive lymphocytes; or, less likely as the infectious agent reported in the literature varies, molecular mimicry, whereby similarities between foreign and self-antigens activates autoreactive lymphocytes. Other systemic autoimmune disorders appear to be uncommon in MOGAD, unlike in NMOSD [37]. Cancer diagnoses associated with MOGAD presentations are very rare (<1%), and likely represent the background malignancy risk rather than a paraneoplastic association [37]. Tumour necrosis factor alpha inhibitor therapy has been rarely associated with the development of MOGAD [38].
The pathophysiology of ON in MOGAD is particularly interesting as MOG is not expressed in the retina[39]. Other mechanisms have been proposed to contribute to retinal ganglion cell degeneration, such as glutamate cytotoxicity [40], as well as the lack of classical blood-brain barrier characteristics of the optic nerve head [41].
Clinical presentation of MOG-ON
MOG-ON presents with typical symptoms of acute ON such as pain and loss of visual acuity. However, certain features, such as bilateral involvement, optic disc swelling and optic perineuritis are characteristic and favour the diagnosis of MOG-ON over other demyelinating aetiologies such as NMOSD and MS. Table 1 highlights the commonalities and differences between ON associated with these three key aetiologies with respect to demographics, clinical and radiological characterisation, investigations, and therapeutic and prognostic features.
Table 1.
Comparison of MOG-ON, AQP4-ON and MS-ON.
| Characteristic | MOG-ON | AQP4-ON | MS-ON |
|---|---|---|---|
| Demographics | |||
| Age at onset | Paediatric and adult onset |
Adult onset Paediatric onset rare |
Adult onset Paediatric onset less common |
| Sex assigned at birth (F:M) | 1:1 | 7–9:1 | 3:1 |
| Ethnic propensity | Caucasian | Afro-Caribbean | Caucasian |
| Disease course | Monophasic or relapsing | Most often relapsing |
Relapsing Secondary progressive Infrequently primary progressive |
| Clinical assessment | |||
| Clinically bilateral at onset | Frequent | May be present | Extremely rare |
| Associated headache | Frequent | Rare | Rare |
| Visual acuity at nadir | Moderate - severely impaired | Moderate - severely impaired | Mild - moderately impaired |
| Fundoscopy at nadir | Moderate - severe optic disc swelling is common; may have associated haemorrhages | May be present | Mild optic disc swelling may be present; rarely moderate-severe |
| Initial recovery | Typically favourable, particularly with corticosteroids | May be poor | Typically favourable |
| Radiological characterisation | |||
| Radiological optic nerve head swelling | May be present | Rare | Rare |
| Longitudinally extensive optic nerve involvement (>½ length of optic nerve) | Frequent | Frequent | Rare; typically focal optic nerve involvement |
| Optic nerve sheath involvement (optic perineuritis) | Frequent | Rare | Not reported |
| Optic nerve oedema | Frequent | Frequent | May be present |
| Optic chiasmal involvement | Less frequent (unless extension of contiguous longitudinally extensive optic neuritis) | May be present | Rare |
| Optic tract involvement | Rare | May be present | Rare |
| Visual pathway involvement | Frequently anterior | Frequently posterior | Frequently anterior |
| Ancillary investigations | |||
| Visual fields |
Variable Peripheral vision loss in optic perineuritis |
Variable | Variable |
| OCT | |||
| Acute | pRNFL thickening; may be significantly swollen | pRNFL thickening | pRNFL thickening |
| Follow-up | pRNFL thinning (MOG > MS) | pRNFL thinning | pRNFL thinning |
| CSF | |||
| Pleocytosis | Frequent; can be very high (>50 cells/μL), especially if accompanying myelitis | Frequent; can be very high (>50 cells/μL) | Variable; rarely very high |
| Elevated protein | May be present | May be present | Less common |
| Oligoclonal bands | May be present | May be present | Very frequent |
| Therapeutic and prognostic features | |||
| Rapid steroid responsiveness and steroid dependence | Frequent | Less frequent | Less frequent |
| Long term visual recovery | Favourable in absence of subsequent relapses | May be poor | Favourable |
OCT optical coherence tomography, pRNFL peripapillary retinal nerve fibre layer, MOG myelin oligodendrocyte glycoprotein, MS multiple sclerosis.
Bilateral optic neuritis
Simultaneous, bilateral ON is a common onset presentation for MOG-ON, occurring in 31–84% of cases [7, 22, 42–45], but is rare in MS-ON [45, 46]. Bilateral ON is described in 13–82% of cases of AQP4-ON, noting variability in sample sizes across studies. Overall, the literature favours a higher frequency of bilateral ON reported in MOG-ON compared to AQP4-ON [44–46].
Optic disc swelling
The presence of severe optic disc swelling and peripapillary haemorrhage in the setting of ON is highly suggestive of MOG-ON. Optic disc swelling visible on fundoscopy has been reported in 45–92% of cases of MOG-ON across various international cohorts [22, 43–45, 47–52], and is an important diagnostic clue when assessing patients with ON. This is far more common than the frequencies reported in AQP4-ON (7–52%) [44, 48, 50, 51], and MS-ON (11–14%) [49, 51]. Optic disc swelling is moderate to severe in many cases of MOG-ON and has been associated with peripapillary haemorrhage [43, 46]. MOG-ON with bilateral optic disc swelling may be mistaken for idiopathic intracranial hypertension, which affects a similar age group and is more common, though does not usually cause the rapid and substantial vision loss or pain on eye movement seen in MOG-ON [53]. MOGAD can also cause papilloedema secondary to raised intracranial pressure, in association with an aseptic meningoencephalitis presentation [54, 55].
Eye pain
Eye pain may be common in MOG-ON, reported in 73–92% of cases internationally [42, 43, 48, 51, 56, 57]. In comparative studies, this proportion has been shown to be greater than in AQP4-ON (28–50%) and seronegative ON, including MS (10–46%) [48, 51, 58]. The majority of eye pain in MOG-ON is pain related to eye movement, with periocular pain being less common [56, 57]. Pain on eye movement may arise from traction on the common tendinous ring caused by retro-orbital pathology, whereas periocular pain is presumed to be governed by the trigeminal nerve. Both types of pain have been shown to be more common when the intra-orbital segment of the optic nerve is inflamed, as is often the case in MOG-ON, and less common when inflammation is restricted to the canalicular or intracranial segments [45, 59]. One study also showed that eye pain occurred more frequently in patients with MOG-ON who had optic nerve sheath enhancement on MRI than in those without, suggesting that spread of inflammation from the optic nerve to the surrounding meningeal nerve sheath containing nociceptive fibres from the trigeminal nerve may be responsible for pain [60]. Associated eye pain can be severe enough to result in a characteristic headache, as reported in adults, which can extend from the ocular region to the periorbital and fronto-temporal areas [61]. Pain usually precedes vision loss, by a median of three days in one study, but does not appear to be related to the severity of visual loss itself [57].
Visual acuity at nadir
Visual acuity deficits at nadir in MOG-ON are generally moderate-to-severe, often logMAR 1.0 (Snellen equivalent 6/60) or worse [1, 43, 57]. Many studies comparing visual acuity and associated visual functional system scores at follow up in MOG-ON and AQP4-ON have shown worse outcomes in AQP4-ON [45, 51, 58], though rates of severe vision loss at nadir, defined as logMAR >1.0 seem to be similar in both conditions [48, 62]. Chen et al. [57] found that visual acuity tended to worsen for a median of four days from onset before reaching nadir in MOG-ON. It is important to note, however, that visual acuity can rarely be normal in MOGAD, in the case of optic perineuritis with sparing of the optic nerve itself [63, 64].
Colour vision loss and relative afferent pupillary defect
Red desaturation and a relative afferent pupillary defect (RAPD) are common findings in ON due to any cause, and in limited studies have been identified in patients with MOG-ON [56]. However, it is important to note that in MOG-ON, where bilateral disease is common, a RAPD may be absent with bilateral, or previous contralateral, optic nerve involvement.
Associations with other ophthalmic presentations
Aside from ON, various other ophthalmic presentations have also been reported in association with MOGAD. These include serum MOG IgG reported in association with uveitis [63, 65], peripheral ulcerative keratitis [63], acute macular neuroretinopathy [66], serous retinal detachment [67], venous stasis retinopathy [68], pre-retinal macular haemorrhage [69], orbital inflammatory syndrome [70] and orbital apex syndrome [71]. Nearly all cases were accompanied by intercurrent, typical MOG-ON, with retinal and orbital manifestations believed to be secondary to the spread of adjacent inflammation and oedema from the optic nerve and nerve head. The association of typical MOG-ON in association with these more atypical ophthalmic findings is important to note. It is not advisable to associate atypical ophthalmic presentations with the presence of low-titre or fixed cell based assay detection of MOG-IgG, as sensitivity and specificity in a clinical context with a low pretest probability is likely to dilute the clinical relevance of this biomarker. Prominent retinal changes are uncommon in MOG-ON and not reported in AQP4-ON but the association between uveitis and MS is well established [72]. An important distinction, is that uveitis in MOGAD has been reported as being anterior [63, 65], posterior [63], or intermediate [34, 63], whereas MS-associated uveitis is predominantly intermediate [73].
Non-ophthalmic clinical associations
It is important to note that the onset of ON in MOGAD may also coincide with other typical MOGAD phenotypes, such as ADEM in children [74], and transverse myelitis in children and adults [20, 75]. Other phenotypes of MOGAD may also be recognised in association with ON, such as cerebral cortical encephalitis with seizures, aseptic meningitis and brainstem and cerebellar syndromes [76].
Investigations
Ancillary investigations play an important role in the early diagnosis and management of ON. MRI is the imaging modality of choice to confirm the presence of optic neuritis and displays characteristic features in MOG-ON that can help distinguish it from other aetiologies. The results of other routine ophthalmological tests such as visual field testing or perimetry, optical coherence tomography (OCT) and visual evoked potentials (VEPs), as well as CSF analysis, are less specific in distinguishing aetiology but may still have a contributory role, particularly in confirming objective evidence of visual pathway involvement or the inflammatory nature of the disease (see Table 1).
MRI
Optic nerve inflammation is best visualised on dedicated orbital MRI with T2 and T1 sequences with fat-suppression and gadolinium enhancement, and thin cuts of 1–3 mm these sequences should be specifically requested in the assessment of all patients with ON [77]. The presence of optic nerve enhancement is often present in ON and may distinguish it from non-inflammatory optic neuropathies [45, 78, 79]. Both gadolinium enhancement and T2 hyperintensity are present in many cases of MOG-ON, and T2 hyperintensity has been shown to be more prominent in MOG-ON and AQP4-ON than MS-ON [45]. Key radiological features of MOG-ON are described below and illustrated in Figs. 1 and 2.
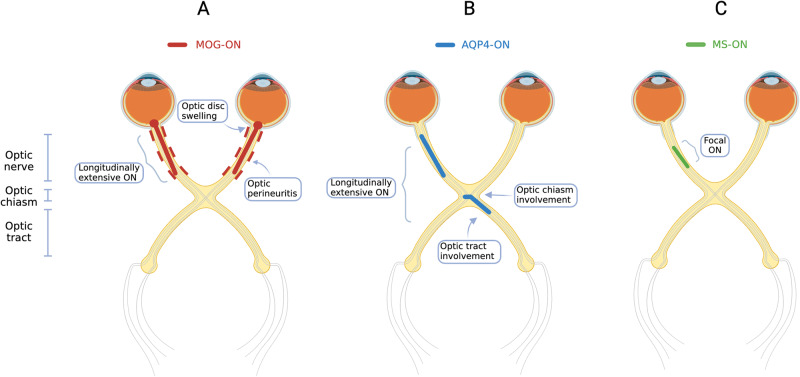
Fig. 1. Localisation of visual pathway involvement in MOG-ON, AQP4-ON and MS-ON.
A MOG-ON (red): Typical features include bilateral longitudinally extensive ON, with optic disc swelling, involvement of the retrobulbar segment of the optic nerve, and optic nerve sheath involvement or optic perineuritis. B AQP4-ON (blue): Longitudinally extensive ON, with optic chiasm +/− optic tract involvement. C MS-ON (green): focal ON. AQP4 aquaporin 4, MOG myelin oligodendrocyte glycoprotein, MS multiple sclerosis, ON optic neuritis.
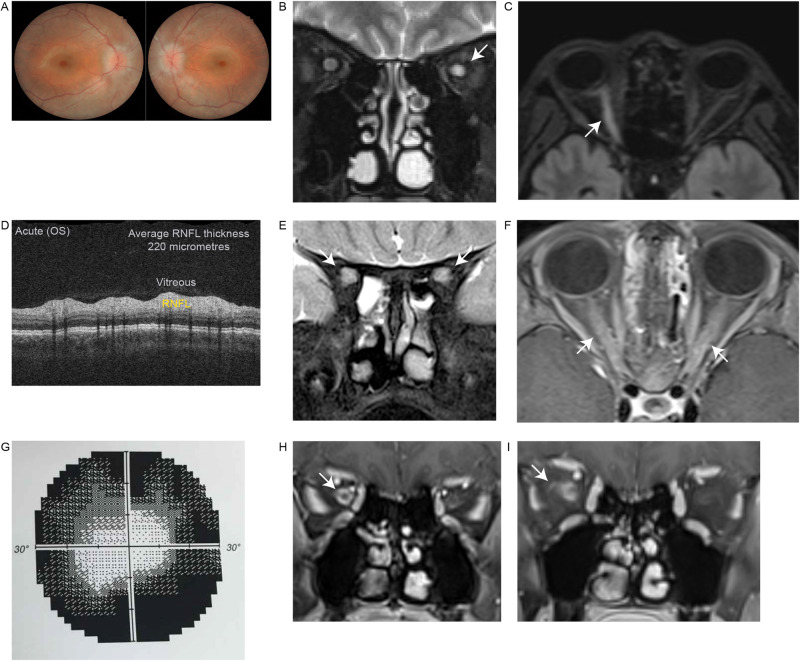
Fig. 2. Clinical and radiological characterisation of MOG antibody-associated optic neuritis.
A Bilateral optic nerve head swelling and oedema visualised on fundoscopy in a patient with a first presentation of MOGAD. B Unilateral left optic nerve T2 hyperintensity (arrow) (Cor T2). C Unilateral right sided longitudinally extensive optic neuritis (arrow) with associated T2 hyperintensity and swelling (Ax T2). D During acute left optic neuritis, swelling resulted in increased periparillary retinal nerve fibre layer (RNFL – yellow) thickness on optical coherence tomography. E Bilateral optic neuritis (arrows) with marked T2 hyperintensity (Cor T2 FS). F Bilateral longitudinally extensive optic neuritis (arrows) with gadolinium enhancement (Ax T1 FS Gad). G Visual field testing with dense peripheral constriction and relative central sparing in a pattern strongly suggestive of optic perineuritis. H Right optic perineuritis (arrow) with optic nerve sheath inflammation and enhancement (Cor T1 FS Gad). I Right optic perineuritis with extension of enhancement into intraorbital fat (arrow) (Cor T1 FS Gad). Figure 1A, G was reproduced from Ramanathan et al. [63] with permission from John Wiley and Sons. Ax axial, Cor coronal, FS fat suppressed, Gad gadolinium enhancement, MOGAD myelin oligodendrocyte glycoprotein antibody-associated disease, OS oculus sinister (left eye), RNFL retinal nerve fibre layer.
Longitudinally extensive lesions
Longitudinally extensive optic neuritis is defined as ON involving more than 50% of the pre-chiasmal optic nerve length. This is characteristic of MOG-ON, occurring in 23–88% of patients, whereas it is rare in MS-ON [45, 77]. Whilst longitudinally extensive optic neuritis is also observed in AQP4-ON, in the range of 50–79%, longer segments are more frequently involved in MOG-ON [77].
Retrobulbar involvement
Multiple studies, including a recent meta-analysis, have established that MOG-ON most commonly affects the retrobulbar segment of the optic nerve [45, 79, 80]. In contrast, AQP4-ON tends to favour the more posterior canalicular and intracranial segments [80]. However, a review of comparative studies concluded that lesion location did not differentiate the two groups overall [77].
Reported rates of optic chiasm involvement vary widely between studies, likely owing to differences in sample size, and ranges from 5 to 17% in MOG-ON and 2 to 64% in AQP4-ON [45, 62, 81–83]. One study [45] detected a statistically significant difference between these rates in MOG-ON and AQP4-ON, whereas other studies did not find a significant difference [62, 81–83]. Importantly, chiasmal involvement, when it occurs in MOG-ON, is more likely to be part of longitudinally extensive disease extending up to the chiasm, in contrast to AQP4-ON where isolated chiasmal involvement can occur [81]. In MS-ON, chiasmal involvement is extremely rare.
Fewer studies have examined the rates of optic tract involvement, and whilst higher rates have been reported in AQP4-ON (4–13%) than MOG-ON (0-5%), this has not been shown to be statistically significant, likely due its rarity in general [62, 82, 83]. However, one study looking at bilateral optic tract involvement specifically, found this to be exclusive to AQP4-ON, where it was present in 45% of cases, compared to not being present in MOG-ON or MS-ON [45].
Optic disc swelling
While more commonly reported on fundoscopy as previously mentioned, or on optical coherence tomography (OCT), optic nerve head swelling can also be visualised on orbital MRI. Radiologically visible optic disc swelling is more common in MOG-ON than AQP4-ON and MS-ON [45, 80].
Optic perineuritis
Optic perineuritis is observed on MRI as circumferential, ‘tram-track’ enhancement of the optic nerve sheath, which may extend into the surrounding orbital fat [84]. This was first reported as a manifestation of MOGAD by Kim et al. in 2015 [85], and, since then, several other reports have emerged in the literature, both with [43, 63], and without [47, 64, 86], accompanying optic nerve involvement. MOGAD is now recognised as an important cause of optic perineuritis, alongside sarcoidosis, which remains the major differential diagnosis for this presentation [87]. Furthermore, very severe cases of MOG-related optic perineuritis leading to orbital apex syndrome, where there is involvement of multiple cranial nerves that traverse the orbital apex, leading to ptosis, proptosis and ophthalmoplegia, have been described [71, 88]. Interestingly, it has been hypothesised that focally elevated pressures in the subarachnoid space due to perineural inflammation may be responsible for the optic disc swelling in MOG-ON, linking these two features [86].
Perimetry
A wide variety of non-specific visual field defects have been reported in MOG-ON including central and paracentral scotomas, temporal field cut and complete visual field loss [56, 89]. Perhaps the only distinctive pattern is that of peripheral vision loss with sparing of central vision, which can occur in MOG antibody-associated optic perineuritis, though this pattern is more commonly seen with alternate aetiologies such as raised intracranial pressure [63, 86]. At follow-up post-ON, the degree of visual field loss, measured as mean deviation from normal, has been reported to be better in MOG-ON than in AQP4-ON in one study [90] but not significantly different from MS-ON in another study [58].
Optical coherence tomography
In the acute stages, MOG-ON leads to prominent thickening of the peripapillary retinal nerve fibre layer (pRNFL) on OCT, reflecting oedema. In fact, the amount of pRNFL thickening in acute MOG-ON has been shown to be significantly greater than in MS-ON (median pRNFL thickness 164 μm vs. 103 μm respectively), consistent with the more frequent observation of fundoscopic optic disc swelling and may be because of its unique predilection for the intra-orbital and longer segments of the optic nerve [91]. OCT may therefore have a place as an early diagnostic tool to differentiate MOG-ON from MS-ON. Chen et al. found that using a pRNFL thickness cut-off of 118 μm afforded a sensitivity and specificity for MOG-ON of 74% and 82% respectively, compared with MS-ON [91]. Similar comparisons have not yet been made with acute AQP4-ON.
Conversely, in the months following acute ON, thinning of the pRNFL as well as of the macular ganglion cell inner plexiform layer (GCIPL) occurs with significantly lower pRNFL and GCIPL thickness found in MOG-ON than in both healthy controls and in MS-ON according to a recent meta-analysis [92, 93]. However, no significant difference was observed in these parameters when compared to AQP4-ON [92].
Relapses may be the main driver of pRNFL atrophy in MOG-ON, with some studies demonstrating a positive correlation between the number of ON episodes and reduction in pRNFL thickness in MOG-ON but not in MS-ON or AQP4-ON [93, 94]. Meanwhile, Akaishi et al. showed that pRNFL thickness was not correlated with months post-ON in MOG-ON, suggesting that retinal thinning in MOG-ON may be primarily driven by acute relapses rather than chronic or progressive atrophy [95].
Furthermore, post-ON pRNFL thinning has also been shown to be linked to functional visual measures in MOG-ON such as worse mean visual field defects and visual acuity [58, 90]. Interestingly, however, functional outcomes such as these seem to be better in MOG-ON than in AQP4-ON despite similar structural damage as described earlier. This mismatch between structure and function may be related to differences in the histological composition of the various retinal layers in these pathologies, which is not captured by OCT, for instance due to differences in the glial population [92]. A floor effect may also be operative, whereby a few, severe attacks of ON are sufficient to lead to marked reductions in pRNFL and GCIPL thickness so that subsequent attacks do not cause appreciable additive atrophy but continue to worsen function [96]. Alternatively, there may be a threshold effect, with significant functional retinal reserve that is maintained despite accumulating structural damage, until a specific threshold. Deschamps et al. [97] found a threshold pRNFL thickness of 50 µm, below which mean deviation of the visual field was significantly worse. Indeed, clinical experience in MOGAD-ON demonstrates that patients may maintain favourable visual acuity after recovery despite pRNFL thinning following early ON episodes.
In addition to conventional OCT, OCT angiography has also emerged as a tool in MOG-ON, to evaluate the retinal microvasculature. Using this technique, reduced peripapillary and parafoveal vessel densities have been observed in MOG-ON compared to healthy controls [98, 99], though comparisons with AQP4-ON have had mixed results, with one study reporting similar vessel densities [98] and others reporting reduced densities in MOG-ON [99]. Furthermore, the reduction in vessel densities has been correlated with other parameters such as the number of ON episodes [100], pRNFL thickness and visual acuity [99]. The mechanism behind retinal vascular rarefaction in MOG-ON is unclear but may be related to reduced metabolic demand following retinal degeneration [99].
Visual evoked potentials
VEPs in the majority of MOG-ON cases can demonstrate both delayed P100 latencies and reduced amplitudes, the former being more common [42, 94]. In severe cases, the evoked potential can be absent. Comparisons to multifocal VEPs in MS-ON and AQP4-ON in one study showed significantly longer latencies in MS-ON than MOG-ON but no significant difference in latencies between AQP4-ON and MOG-ON [93]. There was a trend towards lower amplitudes in AQP4-ON than MOG-ON but this did not reach statistical significance [93]. Overall, the main utility of VEP testing in MOG-ON may be in the confirmation of subtle optic nerve injury in cases where clinical examination findings may be uncertain, and in discriminating organic visual loss from functional visual loss, rather than in discriminating MOG-ON from other demyelinating aetiologies.
CSF analysis
CSF findings in MOG-ON may be contributory but are neither specific nor sensitive for diagnosis. Pleocytosis, which predominantly comprises mononuclear cells, and can be seen in acute attacks, has been reported in 44–72% of patients with MOGAD [42, 43, 101]. CSF pleocytosis appears to be more common in patients with myelitis rather than ON, and very high white cell counts of greater than 50 cells/µL are nearly exclusive to myelitis presentations [101]. Similarly, elevated CSF protein, reflecting dysfunction of the blood-brain barrier, is seen in 32–42% of cases of MOGAD, including some presenting with ON, but is also more common in those with myelitis [43, 101]. The presence of CSF-restricted oligoclonal bands, classically a hallmark of MS, is less common in MOGAD, with reports ranging from 0 to 20% [42, 43, 101]. One potentially differentiating feature is the so-called ‘MRZ reaction’ defined by the presence of intrathecal antibodies to at least two out of three of the measles, rubella and varicella zoster viruses, which has been shown to be common (over 60%) in MS but so far absent in MOGAD [42, 101], although this is not a test that is widely performed. CSF metabolomics and chemokine and cytokine profiles, though non-specific in MOG-ON, may have future use in differentiating MOG-ON from non-inflammatory causes [25].
MOG IgG testing
The detection of serum MOG IgG using a live cell-based assay is highly specific and sensitive for a diagnosis consistent with MOGAD [1]. In contrast, fixed cell-based assays have lower sensitivity and specificity [102–104]. Serum is the recommended biospecimen for testing for MOG IgG. While CSF may be positive for MOG IgG in 40-60% of patients with MOGAD, in many patients the diagnosis would be missed if CSF was tested in isolation [105, 106].
Given that up to 50% of children presenting with demyelination under the age of 10 years will have MOGAD as a final diagnosis, all children in this age group with a first presentation consistent with demyelination, including optic neuritis, should be tested for MOG IgG [1]. In contrast, MOG IgG serological testing should only be performed in adults with demyelination in the context of a suspected clinical or radiological phenotype for MOGAD. Testing large numbers of unselected patients with likely MS-ON in the context of typical MS brain lesions who have a low pre-test probability for MOGAD, will stretch the specificity for even the most specific and sensitive diagnostic biomarker [107].
Finally, interpretation of CSF-restricted MOG IgG (for example in a seronegative patient with ON) requires clinical acumen. While this may be contributory to the diagnosis in seronegative cases where there is a clinicoradiological profile highly associated with MOGAD, in atypical cases this could represent a false positive result [1, 105, 106, 108, 109].
Diagnosis
Diagnostic criteria for MOGAD were recently proposed [1]. ON is considered to be one of the core clinical demyelinating events necessary for the diagnosis of MOGAD, though no distinction is made between definite and possible ON as defined in the recently proposed diagnostic criteria for ON by Petzold et al. [110] In a patient with ON, if clear positive serum MOG IgG antibodies are then detected on a cell-based assay and a better alternate diagnosis is excluded, the diagnosis of MOG-ON can be made. Where there is only a low positive serum MOG IgG, a positive result without a reported titre, or CSF restricted MOG IgG antibodies only, then at least one supportive clinical or radiological feature (bilateral simultaneous involvement, >50% length involvement, perineural optic sheath enhancement or optic disc oedema) and negative serum aquaporin-4 antibodies are additionally required to reduce the likelihood of misdiagnosis, and increase the specificity of conferring a diagnosis of MOGAD.
Differential diagnoses
In addition to AQP4-ON and MS-ON, a range of other conditions can also present with ON and must be considered in the differential diagnosis of MOGAD [111]. These are outlined below and summarised in Table 2.
Table 2.
Differential diagnoses of MOG-ON.
| Differential diagnosis | ON characteristics | Other neurological involvement |
|---|---|---|
| Immune mediated | ||
| NMOSD | Longitudinally extensive, may be bilateral, posterior segment, can have chiasmal +/− optic tract involvement | Longitudinally extensive transverse myelitis, area postrema syndrome, hypothalamic lesions |
| Multiple sclerosis | Focal involvement | Transverse myelitis; typical supratentorial, periventricular, brainstem and cerebellar involvement |
| GFAP antibody | Bilateral optic neuritis, optic disc swelling | Meningoencephalitis, longitudinally extensive transverse myelitis |
| SOX2 antibody | A chronic relapsing inflammatory optic neuropathy | Not specified |
| GlyR antibody | ADEM-ON, a chronic relapsing inflammatory optic neuropathy | Progressive encephalitis with rigidity and myoclonus |
| Paraneoplastic | ||
| CRMP5 antibody | Bilateral optic neuritis with retinitis and vitritis | Limbic encephalitis, cerebellar ataxia, myelopathy, peripheral neuropathy |
| Recoverin antibody | Bilateral optic neuritis, optic disc swelling | Variable |
| Systemic | ||
| Sarcoidosis | Optic perineuritis, bilateral optic neuritis, optic disc swelling | Cranial neuropathies, leptomeningeal involvement, myelopathy, parenchymal disease, muscle disease, peripheral neuropathy |
| Systemic lupus erythematosus | Variable | co-existing NMOSD; longitudinally extensive transverse myelitis |
| Others: Behcet, ANCA vasculitis | Variable | Variable |
|
Infection e.g. T. pallidum, B. henselae, B. burgdorferi, herpes simplex viruses, varicella zoster virus, cytomegalovirus |
Variable | Variable |
| Genetic | ||
| Leber hereditary optic neuropathy | Bilateral, painless, subacute | ‘Leber hereditary optic neuropathy-plus’ – movement disorders, MS-like illness |
| Vascular | ||
| Non-arteritic ischaemic optic neuropathy | Optic disc swelling | Cerebrovascular disease |
NMOSD neuromyelitis optica spectrum disorder, GFAP glial fibrillary acidic protein, GlyR glycine receptor, ADEM-ON acute, disseminated optic neuritis-optic neuritis, CRMP5 collapsin response mediator protein 5.
Other autoantibody-associated ON
Glial Fibrillary Acidic Protein (GFAP) antibody-associated ON
Autoimmune GFAP astrocytopathy is another inflammatory disease of the CNS and typically presents as a meningoencephalitis associated with GFAP-IgG antibodies in the CSF and a characteristic radial, perivascular enhancement pattern on brain MRI [112]. Rarely, in about 6% of all cases, it can present with ON. Like MOG-ON, these patients may also present with bilateral optic disc oedema with normal CSF opening pressures, though the mechanism in this scenario is thought to be related to primary venulitis with prominent vascular leakage [113].
Collapsin Response Mediator Protein 5 (CRMP5) antibody-associated ON
CRMP5-IgG antibodies are associated with a wide range of phenotypes affecting the peripheral and central nervous system including peripheral neuropathy, myelopathy, cerebellar ataxia and optic neuritis, and are typically paraneoplastic in origin, with small cell lung cancer being the most frequently identified tumour [114]. ON is a presentation in some patients with this condition, for whom bilateral optic disc oedema is characteristic, but specific to this condition, is typically accompanied by retinitis and vitreous inflammatory cells [115].
Other autoantibodies
Various other autoantibodies have also been linked with ON, though evidence for their association is less robust. For example, antibodies to recoverin, the photoreceptor protein, which are typically found in paraneoplastic retinopathy have been reported in a case of ON [116]. Additionally, antibodies to SOX2, a transcription factor expressed by glial cells of the optic nerve, have been isolated in a subset of patients with relapsing inflammatory optic neuropathy [117]. Furthermore, anti-glycine receptor antibodies, typically associated with the syndrome of progressive encephalomyelitis with rigidity and myoclonus, have been linked to cases of demyelinating optic neuritis, although these results have not been clearly reproduced [118].
ON associated with systemic conditions
Sarcoidosis
Sarcoidosis is a multisystem autoinflammatory disorder characterised by non-caseous granuloma formation. Ophthalmological manifestations occur in 10–50% of patients, with anterior uveitis being most common [119]. Neurological involvement, which can be peripheral or central and can include ON, is less common and occurs in 5–10% of patients, but is noteworthy because it shares similarities with MOG-ON [120]. For instance, sequential, bilateral involvement may be present in sarcoidosis-related ON, occurring in 28% of cases in one major published case series, although synchronous disease is rare and occurs in only 9% [119]. In the same series, 37% also had visible optic disc swelling and 4% had optic perineuritis. The proportion of cases of optic perineuritis due to sarcoidosis is higher still, at 20% in the largest published case series, and trails only MOGAD as the major secondary cause of optic perineuritis, though idiopathic cases are also well recognised [87]. The finding of non-caseous granulomas on biopsy definitively distinguishes sarcoidosis from MOG-ON, as well as multisystem involvement of sarcoidosis.
Other systemic autoimmune conditions
Many other systemic autoimmune conditions have also been reported to feature ON, including Sjogren’s syndrome, Behcet’s disease, ANCA vasculitis, and systemic lupus erythematosus, the latter often occurring in association with AQP4 IgG antibodies [111, 121].
Infection
Infectious causes of ON include bacteria such as T. pallidum, B. henselae and B. burgdorferi and viruses such as herpes simplex viruses, varicella zoster virus and cytomegalovirus [122]. Importantly, as mentioned earlier, MOG-ON has been reported as occurring alongside, or being triggered by, a number of these infectious aetiologies [34, 35].
Leber hereditary optic neuropathy
Leber hereditary optic neuropathy is a mitochondrial disorder preferentially affecting young males that is characterised by degeneration of the retinal ganglion cell layer, leading to painless, subacute, bilateral central vision loss. Symptom onset is usually in the second or third decade of life, following which occurs progression to visual acuity of 6/60 or worse, over four months on average in one series; with sequential, contralateral eye involvement by two months on average [123]. Thus, Leber hereditary optic neuropathy at first presentation may be considered as a differential for MOG-ON, but the presence of pain and the more rapid visual loss in MOG-ON forms a key distinction. Interestingly, there is one reported case of acute MOG-ON occurring in a known Leber hereditary optic neuropathy carrier [124].
Chronic relapsing inflammatory optic neuropathy
In 2003, Kidd et al. first described a syndrome of unilateral or bilateral, painful, subacute vision loss with initial steroid responsiveness and tendency to relapse on cessation of steroids, termed chronic relapsing inflammatory optic neuropathy (CRION) [125]. Following the discovery of AQP4 IgG and MOG IgG, serum antibody testing of original CRION cohorts demonstrated that subgroups of these patients were in fact seropositive for one of these two antibodies, with up to 22% being AQP4 IgG positive and up to 25% MOG IgG positive [126–128]. This is not surprising given that CRION is by definition a syndromic diagnosis, and one of exclusion, likely comprising a heterogenous group of underlying aetiologies that includes MOG-ON and AQP4-ON.
Disease course, outcomes and relapse prediction of MOGAD
While early reports with median follow-up times of less than two years suggested that MOGAD might be a predominantly monophasic illness [21, 129], a few studies examining patients over longer durations of more than five years have demonstrated relapse rates of up to 70% [42, 130].
It should be considered that patients with disease onset prior to the widespread availability of the MOG IgG antibody test are likely to only be diagnosed with MOGAD if they re-present with relapsing disease, meaning that relapsing patients may be over-represented in retrospective cohort studies [6]. There have only been a few incident studies to address this bias and the largest and longest of these reported a 4-year relapse risk of 32% but included more children, who may have a lower risk of relapse than adults [75, 131]. However, when considering only cases of ON at onset, Cobo-Calvo et al. found no significant difference in relapse rates between children and adults although group means were not reported [75].
ON is the most common relapsing syndrome in both adults and children with MOGAD, occurring in 28-91% of relapsing cases, and may be recurrent unilateral, contralateral or simultaneous bilateral ON [6, 7, 42, 131, 132]. Furthermore, patients with ON at onset may be more likely to develop relapsing disease than those with other clinical presentations, and relapsing ON may be associated with more attacks than other relapsing phenotypes [6, 130]. Patients with ON at onset can also develop other, non-ON relapsing syndromes such as transverse myelitis [131], aseptic meningitis [54] and cerebral cortical encephalitis with seizures [133].
In children, a distinct relapsing phenotype of monophasic ADEM or recurrent/multiphasic ADEM (MDEM) followed by ON after at least three months, termed ADEM-ON, is well recognised [134]. Though ADEM-ON may be rare overall when considering all acquired demyelinating syndromes [135], it is seen in up to 40% of children with relapsing MOGAD with an initial presentation of ADEM [7, 74]. A median of three relapses per patient over a median follow-up time of 5.3 years, of which 94% of relapses are ON, has been described in one ADEM-ON cohort [134]. Importantly, unlike the high level of functional recovery previously reported in children [75], 71% of ADEM-ON patients in this cohort had residual deficits, most commonly visual impairment.
MOGAD patients at disease onset may be prone to ‘very early relapses’ (<3 months after onset) and ‘delayed early relapses’ (3–12 months after onset). Chen et al. demonstrated that very early relapses in adults, and delayed early relapses in children and adults, were associated with an increased risk of long-term relapsing disease [136]. This provides a compelling reason to consider whether therapeutic choices at disease onset have the potential to modify natural history of MOGAD.
Overall, despite frequent relapses, patients with MOGAD are reported to have comparatively favourable outcomes, with expanded disability status scale (EDSS) scores of 1–2 reported at last follow-up [7, 130], compared to AQP4-IgG positive NMOSD where follow-up EDSS can often be >3 [21, 129, 137]. However, it is important to note that the EDSS was developed for use in classical MS and is strongly weighted towards locomotor deficits, making visual disability likely to be underestimated. In one study, severe visual impairment, defined as visual acuity worse than 50% of normal in one or both eyes, was present at last follow-up in 36% of patients with MOGAD [7, 42] whereas other studies have reported lower rates of 16–24% [6, 7, 138]. Unlike in MS, thus far, only relapse-related stepwise progression of disability has been observed in MOGAD, without clear evidence to support relapse-independent progression [139].
A means to predict relapse is of significant interest. A number of studies have observed the conversion of MOG IgG to seronegativity over time in monophasic patients, whereas relapsing patients tend to remain persistently MOG IgG seropositive but with lower titres during periods of remission than during active disease [130, 140]. However, many patients who display persistently positive MOG IgG can still remain monophasic [3, 6] and, conversely, albeit rarely, patients can still relapse after seroconversion, possibly related to assay sensitivity or patient treatment timing relative to serological testing [137]. Prospective studies are needed to elucidate the exact role of MOG IgG titres in predicting relapsing disease. Recent publications identified that adults with MOG IgG recognising a non-proline 42 epitope were at a higher risk of relapse, suggesting that epitope profiling at disease onset may also assist with prediction of a relapsing course [103, 141].
Treatment
Acute therapy
The use of high dose intravenous methylprednisolone for 3–5 days is common practice internationally for the treatment of acute attacks of ON [142]. This is in line with the results of the landmark Optic Neuritis Treatment Trial (ONTT) evaluating 448 adults with ON and supporting intravenous methylprednisolone, though only three of the patients in this study of a subgroup of 177 in whom archival sera was available for testing, were subsequently found to be seropositive for MOG IgG [143, 144]. In a large, retrospective study of MOGAD patients by Jarius et al. [42], intravenous methylprednisolone treatment was associated with complete recovery in half and partial recovery in 44% of patients.
MOGAD appears to be highly steroid sensitive, with rapid recovery following initial administration of intravenous and/or oral corticosteroids [7, 22], but also to some extent steroid dependent. In one study, 70% of episodes treated with oral prednisone relapsed, particularly at doses of <10 mg a day in adults (or a weight based equivalent in children) or within two months of cessation, cautioning against rapid tapers [7]. A number of further studies have confirmed the modulating effect of oral corticosteroids on inducing disease remission, with suggested treatment durations ranging from five weeks to six months, and significant variability depending on clinician experience and preference, highlighting that this is a matter of notable equipoise [6, 42, 142, 145]. Identifying the minimum dose and duration of corticosteroid treatment at disease onset which will delay time to first relapse, while limiting steroid related adverse effects, will be a significant step forward in the acute management of this condition.
In terms of treatment timing, after adjusting for initial visual acuity, Chen et al. [57] found no difference in outcomes between MOG-ON patients treated with intravenous methylprednisolone within 3 days of onset and those treated later, however, better outcomes were reported in a smaller cohort with treatment initiation prior to 7 days [146]. Rode et al. found that treatment initiation after 10 days was significantly associated with failure to recover visual acuity at three months and reduced pRNFL thickness (i.e. structure and function) [147].
There is limited data on the efficacy of second-line agents for acute attacks, such as intravenous immunoglobulin and plasmapheresis, when the initial response to steroids is poor, but this scenario is less common [42, 57]. In a survey of international experts on MOGAD, the most popular second-line agent was plasmapheresis, with 81% of respondents in favour and others preferring to retrial high dose corticosteroid treatment [142]. Certainly, intravenous immunoglobulin and plasmapheresis are well established first line therapeutic agents in neuroglial cell surface antibody syndromes including NMOSD and autoimmune encephalitis, and likely to be of benefit in MOGAD.
Maintenance therapy
There are no published randomised, controlled clinical trials of treatment to prevent relapses in MOGAD, although such studies are currently recruiting for novel immunotherapeutic agents.
Importantly, short ‘pulses’ of high dose corticosteroids alone appear to be insufficient for lasting disease activity control following acute MOG-ON. In one cohort, 70% of MOGAD episodes treated with oral prednisolone relapsed, particularly at doses under 10 mg or within two months of cessation [7]. Another study found similar results and also showed that the risk of relapse was significantly greater in patients with corticosteroid treatment durations of less than three months compared to those that received more [6]. However, there are heightened concerns about prolonged steroid tapers, especially in children, because of the neuropsychiatric, metabolic and infection risks associated with long-term steroid exposure [136, 148].
The role of steroid-sparing maintenance therapy after the first acute MOG-ON attack is unclear. In one study, maintenance steroid therapy was superior in efficacy to non-steroidal maintenance immunotherapy, including intravenous immunoglobulin, rituximab and mycophenolate, with respect to relapse prevention [7], however concerns related to the long-term side effects of steroid based regimes make this less viable as a long-term maintenance treatment option. A large, international retrospective cohort study found that maintenance intravenous immunoglobulin treatment was associated with a significant reduction in annualised relapse rates of MOGAD, and displayed a dose-response relationship with patients who received at least 1 g/kg of intravenous immunoglobulin every 4 weeks experiencing significantly less relapses than those who received lower or less frequent doses [149]. The role of rituximab in relapse prevention for MOGAD remains less clear. Evaluation of rituximab in MOGAD showed that it reduced relapse rates, though some patients still experienced relapses despite apparent B-cell depletion [150]. In a prospective study, a significant proportion of MOGAD relapses on rituximab treatment occurred despite effective B-cell depletion compared to AQP4-IgG positive NMOSD relapses, implicating less biological efficacy in MOGAD [151]. A meta-analysis of nineteen studies found a significantly greater reduction in annualised relapse rates post-rituximab in AQP4-IgG positive NMOSD compared to MOGAD [152].
Other commonly used immunomodulatory agents such as methotrexate, azathioprine and cyclophosphamide have also been retrospectively studied in MOGAD, with benefits when compared to a ‘no treatment’ group, but none have been shown to be completely effective in preventing relapses [42]. Traditional disease-modifying therapies used in MS do not appear to be effective in MOGAD, and may exacerbate disease, as can be seen in NMOSD [42, 131, 153]. There is no clear hierarchy amongst these different steroid-sparing agents. However, switching maintenance immunotherapy following treatment failure, irrespective of the initial agent, has been shown to reduce relapse rates in MOGAD, supporting a dynamic and multifaceted treatment approach which may need to be individualised [7].
Relapse prevention is a key goal in the management of MOGAD as relapse-associated disability, particularly in relation to visual (following ON) and sphincter (following TM) dysfunction [6, 42]. While relapse rates are reported as 30–60% in MOGAD, this may increase to the order of up to 70% when patients are followed up for over five years [42]. This brings to discussion whether there is a role for introducing corticosteroid-sparing maintenance immunosuppression at ON onset. However, while the treatment of MS and NMOSD highlights the need for ongoing immunotherapy from diagnosis, in MOGAD there remains a proportion of patients who have a monophasic illness who may be unnecessarily exposed to long-term immunosuppression if this approach was uniformly adopted at disease onset.
Whilst the therapeutic landscape remains under investigation, novel agents including monoclonal antibodies targeting the neonatal Fc receptor (rozanolixizumab; NCT05063162) and IL-6 receptor (satralizumab; NCT05271409) are currently undergoing phase III double blind placebo-controlled randomised controlled clinical trials.
Conclusion
MOG-ON is an increasingly recognised condition affecting both children and adults that deserves specific attention because of its distinction from ON associated with MS and NMOSD, and the therapeutic and prognostic implications of an early and accurate diagnosis. Characteristic clinical and radiological features can expedite diagnosis and facilitate initiation of appropriate therapeutic pathways. Confirmation of the diagnosis ideally relies on the detection of specific serum MOG IgG antibodies on a live cell-based assay, in association with a typical clinical phenotype. Although a relapsing disease course is common, and outcomes with treatment can be favourable, residual disability may be present. Future research priorities include better elucidating the underlying pathophysiological mechanisms in this condition, identifying prognostic biomarkers, and establishing optimal and well-tolerated long-term therapeutic strategies.
Author contributions
NJ, RCD and SR all contributed to study conception and design. NJ, ML, and SR drafted the manuscript. All authors reviewed the manuscript and provided intellectual input and direction.
Funding
SR is supported by an NHMRC Investigator Grant (GNT2008339). Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
As this is an invited review, there is no original data. All sources have been referenced.
Competing interests
NJ and ML have no financial disclosures to report. RD has received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation and the NHMRC (Australia; Investigator Grant). He has also received honoraria from Biogen Idec as an invited speaker, and is on the IDMC for a Roche RCT in paediatric MS. He is on the medical advisory board (non-remunerated position) of The MOG Project. SR has received research funding from the National Health and Medical Research Council (NHMRC, Australia), the Petre Foundation, the Brain Foundation, the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for educational/research sessions coordinated by Biogen, Alexion, Novartis, Excemed and Limbic Neurology. She is on the medical advisory boards (non-remunerated positions) of The MOG Project and the Sumaira Foundation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023;22:268–82. 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 2.Linnington C, Webb M, Woodhams PL. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol. 1984;6:387–96. 10.1016/0165-5728(84)90064-X [DOI] [PubMed] [Google Scholar]
- 3.de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 2020;26:806–14. 10.1177/1352458519845112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell K, Hamilton-Shield A, Woodhall M, Messina S, Mariano R, Waters P, et al. Prevalence and incidence of neuromyelitis optica spectrum disorder, aquaporin-4 antibody-positive NMOSD and MOG antibody-positive disease in Oxfordshire, UK. J Neurol Neurosurg Psychiatry. 2020;91:1126–8. 10.1136/jnnp-2020-323158 [DOI] [PubMed] [Google Scholar]
- 5.Hor JY, Fujihara K. Epidemiology of myelin oligodendrocyte glycoprotein antibody-associated disease: a review of prevalence and incidence worldwide. Front Neurol. 2023;14:1260358. 10.3389/fneur.2023.1260358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–38. 10.1093/brain/awx276 [DOI] [PubMed] [Google Scholar]
- 7.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89:127–37. 10.1136/jnnp-2017-316880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters P, Fadda G, Woodhall M, O’Mahony J, Brown RA, Castro DA, et al. Serial Anti-Myelin Oligodendrocyte Glycoprotein Antibody Analyses and Outcomes in Children With Demyelinating Syndromes. JAMA Neurol. 2020;77:82–93. 10.1001/jamaneurol.2019.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro JD, Beukelman T, Hemingway C, Hokkanen SRK, Tennigkeit F, Chitnis T. Attack phenotypes and disease course in pediatric MOGAD. Ann Clin Transl Neurol. 2023;10:672–85. 10.1002/acn3.51759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaertner S, de Graaf KL, Greve B, Weissert R. Antibodies against glycosylated native MOG are elevated in patients with multiple sclerosis. Neurology. 2004;63:2381–3. 10.1212/01.WNL.0000147259.34163.33 [DOI] [PubMed] [Google Scholar]
- 11.Kuhle J, Lindberg RL, Regeniter A, Mehling M, Hoffmann F, Reindl M, et al. Antimyelin antibodies in clinically isolated syndromes correlate with inflammation in MRI and CSF. J Neurol. 2007;254:160–8. 10.1007/s00415-006-0299-4 [DOI] [PubMed] [Google Scholar]
- 12.Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–45. 10.1056/NEJMoa022328 [DOI] [PubMed] [Google Scholar]
- 13.Rauer S, Euler B, Reindl M, Berger T. Antimyelin antibodies and the risk of relapse in patients with a primary demyelinating event. J Neurol Neurosurg Psychiatry. 2006;77:739–42. 10.1136/jnnp.2005.077784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, et al. Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci USA. 2003;100:9446–51. 10.1073/pnas.1133443100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Graaf KL, Albert M, Weissert R. Autoantigen conformation influences both B- and T-cell responses and encephalitogenicity. J Biol Chem. 2012;287:17206–13. 10.1074/jbc.M111.304246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: The history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev. 2016;15:307–24. 10.1016/j.autrev.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 17.O’Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, Xu C, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–7. 10.1038/nm1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin KA, Chitnis T, Newcombe J, Franz B, Kennedy J, McArdel S, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J Immunol. 2009;183:4067–76. 10.4049/jimmunol.0801888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brilot F, Dale RC, Selter RC, Grummel V, Kalluri SR, Aslam M, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. 2009;66:833–42. 10.1002/ana.21916 [DOI] [PubMed] [Google Scholar]
- 20.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–7. 10.1212/WNL.0b013e31826aac4e [DOI] [PubMed] [Google Scholar]
- 21.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–81. 10.1212/WNL.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan S, Reddel SW, Henderson A, Parratt JD, Barnett M, Gatt PN, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2014;1:e40. 10.1212/NXI.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139:875–92. 10.1007/s00401-020-02132-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. 2020;143:1431–46. 10.1093/brain/awaa102 [DOI] [PubMed] [Google Scholar]
- 25.Kothur K, Wienholt L, Tantsis EM, Earl J, Bandodkar S, Prelog K, et al. B Cell, Th17, and Neutrophil Related Cerebrospinal Fluid Cytokine/Chemokines Are Elevated in MOG Antibody Associated Demyelination. PLoS One. 2016;11:e0149411. 10.1371/journal.pone.0149411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): A case-series and review. Mult Scler Relat Disord. 2021;48:102696. 10.1016/j.msard.2020.102696 [DOI] [PubMed] [Google Scholar]
- 27.Winklmeier S, Schlüter M, Spadaro M, Thaler FS, Vural A, Gerhards R, et al. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol Neuroimmunol Neuroinflamm. 2019;6:625. 10.1212/NXI.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Wang L, Zhou L, ZhangBao J, Miao MZ, Lu C, et al. The imbalance between regulatory and memory B cells accompanied by an increased number of circulating T-follicular helper cells in MOG-antibody-associated demyelination. Mult Scler Relat Disord. 2019;36:101397. 10.1016/j.msard.2019.101397 [DOI] [PubMed] [Google Scholar]
- 29.Hofer LS, Ramberger M, Gredler V, Pescoller AS, Rostásy K, Sospedra M, et al. Comparative Analysis of T-Cell Responses to Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein in Inflammatory Demyelinating Central Nervous System Diseases. Front Immunol. 2020;11:1188. 10.3389/fimmu.2020.01188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spadaro M, Winklmeier S, Beltrán E, Macrini C, Höftberger R, Schuh E, et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol. 2018;84:315–28. 10.1002/ana.25291 [DOI] [PubMed] [Google Scholar]
- 31.Keller CW, Lopez JA, Wendel E-M, Ramanathan S, Gross CC, Klotz L, et al. Complement Activation Is a Prominent Feature of MOGAD. Ann Neurol. 2021;90:976–82. 10.1002/ana.26226 [DOI] [PubMed] [Google Scholar]
- 32.Mader S, Ho S, Wong HK, Baier S, Winklmeier S, Riemer C, et al. Dissection of complement and Fc-receptor-mediated pathomechanisms of autoantibodies to myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci USA. 2023;120:e2300648120. 10.1073/pnas.2300648120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramanathan S, Brilot F, Irani SR, Dale RC. Origins and immunopathogenesis of autoimmune central nervous system disorders. Nat Rev Neurol. 2023;19:172–90. 10.1038/s41582-023-00776-4 [DOI] [PubMed] [Google Scholar]
- 34.Jeyakumar N, Waller S, Mahant N, Ramanathan S, Henderson APD. A case of CSF anti-MOG antibody-positive papillitis with intermediate uveitis in the setting of acute neurosyphilis. Neuroimmunol Rep. 2021;1:100033. 10.1016/j.nerep.2021.100033 [DOI] [Google Scholar]
- 35.Rusescu B-V, Diederich NJ, Tsobo C, Marignier R, Kerschen P. MOG antibody-associated optic neuritis in the setting of acute CMV infection. J Neurological Sci. 2017;382:44–6. 10.1016/j.jns.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 36.Mutete F, Mwendaweli N, Asukile M. mulubwa s, Mumbi W, Saylor D. MOG Antibody-Associated Bilateral Optic Neuritis Associated with Johnson and Johnson COVID-19 Vaccination (P12-3.009). Neurology. 2023;100:2691. 10.1212/WNL.0000000000202726 [DOI] [Google Scholar]
- 37.Molazadeh N, Bose G, Lotan I, Levy M. Autoimmune diseases and cancers overlapping with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A systematic review. Mult Scler J Exp Transl Clin. 2022;8:20552173221128170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redenbaugh V, Flanagan EP, Floris V, Zara P, Bhatti MT, Sanchez F, et al. Exposure to TNF inhibitors is rare at MOGAD presentation. J Neurol Sci. 2022;432:120044. 10.1016/j.jns.2021.120044 [DOI] [PubMed] [Google Scholar]
- 39.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 40.Boccuni I, Bas-Orth C, Bruehl C, Draguhn A, Fairless R. Glutamate transporter contribution to retinal ganglion cell vulnerability in a rat model of multiple sclerosis. Neurobiol Dis. 2023;187:106306. 10.1016/j.nbd.2023.106306 [DOI] [PubMed] [Google Scholar]
- 41.Hofman P, Hoyng P, vanderWerf F, Vrensen GF, Schlingemann RO. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42:895–901. [PubMed] [Google Scholar]
- 42.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JJ, Flanagan EP, Jitprapaikulsan J, López-Chiriboga ASS, Fryer JP, Leavitt JA, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol. 2018;195:8–15. 10.1016/j.ajo.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rempe T, Tarhan B, Rodriguez E, Viswanathan VT, Gyang TV, Carlson A, et al. Anti-MOG associated disorder-Clinical and radiological characteristics compared to AQP4-IgG+ NMOSD-A single-center experience. Mult Scler Relat Disord. 2021;48:102718. 10.1016/j.msard.2020.102718 [DOI] [PubMed] [Google Scholar]
- 45.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson APD, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler J. 2015;22:470–82. 10.1177/1352458515593406 [DOI] [PubMed] [Google Scholar]
- 46.Bennett JL, Costello F, Chen JJ, Petzold A, Biousse V, Newman NJ, et al. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol. 2023;22:89–100. 10.1016/S1474-4422(22)00187-9 [DOI] [PubMed] [Google Scholar]
- 47.Shor N, Aboab J, Maillart E, Lecler A, Bensa C, Le Guern G, et al. Clinical, imaging and follow-up study of optic neuritis associated with myelin oligodendrocyte glycoprotein antibody: a multicentre study of 62 adult patients. Eur J Neurol. 2020;27:384–91. 10.1111/ene.14089 [DOI] [PubMed] [Google Scholar]
- 48.Narongkhananukul C, Padungkiatsagul T, Jindahra P, Khongkhatithum C, Thampratankul L, Vanikieti K. MOG-IgG- versus AQP4-IgG-Positive Optic Neuritis in Thailand: Clinical Characteristics and Long-Term Visual Outcomes Comparison. Clin Ophthalmol. 2020;14:4079–88. 10.2147/OPTH.S288224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ducloyer JB, Caignard A, Aidaoui R, Ollivier Y, Plubeau G, Santos-Moskalyk S, et al. MOG-Ab prevalence in optic neuritis and clinical predictive factors for diagnosis. Br J Ophthalmol. 2020;104:842–5. 10.1136/bjophthalmol-2019-314845 [DOI] [PubMed] [Google Scholar]
- 50.Kang H, Qiu H, Hu X, Wei S, Tao Y. Differences in Neuropathic Pain and Radiological Features Between AQP4-ON, MOG-ON, and IDON. Front Pain Res. 2022;3:870211. 10.3389/fpain.2022.870211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambika S, Durgapriyadarshini S, Padmalakshmi K, Noronha V, Arjundas D. Clinical profile, imaging features and short term visual outcomes of Indian optic neuritis patients with and without seromarkers for myelin oligodendrocyte glycoprotein and neuromyelitis optica. Indian J Ophthalmol. 2022;70:194–200. 10.4103/ijo.IJO_887_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dauby S, Dive D, Lutteri L, Andris C, Hansen I, Maquet P, et al. Comparative study of AQP4-NMOSD, MOGAD and seronegative NMOSD: a single-center Belgian cohort. Acta Neurol Belg. 2022;122:135–44. 10.1007/s13760-021-01712-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lotan I, Brody J, Hellmann MA, Bialer O, Ganelin-Cohen E, Michaeli N, et al. Myelin oligodendrocyte glycoprotein-positive optic neuritis masquerading as pseudotumor cerebri at presentation. J Neurol. 2018;265:1985–8. 10.1007/s00415-018-8956-y [DOI] [PubMed] [Google Scholar]
- 54.Narayan RN, Wang C, Sguigna P, Husari K, Greenberg B. Atypical Anti-MOG syndrome with aseptic meningoencephalitis and pseudotumor cerebri-like presentations. Mult Scler Relat Disord. 2019;27:30–3. 10.1016/j.msard.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 55.Wong WK, Troedson C, Peacock K, Brilot-Turville F, Menezes MP, Dale RC, et al. Steroid-responsive aseptic meningitis with raised intracranial pressure syndrome associated with myelin oligodendrocyte glycoprotein autoantibodies. J Paediatr Child Health. 2022;58:2322–6. 10.1111/jpc.16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murtagh P, Coman A, Stephenson K, Gaughan M, Ryan D, McNeill G, et al. Neuromyelitis optica spectrum disorders and anti-myelin oligodendrocyte glycoprotein positive optic neuropathies. Int J Ophthalmol. 2022;15:1095–107. 10.18240/ijo.2022.07.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen JJ, Flanagan EP, Bhatti MT, Tisavipat N, Jamali S, Kunchok A, et al. Details and outcomes of a large cohort of MOG-IgG associated optic neuritis. Mult Scler Relat Disord. 2022;68:104237. 10.1016/j.msard.2022.104237 [DOI] [PubMed] [Google Scholar]
- 58.Vicini R, Brügger D, Abegg M, Salmen A, Grabe HM. Differences in morphology and visual function of myelin oligodendrocyte glycoprotein antibody and multiple sclerosis associated optic neuritis. J Neurol. 2021;268:276–84. 10.1007/s00415-020-10097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fazzone HE, Lefton DR, Kupersmith MJ. Optic neuritis: correlation of pain and magnetic resonance imaging. Ophthalmology. 2003;110:1646–9. 10.1016/S0161-6420(03)00477-9 [DOI] [PubMed] [Google Scholar]
- 60.Jang Y, Kim SM, Yun YI, Lee HJ, Kim SJ, Jung JH, et al. Comparison between optic neuritis associated with antibody against myelin oligodendrocyte glycoprotein and presumed idiopathic optic perineuritis. Neurol Sci. 2020;41:2755–60. 10.1007/s10072-020-04371-z [DOI] [PubMed] [Google Scholar]
- 61.Asseyer S, Hamblin J, Messina S, Mariano R, Siebert N, Everett R, et al. Prodromal headache in MOG-antibody positive optic neuritis. Mult Scler Relat Disord. 2020;40:101965. 10.1016/j.msard.2020.101965 [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Tan S, Chan TCY, Xu Q, Zhao J, Teng D, et al. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol. 2018;102:1372–7. 10.1136/bjophthalmol-2017-311177 [DOI] [PubMed] [Google Scholar]
- 63.Ramanathan S, Fraser C, Curnow SR, Ghaly M, Leventer RJ, Lechner-Scott J, et al. Uveitis and optic perineuritis in the context of myelin oligodendrocyte glycoprotein antibody seropositivity. Eur J Neurol. 2019;26:1137–e75. 10.1111/ene.13932 [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Chiriboga AS, Van Stavern G, Flanagan EP, Pittock SJ, Fryer J, Bhatti MT, et al. Myelin Oligodendrocyte Glycoprotein Antibody (MOG-IgG)-Positive Optic Perineuritis. Neuroophthalmology. 2020;44:1–4. 10.1080/01658107.2019.1607883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta P, Goyal V, Srivastava AK, Pandit AK, Prasad K. Uveitis, optic neuritis and MOG. Mult Scler J Exp Transl Clin. 2020;6:2055217320925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deschamps R, Vasseur V, Shor N, Vignal C, Salomon L, Gout O, et al. A new association: acute macular neuroretinopathy in acute optic neuritis. Acta Ophthalmologica. 2019;97:e753–e6. 10.1111/aos.14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kon T, Hikichi H, Ueno T, Suzuki C, Nunomura J, Kaneko K, et al. Myelin Oligodendrocyte Glycoprotein-IgG-positive Recurrent Bilateral Optic Papillitis with Serous Retinal Detachment. Intern Med. 2018;57:3307–12. 10.2169/internalmedicine.9840-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mittal A, Baig IF, Merchant AG, Chen JJ, Choi JJ, Goldberg A, et al. Sjögren Disease and Myelin Oligodendrocyte Glycoprotein Antibody–Associated Optic Neuritis. J Neuro Ophthalmol. 2021;41:e48–e50. [DOI] [PubMed]
- 69.Budoff G, Bhagat N, Frohman L. Anti-Myelin Oligodendrocyte Glycoprotein Optic Neuritis Presenting with Dense Premacular Hemorrhage. Ophthalmology. 2019;126:1639. 10.1016/j.ophtha.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 70.Wagley S, Wang Y, McClelland CM, Chen JJ, Lee MS. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis Presenting as Idiopathic Orbital Inflammatory Syndrome. J Neuro Ophthalmol. 2021;41:e46–e47. [DOI] [PubMed]
- 71.Kurathong S, Sanpatchayapong A, Apiwattanakul M. Case Report: Anti-myelin Oligodendrocyte Glycoprotein (MOG) Positive in a Patient Presented with Orbital Apex Syndrome. Neuroophthalmology. 2019;44:255–7. 10.1080/01658107.2019.1571089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valérie B, Claudine T, Etienne B-M, Etienne R. Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology. 1999;52:179. 10.1212/WNL.52.1.179 [DOI] [PubMed] [Google Scholar]
- 73.Shugaiv E, Tüzün E, Kürtüncü M, Kıyat-Atamer A, Çoban A, Akman-Demir G, et al. Uveitis as a prognostic factor in multiple sclerosis. Mult Scler. 2015;21:105–7. 10.1177/1352458514539782 [DOI] [PubMed] [Google Scholar]
- 74.Hacohen Y, Wong YY, Lechner C, Jurynczyk M, Wright S, Konuskan B, et al. Disease Course and Treatment Responses in Children With Relapsing Myelin Oligodendrocyte Glycoprotein Antibody–Associated Disease. JAMA Neurol. 2018;75:478–87. 10.1001/jamaneurol.2017.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody–Associated Disease. Ann Neurol. 2021;89:30–41. 10.1002/ana.25909 [DOI] [PubMed] [Google Scholar]
- 76.Parrotta E, Kister I. The Expanding Clinical Spectrum of Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Associated Disease in Children and Adults. Front Neurol. 2020;11:960. 10.3389/fneur.2020.00960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winter A, Chwalisz B. MRI Characteristics of NMO, MOG and MS Related Optic Neuritis. Semin Ophthalmol. 2020;35:333–42. 10.1080/08820538.2020.1866027 [DOI] [PubMed] [Google Scholar]
- 78.Rizzo JF, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2002;109:1679–84. 10.1016/S0161-6420(02)01148-X [DOI] [PubMed] [Google Scholar]
- 79.Salama S, Khan M, Levy M, Izbudak I. Radiological characteristics of myelin oligodendrocyte glycoprotein antibody disease. Mult Scler Relat Disord. 2019;29:15–22. 10.1016/j.msard.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carandini T, Sacchi L, Bovis F, Azzimonti M, Bozzali M, Galimberti D, et al. Distinct patterns of MRI lesions in MOG antibody disease and AQP4 NMOSD: a systematic review and meta-analysis. Mult Scler Relat Disord. 2021;54:103118. 10.1016/j.msard.2021.103118 [DOI] [PubMed] [Google Scholar]
- 81.Tajfirouz D, Padungkiatsagul T, Beres S, Moss HE, Pittock S, Flanagan E, et al. Optic chiasm involvement in AQP-4 antibody-positive NMO and MOG antibody-associated disorder. Mult Scler. 2022;28:149–53. 10.1177/13524585211011450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C, Liu C, Fang L, Zou Y, Ruan H, Wang Y, et al. Different magnetic resonance imaging features between MOG antibody- and AQP4 antibody-mediated disease: A Chinese cohort study. J Neurol Sci. 2019;405:116430. 10.1016/j.jns.2019.116430 [DOI] [PubMed] [Google Scholar]
- 83.Salama S, Khan M, Shanechi A, Levy M, Izbudak I. MRI differences between MOG antibody disease and AQP4 NMOSD. Mult Scler. 2020;26:1854–65. 10.1177/1352458519893093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol. 2001;119:1299–306. 10.1001/archopht.119.9.1299 [DOI] [PubMed] [Google Scholar]
- 85.Kim S-M, Woodhall MR, Kim J-S, Kim S-J, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflammation. 2015;2:e163-e. 10.1212/NXI.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yanagidaira M, Hattori T, Emoto H, Kiyosawa M, Yokota T. Optic perineuritis with anti-myelin oligodendrocyte glycoprotein antibody. Mult Scler Relat Disord. 2020;38:101444. 10.1016/j.msard.2019.101444 [DOI] [PubMed] [Google Scholar]
- 87.Xie JS, Donaldson L, Margolin E. Optic perineuritis: A Canadian case series and literature review. J Neurol Sci. 2021;430:120035. 10.1016/j.jns.2021.120035 [DOI] [PubMed] [Google Scholar]
- 88.Sulaiman FN, Kamardin NF, Sultan Abdul Kader MI, Ch’ng H, Wan Abdul Halim WH. Myelin Oligodendrocyte Glycoprotein Optic Neuritis Presenting With Orbital Apex Syndrome. Cureus. 2023;15:e38975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuda R, Kezuka T, Umazume A, Okunuki Y, Goto H, Tanaka K. Clinical Profile of Anti-Myelin Oligodendrocyte Glycoprotein Antibody Seropositive Cases of Optic Neuritis. Neuro Ophthalmol. 2015;39:213–9. 10.3109/01658107.2015.1072726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stiebel-Kalish H, Lotan I, Brody J, Chodick G, Bialer O, Marignier R, et al. Retinal Nerve Fiber Layer May Be Better Preserved in MOG-IgG versus AQP4-IgG Optic Neuritis: A Cohort Study. PloS One. 2017;12:e0170847-e. 10.1371/journal.pone.0170847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen JJ, Sotirchos ES, Henderson AD, Vasileiou ES, Flanagan EP, Bhatti MT, et al. OCT retinal nerve fiber layer thickness differentiates acute optic neuritis from MOG antibody-associated disease and Multiple Sclerosis: RNFL thickening in acute optic neuritis from MOGAD vs MS. Mult Scler Relat Disord. 2022;58:103525. 10.1016/j.msard.2022.103525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES. AQP4-IgG and MOG-IgG Related Optic Neuritis—Prevalence, Optical Coherence Tomography Findings, and Visual Outcomes: A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:540156. 10.3389/fneur.2020.540156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barnes S, You Y, Shen T, Hardy TA, Fraser C, Reddel SW, et al. Structural and functional markers of optic nerve damage in myelin oligodendrocyte glycoprotein antibody-associated optic neuritis. Mult Scler J Exp Transl Clin. 2021;7:20552173211063126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13:282. 10.1186/s12974-016-0720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akaishi T, Himori N, Takeshita T, Misu T, Takahashi T, Takai Y, et al. Follow-up of retinal thickness and optic MRI after optic neuritis in anti-MOG antibody-associated disease and anti-AQP4 antibody-positive NMOSD. J Neurol Sci. 2022;437:120269. 10.1016/j.jns.2022.120269 [DOI] [PubMed] [Google Scholar]
- 96.Costello F. Optical Coherence Tomography in Neuro-ophthalmology. Neurol Clin. 2017;35:153–63. 10.1016/j.ncl.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 97.Deschamps R, Philibert M, Lamirel C, Lambert J, Vasseur V, Gueguen A, et al. Visual field loss and structure-function relationships in optic neuritis associated with myelin oligodendrocyte glycoprotein antibody. Mult Scler. 2021;27:855–63. 10.1177/1352458520937281 [DOI] [PubMed] [Google Scholar]
- 98.Yu J, Huang Y, Zhou L, ZhangBao J, Zong Y, Quan C, et al. Comparison of the retinal vascular network and structure in patients with optic neuritis associated with myelin oligodendrocyte glycoprotein or aquaporin-4 antibodies: an optical coherence tomography angiography study. J Neurol. 2021;268:4874–81. 10.1007/s00415-021-10609-3 [DOI] [PubMed] [Google Scholar]
- 99.Yao Y, Li X, Xu Y, Liang X, Yang L, Shi FD, et al. The difference of the retinal structural and microvascular characteristics in patients with MOGAD-ON and AQP4-ON. BMC Neurol. 2022;22:323. 10.1186/s12883-022-02848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu J, Huang Y, Quan C, Zhou L, ZhangBao J, Wu K, et al. Alterations in the Retinal Vascular Network and Structure in MOG Antibody-Associated Disease: An Optical Coherence Tomography Angiography Study. J Neuroophthalmol. 2021;41:e424–e32. 10.1097/WNO.0000000000001116 [DOI] [PubMed] [Google Scholar]
- 101.Jarius S, Pellkofer H, Siebert N, Korporal-Kuhnke M, Hummert MW, Ringelstein M, et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: Results from 163 lumbar punctures in 100 adult patients. J Neuroinflammation. 2020;17:261. 10.1186/s12974-020-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflammation. 2020;7:e674-e. 10.1212/NXI.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tea F, Lopez JA, Ramanathan S, Merheb V, Lee FXZ, Zou A, et al. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathologica Commun. 2019;7:145. 10.1186/s40478-019-0786-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopez JA, Houston SD, Tea F, Merheb V, Lee FXZ, Smith S, et al. Validation of a Flow Cytometry Live Cell-Based Assay to Detect Myelin Oligodendrocyte Glycoprotein Antibodies for Clinical Diagnostics. J Appl Lab Med. 2022;7:12–25. 10.1093/jalm/jfab101 [DOI] [PubMed] [Google Scholar]
- 105.Kwon YN, Kim B, Kim JS, Mo H, Choi K, Oh SI, et al. Myelin Oligodendrocyte Glycoprotein-Immunoglobulin G in the CSF: Clinical Implication of Testing and Association With Disability. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1095. [DOI] [PMC free article] [PubMed]
- 106.Pace S, Orrell M, Woodhall M, Palace J, Leite MI, Irani SR, et al. Frequency of MOG-IgG in cerebrospinal fluid versus serum. J Neurol Neurosurg Psychiatry. 2022;93:334–5. 10.1136/jnnp-2021-326779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dale RC, Ramanathan S. Clinical decision making in MOG antibody-associated disease. Lancet Neurol. 2021;20:695–7. 10.1016/S1474-4422(21)00247-7 [DOI] [PubMed] [Google Scholar]
- 108.Matsumoto Y, Kaneko K, Takahashi T, Takai Y, Namatame C, Kuroda H, et al. Diagnostic implications of MOG-IgG detection in sera and cerebrospinal fluids. Brain. 2023;146:3938–48. 10.1093/brain/awad122 [DOI] [PubMed] [Google Scholar]
- 109.Carta S, Cobo Calvo Á, Armangué T, Saiz A, Lechner C, Rostásy K, et al. Significance of Myelin Oligodendrocyte Glycoprotein Antibodies in CSF: A Retrospective Multicenter Study. Neurology. 2023;100:e1095–e108. 10.1212/WNL.0000000000201662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petzold A, Fraser CL, Alroughani R, Alshowaeir D, Alvarenga R, Andris C, et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022;21:1120–34. 10.1016/S1474-4422(22)00200-9 [DOI] [PubMed] [Google Scholar]
- 111.Greco G, Colombo E, Gastaldi M, Ahmad L, Tavazzi E, Bergamaschi R, et al. Beyond Myelin Oligodendrocyte Glycoprotein and Aquaporin-4 Antibodies: Alternative Causes of Optic Neuritis. Int J Mol Sci. 2023;24:15986. 10.3390/ijms242115986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fang B, McKeon A, Hinson SR, Kryzer TJ, Pittock SJ, Aksamit AJ, et al. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol. 2016;73:1297–307. 10.1001/jamaneurol.2016.2549 [DOI] [PubMed] [Google Scholar]
- 113.Chen JJ, Aksamit AJ, McKeon A, Pittock SJ, Weinshenker BG, Leavitt JA, et al. Optic Disc Edema in Glial Fibrillary Acidic Protein Autoantibody–Positive Meningoencephalitis. J Neuro Ophthalmol. 2018;38:276–81. [DOI] [PubMed]
- 114.Wang S, Hou H, Tang Y, Zhang S, Wang G, Guo Z, et al. An overview on CV2/CRMP5 antibody-associated paraneoplastic neurological syndromes. Neural Regen Res. 2023;18:2357–64. [DOI] [PMC free article] [PubMed]
- 115.Cross SA, Salomao DR, Parisi JE, Kryzer TJ, Bradley EA, Mines JA, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. 2003;54:38–50. 10.1002/ana.10587 [DOI] [PubMed] [Google Scholar]
- 116.Kaushik M, Virdee J, Giridharan S, Chavda SV, Batra R. Response of Recoverin-Positive Optic Neuritis to Chemotherapy, Steroid, and Plasma Exchange. Journal of neuro-ophthalmology. 2024;44:e79–e81. [DOI] [PubMed]
- 117.Erdağ E, Emekli AS, Gündüz T, Küçükali Cİ, Kürtüncü M, Tüzün E. Serum IgG of patients with relapsing inflammatory optic neuropathy immunoreacts with Sox2-positive glial cells of the optic nerve. Mult Scler Relat Disord. 2023;73:104694. 10.1016/j.msard.2023.104694 [DOI] [PubMed] [Google Scholar]
- 118.Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137:2178–92. 10.1093/brain/awu142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Desmond PK, Ben JB, Elizabeth MG, Gordon TP. Optic neuropathy associated with systemic sarcoidosis. Neurol Neuroimmunol Neuroinflammation. 2016;3:e270. 10.1212/NXI.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kidd D, Beynon HL. The neurological complications of systemic sarcoidosis. Sarcoidosis Vasc Diffus Lung Dis. 2003;20:85–94. [PubMed] [Google Scholar]
- 121.Asgari N, Jarius S, Laustrup H, Skejoe HPB, Lillevang ST, Weinshenker BG, et al. Aquaporin-4-autoimmunity in patients with systemic lupus erythematosus: A predominantly population-based study. Mult Scler. 2018;24:331–9. 10.1177/1352458517699791 [DOI] [PubMed] [Google Scholar]
- 122.Kahloun R, Abroug N, Ksiaa I, Mahmoud A, Zeghidi H, Zaouali S, et al. Infectious optic neuropathies: a clinical update. Eye Brain. 2015;7:59–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newman NJ, Lott MT, Wallace DC. The clinical characteristics of pedigrees of Leber’s hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol. 1991;111:750–62. 10.1016/S0002-9394(14)76784-4 [DOI] [PubMed] [Google Scholar]
- 124.Raviskanthan S, Perera N, Fok A. Recurrent Bilateral Myelin Oligodendrocyte Glycoprotein Antibody Optic Neuritis in a Leber Hereditary Optic Neuropathy Carrier. J Neuro Ophthalmol. 2020;40:246–7. 10.1097/WNO.0000000000000853 [DOI] [PubMed] [Google Scholar]
- 125.Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain. 2003;126:276–84. 10.1093/brain/awg045 [DOI] [PubMed] [Google Scholar]
- 126.Petzold A, Woodhall M, Khaleeli Z, Tobin WO, Pittock SJ, Weinshenker BG, et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies in immune-mediated optic neuritis at long-term follow-up. J Neurol Neurosurg Psychiatry. 2019;90:1021–6. 10.1136/jnnp-2019-320493 [DOI] [PubMed] [Google Scholar]
- 127.Lee H-J, Kim B, Waters P, Woodhall M, Irani S, Ahn S, et al. Chronic relapsing inflammatory optic neuropathy (CRION): a manifestation of myelin oligodendrocyte glycoprotein antibodies. J Neuroinflammation. 2018;15:302. 10.1186/s12974-018-1335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petzold A, Pittock S, Lennon V, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J Neurol Neurosurg Psychiatry. 2010;81:109–11. 10.1136/jnnp.2008.146894 [DOI] [PubMed] [Google Scholar]
- 129.Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, et al. Neuromyelitis Optica Spectrum Disorders With Aquaporin-4 and Myelin-Oligodendrocyte Glycoprotein Antibodies: A Comparative Study. JAMA Neurol. 2014;71:276–83. 10.1001/jamaneurol.2013.5857 [DOI] [PubMed] [Google Scholar]
- 130.Oliveira LM, Apóstolos-Pereira SL, Pitombeira MS, Bruel Torretta PH, Callegaro D, Sato DK. Persistent MOG-IgG positivity is a predictor of recurrence in MOG-IgG-associated optic neuritis, encephalitis and myelitis. Mult Scler. 2019;25:1907–14. 10.1177/1352458518811597 [DOI] [PubMed] [Google Scholar]
- 131.Satukijchai C, Mariano R, Messina S, Sa M, Woodhall MR, Robertson NP, et al. Factors Associated With Relapse and Treatment of Myelin Oligodendrocyte Glycoprotein Antibody–Associated Disease in the United Kingdom. JAMA Netw Open. 2022;5:e2142780-e. 10.1001/jamanetworkopen.2021.42780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wendel EM, Thonke HS, Bertolini A, Baumann M, Blaschek A, Merkenschlager A, et al. Temporal Dynamics of MOG Antibodies in Children With Acquired Demyelinating Syndrome. Neurol Neuroimmunol Neuroinflammation. 2022;9:e200035. 10.1212/NXI.0000000000200035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Budhram A, Kunchok A, Flanagan E. Adding FUEL to the FLAMES: FLAIR-variable Unilateral Enhancement of the Leptomeninges (FUEL) in MOG-IgG-Associated Disease (862). Neurology. 2020;94:862. 15_supplement32321765 10.1212/WNL.94.15_supplement.862 [DOI] [Google Scholar]
- 134.Wong YYM, Hacohen Y, Armangue T, Wassmer E, Verhelst H, Hemingway C, et al. Paediatric acute disseminated encephalomyelitis followed by optic neuritis: disease course, treatment response and outcome. Eur J Neurol. 2018;25:782–6. 10.1111/ene.13602 [DOI] [PubMed] [Google Scholar]
- 135.Ketelslegers IA, Catsman-Berrevoets CE, Neuteboom RF, Boon M, van Dijk KG, Eikelenboom MJ, et al. Incidence of acquired demyelinating syndromes of the CNS in Dutch children: a nationwide study. J Neurol. 2012;259:1929–35. 10.1007/s00415-012-6441-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen B, Gomez-Figueroa E, Redenbaugh V, Francis A, Satukijchai C, Wu Y, et al. Do Early Relapses Predict the Risk of Long-Term Relapsing Disease in an Adult and Paediatric Cohort with MOGAD? Ann Neurol. 2023;94:508–17. 10.1002/ana.26731 [DOI] [PubMed] [Google Scholar]
- 137.Cobo-Calvo Á, Sepúlveda M, Bernard-Valnet R, Ruiz A, Brassat D, Martínez-Yélamos S, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: Clinical and prognostic implications. Mult Scler. 2016;22:312–9. 10.1177/1352458515591071 [DOI] [PubMed] [Google Scholar]
- 138.Sepúlveda M, Armangue T, Martinez-Hernandez E, Arrambide G, Sola-Valls N, Sabater L, et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016;263:1349–60. 10.1007/s00415-016-8147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akaishi T, Misu T, Takahashi T, Takai Y, Nishiyama S, Fujimori J, et al. Progression pattern of neurological disability with respect to clinical attacks in anti-MOG antibody-associated disorders. J Neuroimmunol. 2021;351:577467. 10.1016/j.jneuroim.2020.577467 [DOI] [PubMed] [Google Scholar]
- 140.Hyun JW, Woodhall MR, Kim SH, Jeong IH, Kong B, Kim G, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88:811–7. 10.1136/jnnp-2017-315998 [DOI] [PubMed] [Google Scholar]
- 141.Liyanage G, Trewin BP, Lopez JA, Andersen J, Tea F, Merheb V, et al. The MOG antibody non-P42 epitope is predictive of a relapsing course in MOG antibody-associated disease. J Neurol Neurosurg Psychiatry. 2024. 10.1136/jnnp-2023-332851. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 142.Whittam DH, Karthikeayan V, Gibbons E, Kneen R, Chanatre S, Ciccarelli O, et al. Treatment of MOG antibody associated disorders: results of an international survey. J Neurol. 2020;267:3565–77. 10.1007/s00415-020-10026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326:581–8. 10.1056/NEJM199202273260901 [DOI] [PubMed] [Google Scholar]
- 144.Chen JJ, Tobin WO, Majed M, Jitprapaikulsan J, Fryer JP, Leavitt JA, et al. Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4-IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol. 2018;136:419–22. 10.1001/jamaophthalmol.2017.6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nosadini M, Eyre M, Giacomini T, Valeriani M, Della Corte M, Praticò AD, et al. Early Immunotherapy and Longer Corticosteroid Treatment Are Associated With Lower Risk of Relapsing Disease Course in Pediatric MOGAD. Neurol Neuroimmunol Neuroinflamm. 2023;10:e200065. [DOI] [PMC free article] [PubMed]
- 146.Stiebel-Kalish H, Hellmann MA, Mimouni M, Paul F, Bialer O, Bach M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflammation. 2019;6:e572. 10.1212/NXI.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rode J, Pique J, Maarouf A, Ayrignac X, Bourre B, Ciron J, et al. Time to steroids impacts visual outcome of optic neuritis in MOGAD. J Neurol Neurosurg Psychiatry. 2023;94:309–13. 10.1136/jnnp-2022-330360 [DOI] [PubMed] [Google Scholar]
- 148.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma Clin Immunol. 2013;9:30. 10.1186/1710-1492-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen JJ, Huda S, Hacohen Y, Levy M, Lotan I, Wilf-Yarkoni A, et al. Association of Maintenance Intravenous Immunoglobulin With Prevention of Relapse in Adult Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. JAMA Neurol. 2022;79:518–25. 10.1001/jamaneurol.2022.0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS, Pardo S, Gornall M, Cicconi S, et al. Treatment of MOG-IgG-associated disorder with rituximab: An international study of 121 patients. Mult Scler Relat Disord. 2020;44:102251. 10.1016/j.msard.2020.102251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Durozard P, Rico A, Boutiere C, Maarouf A, Lacroix R, Cointe S, et al. Comparison of the Response to Rituximab between Myelin Oligodendrocyte Glycoprotein and Aquaporin-4 Antibody Diseases. Ann Neurol. 2020;87:256. 10.1002/ana.25648 [DOI] [PubMed] [Google Scholar]
- 152.Spagni G, Sun B, Monte G, Sechi E, Iorio R, Evoli A, et al. Efficacy and safety of rituximab in myelin oligodendrocyte glycoprotein antibody-associated disorders compared with neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2023;94:62–9. 10.1136/jnnp-2022-330086 [DOI] [PubMed] [Google Scholar]
- 153.Seneviratne SO, Marriott M, Ramanathan S, Yeh W, Brilot-Turville F, Butzkueven H, et al. Failure of alemtuzumab therapy in three patients with MOG antibody associated disease. BMC Neurol. 2022;22:84. 10.1186/s12883-022-02612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this is an invited review, there is no original data. All sources have been referenced.